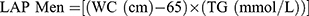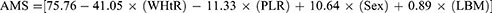Back to Journals » Clinical Interventions in Aging » Volume 18
Predictive Models of Muscle Strength in Older People with Type 2 Diabetes Mellitus
Authors Leite MM , de Sousa Neto IV , Dutra MT , Funghetto SS, de Oliveira Silva A , da Silva ICR , Ramos de Lima L , Morato Stival M
Received 29 March 2023
Accepted for publication 3 August 2023
Published 14 September 2023 Volume 2023:18 Pages 1535—1546
DOI https://doi.org/10.2147/CIA.S414620
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Maddalena Illario
Mateus Medeiros Leite,1 Ivo Vieira de Sousa Neto,2 Maurílio Tiradentes Dutra,3 Silvana Schwerz Funghetto,1 Alessandro de Oliveira Silva,4 Izabel Cristina Rodrigues da Silva,1 Luciano Ramos de Lima,5 Marina Morato Stival1
1Graduate Program in Health Sciences and Technologies, University of Brasilia, Faculty of Ceilândia, Brasilia, Brazil; 2School of Physical Education and Sport of Ribeirão Preto, University of São Paulo (USP), São Paulo, Brazil; 3Federal Institute of Education, Science and Technology of Brasília, Brasilia, Brazil; 4Physical Education Department, University Center of Brasilia - UniCEUB, Brasilia, Brazil; 5Nursing Course, University of Brasilia, Faculty of Ceilândia, Brasilia, Brazil
Correspondence: Mateus Medeiros Leite, University of Brasilia, Graduate Program in Health Sciences and Technologies, Faculty of Ceilândia, Brasilia, Brasilia, Federal District, Brazil – Campus Universitario, s/n, Centro Metropolitano, Brasília, 72220-275, Brazil, Tel + 55 61 998541966, Email [email protected]
Purpose: To propose predictive models for absolute muscle strength (AMS) of elderly people with type 2 Diabetes Mellitus (DM2) in primary health care.
Patients and Methods: The cross-sectional study was conducted with 138 elderly diabetics. The AMS was measured by a JAMAR® hydraulic handgrip dynamometer, determined by the sum of both hands. The following indices were evaluated: waist-to-height ratio (WHtR), body mass index (BMI), Lipid Accumulation Product (LAP), Triglyceride/High Density Lipoprotein (TG/HDL) ratio and platelet/lymphocyte ratio (PLR). Multiple linear regression was used in the statistical analysis.
Results: The final regression model indicated 66.4% (R²=0.66) of the variation in AMS. WHtR decreased AMS by 41.1% (β = − 0.19; t = − 3.70; p < 0.001), while PLR by 11.3% (β = − 0.12; t = − 2.36; p = 0.020). Male sex increased AMS by 10.6% (β = 0.32; t = 4.16; p < 0.001), and lean mass (LM) by 0.89% (β = 0.46; t = 6.03; p < 0.001).
Conclusion: WHtR and PLR predicted a decrease, while male sex and LM predicted an increase in AMS. It is suggested that these markers be used as screening measures for variation in AMS in older adults with DM2. These results have relevant practical application in primary health care since the markers are easy to use.
Keywords: handgrip strength, obesity, diabetes mellitus, inflammation, primary health care
Introduction
Physiological changes that occur with aging are usually accompanied by multifactorial complications that make the elderly more susceptible to developing diseases such as cancer, neurodegenerative, cardiovascular, and metabolic diseases.1,2 Among these, type 2 diabetes mellitus (DM2) is of concern. Population-based studies show a variation in the prevalence of diabetes mellitus in the elderly, depending on the verification method, between 14.24% and 22.66%.3 Data from the national health survey from 2013 to 2019 point to a prevalence between 17.7% and 19.9% for elderly aged 65 to 74 and 19.5% and 21.1% for those over 75 years old.4
Several studies have demonstrated the deleterious effects of DM2 on human health in the long term, such as vision loss associated with diabetic retinopathy, chronic kidney disease, amputations, and poorer quality of life.5–7 DM2 is characterized by hyperglycemia resulting from a progressive dysfunction in insulin secretion by pancreatic β-cells, leading to insulin resistance (IR).8,9 Generally, the IR process during aging is accompanied by a metabolic disorder, which can be potentiated by a sedentary lifestyle. It was demonstrated that physical inactivity contributes to excess adiposity while decreased muscle mass and strength.8–10
Of note, changes in the musculoskeletal system, as well as other alterations that impact autonomy or the ability to perform daily living activities, are associated with DM2 in the elderly.11 The interaction of these two conditions in a bidirectional manner can lead to a high prevalence of reduced mobility, central obesity, sarcopenia,12 worse physical performance, increased falls risk and lower muscle strength (MS).13–16 Furthermore, in patients with DM2, muscle quality is significantly reduced, which can lead to incapacitation, dependence, and even death.17 In an elegant study, it was observed that MS declined by one-third more over 3 years in older patients with DM2 compared with those without diabetes after controlling covariates, such as demographics, body composition, physical activity and combined chronic diseases.18
Additionally, clinical follow-up studies have proposed new metabolic and inflammatory markers associated with disease progression. Among these markers, the Lipid Accumulation Product (LAP) and the Triglyceride/High Density Lipoprotein (TG/HDL) ratio are noteworthy, both with predictive capacity for pre-diabetes, diabetes, and visceral obesity. Accumulating reports show an association of these indices with metabolic syndrome and sarcopenia.19–23 Furthermore, elevated platelet/lymphocyte ratio (PLR), which is considered an inflammatory biomarker, has been associated with unfavorable outcomes and poorer glycemic control in patients with DM2.24,25
Evidence indicates that DM2 may influence the reduction of MS by increasing the inflammatory cytokine levels, which is commonly observed in IR. Moreover, some skeletal muscle changes typical of DM2, such as decreased type II fibers and decreased glucose uptake capacity may generate IR, in addition to the accumulation of senescent cells and decreased autophagy capacity and enzyme activity.10,26,27 Despite these DM2 alterations may contribute to the decline in MS, reduced MS is not completely explained by the biological markers. The care for elderly with DM2 must be integrated and holistic, being essential a multidimensional approach for better understanding of disease-related complications. Thus, Handgrip strength test is strong and independent predictor of short-term mortality, functional impairment, chronic disease incidence such as DM2, and polypharmacy.28
Addressing the multiple predictors of MS in DM2 has significant functional implications for clinical outcomes. However, the intrinsic and extrinsic factors that negatively affect muscle strength in the elderly with DM2 have not yet been fully investigated. To the best of our knowledge, there are no studies in the literature in which muscle strength, anthropometric, metabolic, and inflammatory parameters were treated simultaneously in specific population with DM2. Since the impact of diabetes on neuromuscular function has been related to the co-existence of long-term complications, our findings may help health professionals in designing interventions targeted at MS, besides providing meaningful and realistic goals to progress geriatric management. Better understand the predictors of MS could offer fascinating insights for practical diagnostic and treatment guidance related to health status in DM2. Hence, this study aimed to propose predictive models for absolute MS in the elderly, besides analyzing the relationship between body composition, anthropometric, metabolic, and inflammatory indices with muscle strength in the elderly with DM2.
Materials and Methods
This is a cross-sectional study conducted with elderly individuals seen at the Primary Health Care system (PHC) of the capital of Brazil. The project was approved by the Institutional Ethical Review Board (Health Department of the Federal District) under the number 1.355.211. All participants were informed about the research and signed the Informed Consent Form. All procedures followed the guidelines of the National Research Council according to the current legislation in Brazil (Resolution No. 46/2012) and are in agreement with the Declaration of Helsinki.
Participants and Study Design
To calculate the sample size, a confidence level of 95%, a statistical error of 5% and the total number of older adults enrolled in the diabetic follow-up group of the PHC (N = 300) was adopted. The sampling was random, by means of a conventional drawing carried out according to the number of people enrolled in the group. The sample was initially composed of 152 elderly subjects of both genders who met the following inclusion criteria: age ≥60 years; being able to verbalize and answer the proposed questions (assessed by the Mini Mental State Examination (MMSE));29 have been diagnosed with DM2 for at least one year. Study participants were invited by telephone calls as registered in the PHC diabetic follow-up group.
Fourteen subjects were excluded due to the following reasons: presence of inflammatory, rheumatic, or autoimmune diseases that made it impossible to perform the procedures; wheelchair users; amputees or any other physical disability that prevented them from performing the muscle strength tests and/or the physical performance test; and those who did not comply with the study steps. The cutoff scores used for exclusion in the MMSE were as follows: 17 for illiterates; 22 for seniors with 1 to 4 years of schooling; 24 for those with between 5 and 8 years of study and 26 for those with 9 or more years of study.29
Thus, 138 participants completed the study. Data collection occurred from June to August 2019 in two moments. The first moment was at the university where participants underwent questionnaires, anthropometrics, and Dual-energy X-ray Absorptiometry (DEXA) measurements. In the second moment, at the PHC, blood samples were collected, and the muscle strength test was performed.
Procedures, Tests, and Measurements
Initially, a structured questionnaire was applied to verify the demographic and clinical data of the participants, as well as life habits. Changes in sleep patterns were reported by the elderly as normal sleep or difficulty sleeping.30 The level of physical activity was assessed by the International Physical Activity Questionnaire (IPAq) short version.31 Blood pressure (BP) was measured following the criteria established by the Brazilian Guideline on Hypertension.32
Anthropometric characteristics were measured by body mass, height, and waist circumference (WC). Central obesity was considered when WC measurements were ≥88 cm (women) and ≥102 cm (men).33 Fat Mass (FM), lean body mass (LBM), fat-free mass (FFM) and the percentage of body fat (%BF) were determined using DEXA (General Electric-GE, 8548 BX1L, 2005, type Lunar DPX-Encore 2005).34 The %BF ≥38% (women) and ≥25% (men) were considered to be high.35
Subsequently, participants were scheduled to attend the PHC for blood collection after fasting for 12 hours. A biomedical researcher from the research group collected 15 mL of blood using the venipuncture method, preferably in the antecubital fossa. The following metabolic markers were evaluated: glycated hemoglobin (HbA1c), glycaemia, total cholesterol, triglycerides, high-density lipoprotein (HDL-C), and low-density lipoprotein (LDL). Inflammatory markers evaluated were Tumor Necrosis Factor-alpha (TNF-α), Interleukin-6 (IL-6), and Interleukin-10 (IL-10). Cytokines were evaluated by Enzyme-Linked Immunosorbent Assay (ELISA) according to the specifications of the high-sensitivity R&D Systems Quantikine kit. The coefficient of variation (CV) and intra-assay sensitivity were determined. Measurements were performed in triplicate with mean values being reported, as previously described.36 For hematological parameters, platelet and lymphocyte counts were determined using a MICROS ABX analyzer according to the manufacturer’s protocol.37
Anthropometric, Metabolic, and Inflammatory Indices
The body mass index (BMI) and the waist-to-height ratio (WHtR) were used as anthropometric indices based on the following equations:
The following metabolic indices were adopted:
The Lipid Accumulation Product (LAP) calculated using sex-specific formulas with TG expressed in mmol/L and the appropriate conversions made40 as well as the Triglyceride to high-density lipoprotein ratio (TG/HDL) were adopted as metabolic indices. They were calculated according to the following equations:
As an inflammatory index, we used the platelet/lymphocyte ratio (PLR) with Logarithm transformed (log-trans), calculated using the equation:
Absolute Muscle Strength
Strength was measured using a calibrated JAMAR® hydraulic dynamometer. In the sitting position and holding the dynamometer in a 90-degree elbow flexion, the participants did three measurements in each hand, with a one-minute interval between each measurement. The maximum readings were recorded. Verbal encouragement was provided during measurements, and absolute handgrip strength (AMS) was the sum of the maximum readings from both hands.44
Statistical Analysis
All analyses were performed using the Statistical Package for Social Sciences (IBM SPSS, IBM Corporation, Armonk, NY, USA, 25.0). The Kolmogorov–Smirnov Test was used to verify data distribution. Descriptive analysis was used to present the data as minimum and maximum values, “mean ± standard deviation” (when parametric), “median and interquartile range-IQR” (when nonparametric) or “percentage of total” (when categorical). Comparisons were made with Student’s t-test for parametric variables and the Mann–Whitney test for non-parametric variables.
Subsequently, a multiple linear regression analysis was performed considering the following prerequisites: minimum number of 20 subjects per independent variable, normally distributed residuals, absence of outliers, absence of multicollinearity, linear relationship between the independent variables and the dependent variable, and presence of homogeneity. The initial model included ten variables and the Stepwise Backward method was used in the subsequent models. From the initial model, the variables that contributed less to the prediction of AMS were removed, according to the p-value. The final model presented the unstandardized coefficients (NSC), regression coefficient (β), t-test and p-value, which identifies the contribution of each variable in the model prediction. The multiple determination coefficients (R2) of the models were presented. Significance was set at p < 0.05.
Results
We assessed 138 elderly subjects (66.7% women) with a mean age of 68.3 ± 6.2 years. Based on BMI, most of the sample (64.5%) presented obesity, with a prevalence of 67.4% in women and 58.7% in men. Moreover, 84.1% of the sample presented high %BF, while 65.2% presented central obesity with increased WC in 82.6% of women and 30.4% of men. Most of the study volunteers had systemic arterial hypertension (SAH) (84.8%), more than 10 years of DM2 diagnosis (54.4%), no insulin use (73.9%), and sleep pattern changes (51.5%). According to IPAq, 57.3% of the sample was considered irregularly active or sedentary (59.8% of women and 52.2% of men).
Women presented lower body mass, height, LBM and FFM (p < 0.001), as well as lower TG/HDL ratio (p = 0.016) compared to men. On the other hand, higher values of %BF and FM (p < 0.001), WHtR (p = 0.025), HDL (p < 0.001), and IL-6 was observed in women (p = 0.011). The mean AMS in the sample was 46.7 ± 15.5 (KgF), being lower in females (p < 0.001), as shown in Table 1.
 |
Table 1 Baseline Characteristics of the Sample According to Sex (n = 138) |
Lower AMS was observed in participants older than 70 years (p = 0.049), with central obesity (p < 0.001), with SAH (p = 0.010), with altered sleep (p = 0.015), and irregularly active/sedentary (p = 0.017). When divided by sex, lower AMS was found in irregularly active/sedentary women (p = 0.035), as shown in Table 2.
 |
Table 2 Comparison of AMS According to the Clinical Characterization and Physical Activity (n = 138) |
In the multiple linear regression analysis, seven predictive models of AMS were generated with the p-values of the variables included in each model according to the regression coefficients (β) of the variables (Table 3).
 |
Table 3 Presentation of the p-value of the Predictive Variables Included in the Predictive Models of AMS of the Sample |
The model for predicting AMS showed a coefficient of determination R² = 0.664, indicating that 66% of the variation of the AMS in the elderly can be explained by the determination and the variables in the model. The absence of multicollinearity was verified among the predictor variables, with standardized residuals within normal range. The negative regression coefficients indicated a decrease in AMS. Thus, WHtR decreases AMS by 41.1% (β = −0.193; t = −3.702; p < 0.001), while PLR decreases it by 11.3% (β = −0.121; t = −2.362; p = 0.020). Male sex, on the other hand, increased AMS by 10.6% (β = 0.326; t = 4.158; p < 0.001), and LBM increased it by 0.89% (β = 0.466; t = 6.029; p < 0.001) (Table 4).
 |
Table 4 Regression Coefficients of the Predictive Variables of AMS of the Elderly Included in the Final Model |
Thus, the regression function for Model 7 is presented:
Discussion
The aim of the present study was to analyze the relationship of body composition, anthropometric, metabolic and inflammatory indices with MS in elderly people with DM2. The initial hypothesis of the study was partially confirmed, since the final prediction model pointed to WHtR, PLR and LBM as predictor variables of AMS. Regarding body composition, measurements, and anthropometric indices, it was found that men had higher body mass, height, LBM, and FFM, whereas women had higher %BF, FM, and WHtR. Collectively, our findings support the idea that MS decline in DM2 can be explained by multifactorial causes.
Our results are similar to those of other studies.45,46 During aging, these changes are mainly characterized by the accumulation of abdominal and intramuscular fat, with increased pro-inflammatory status and IR.47 Such results are related to the decrease in MS taking into consideration the other substantial age-related changes, especially in mechanical and neuromuscular factors, such as reduction in the number and size of muscle fibers, especially type II, which are responsible for force production48 and increased muscle stiffness, caused by changes in the composition of muscle connective tissue.49 An early screening of MS will identify the physical disability risk, besides facilitating the planning of appropriate interventions that prevent deficits in functional capacity during aging.
On the other hand, body and intramuscular lipid accumulation, identified in %BF, LBM and WHtR measurements, explains, at least in part, the influence of obesity on the relationship between MS and LBM.50,51 Furthermore, lipid accumulation is known to be associated with IR, inflammation, and functional deficits in skeletal muscle,52,53 characteristics that were observed in the participants of this study. Since the sample was composed of diabetics, the low MS results are in line with the existing relationships between increased pro-inflammatory status and IR, with a specific increase in visceral fat in the abdomen,47 as demonstrated by the WHtR.54
It was observed that WHtR presented higher values than those observed in a study carried out with Brazilian elderly whose objective was to identify cut-off points for WHtR and identify overweight elderly individuals based on three BMI criteria, which was used as anthropometric reference. In that study, the WHtR cut points were 0.57, 0.58, and 0.59 for women, and 0.56, 0.58, and 0.59 for men, which shows, therefore, a high WHtR in the sample of the present study.55 The high WHtR values found in the present study are associated with the high prevalence of obesity observed, similarly to the results of previous studies carried out in the PHC in the capital of Brazil.56,57
The relation of obesity with decreased MS can be explained, in part, by the increase in adipose tissue and its metabolic alterations, causing tissue fibrosis, hypoxia, and necrosis of adipocytes, which elevates chronic inflammation and IR.58 In a recent meta-analysis, involving 16,800 individuals with DM2 observed that elder age, male gender, and chronic hyperglycemia were predictors for sarcopenia, whereas patients with lower BMI were not prone to get sarcopenia,59 which suggests that obesity is often the most important risk factor in the pathological progression and DM2 development. Also, it is associated with metabolic abnormalities that result in IR and a progressive decrease in MS.60 Thus, the relationship between predictive factors related to obesity must be established to serve as a guideline for clinical protocols that utilized MS.
In the present study, the high values of fasting glucose and biochemical and metabolic markers observed were expected, since it is a sample composed of diabetics and most of them were overweight. Individuals with DM2 usually present dyslipidemia with changes in biochemical and metabolic markers, such as increased triglycerides and LDL and low HDL levels, which increases blood glucose and generates IR.9,61 Some metabolic indices have been shown to be associated with DM2 and poor glycemic control.21 These indices may be related to low MS, given that IR present in DM2 induces an additional accumulation of extracellular matrix, which is related to increased muscle stiffness and impaired muscle functions, especially low strength.62
In the analysis of inflammatory parameters, higher levels of IL-6 were observed in women compared to men. It is known that in the elderly, changes in the immune system and inflammation response are influenced by factors such as gender, changes in hormone concentrations, age, health status, and drug treatments.63 IL-6 is a cytokine characterized especially by its pro-inflammatory action that may be related to IR, which promotes the accumulation of M1 macrophages and stimulates inflammation, being directly linked to the decrease in MS.64,65 In the initial prediction models of strength that were used, IL-6 was included, although it did not show statistical significance. For this reason, it was excluded in the final model.
The volunteers who reported altered sleep had lower AMS. Sleep disorders are related to a lower level of physical activity,66,67 thus corroborating the present study, which observed lower AMS in the irregularly active/sedentary elderly. Noteworthy, insufficient physical activity is a known factor for lower MS.68 In this context, physical inactivity in diabetic elderly may be associated with sleep disorders and physical, emotional, and cognitive changes, especially physical and cognitive disability, as well as depressive symptoms.69 All these factors are related to the high prevalence of altered sleep observed in this study. In addition, physical inactivity contributes to the loss of lean body mass, increased adipose tissue and obesity, a fact verified in most of the participants in this study, since most of them were physically inactive and had a high body fat percentage.
Regarding the relationship of TG/HDL with MS, there are still no consistent results in the literature, as observed in studies that look for a relationship between TG/HDL and sarcopenia, presenting conflicting findings. In a study with 879 elderly from Korea, it was concluded that TG/HDL was positively associated with a higher risk of sarcopenia.70 In a study that stratified the participants by quartiles of TG/HDL, it was observed that sarcopenia decreased with increasing TG/HDL ratio, while those classified with higher TG/HDL were mostly male and had higher LBM.23
The differences found in these results depend on other factors such as genetics, lifestyle, and different populations, which contribute to interindividual variations in serum cholesterol and TG levels. In addition, the sarcopenia criterion adopted must be considered. Above all, the use of the TG/HDL ratio is an easy-to-apply method which has become a measure of insulin resistance used,21 where insulin resistance, characterized by a higher TG/HDL ratio, can be significantly associated with an increased risk of sarcopenia in the elderly.70 However, further studies are needed to elucidate this relationship, to conclude whether the TG/HDL ratio is a risk factor actively involved in the development of sarcopenia or just a casual relationship.
In the present investigation, the predictive model of AMS showed R²=0.664, in which the variation of AMS was explained by the variables WHtR, PLR, Sex and LBM. It is emphasized that WHtR and PLR predicted the decrease in AMS, while male sex and LBM predicted increased AMS. In a cohort of geriatric outpatients, no influence of WHtR on MS was found. However, the same study indicated worse physical performance associated with higher WHtR values.71 Therefore, WHtR is an effective measure of central obesity in predicting risk factors for cardiovascular disease, DM, and high metabolic risk.72,73 Understanding the predictors of AMS is relevant to assessing the need for assistance, care, and support for the elderly. Also, it may have greater relevance in self-rated health, mainly when they are matched with other persons of the same age.
It is believed that the high influence of WHtR on the variation of AMS in the present study is due to its association with metabolic syndrome (METS). Although the classification of METS was not performed, most of the sample in the present study presented components that are characteristic of the syndrome, such as DM, high prevalence of SAH, high WC, increased glycemia and TG.74 It is known that hyperglycemia and systemic inflammation, mechanisms associated with METS, are related to muscle fiber composition, insulin sensitivity, mitochondrial function and MS.75 A study of 1244 elderly Koreans found that relative MS correlated negatively with metabolic parameters, including components of METS. The study further suggests that maintaining a high level of MS may reduce the incidence of METS.76
In the final prediction model, PLR was able to predict AMS. This variable has been suggested as an indicator of systemic inflammation.77 Platelets secrete inflammatory substances and interact with various cell types that can initiate or exacerbate arterial wall inflammation. In addition, a study of 3671 elderly people concluded that elevated PLR was significantly associated with sarcopenia.78 In addition, PLR has been related to other disorders affecting the elderly, such as DM2, atherosclerosis, high visceral fat, and osteoporosis. In this sense, PLR is considered an easy-to-use marker, since it can be determined from routine tests in PHC.78,79 It should be considered that obesity and DM elevate this marker. Overweight individuals have higher lymphocyte counts, confirming the positive correlation between lymphocyte counts and BMI.80 Furthermore, PLR is an independent predictor for METS occurrence.81
Another significant variable in the prediction equation was LBM. Although the relationship between LBM and MS is well established in the literature, in this study, LBM explained only 0.89% of the AMS. This result may be related to the obesity profile identified in the sample, since LBM better predicts MS in non-obese elderly, indicating that obesity classification plays an important role in the relationship between MS and LBM.50 Furthermore, men generally have higher LBM compared to women, which explains lower AMS in women compared to men, as found in previous studies.44,82 Thus, in the present study, male gender was a predictor variable of increased AMS. This finding is related to the factors that decrease MS in women, such as a greater tendency to accumulate fat and changes in the type of body fat distribution in women, especially after menopause, when hormone levels and muscle protein synthesis decrease, while inflammation and catabolic pathways increase.83,84
The results of the present study demonstrate that the significant variables in predicting AMS in older people are related to the obesity profile. Furthermore, in order to develop strategies to promote the health, the determinants of obesity, such as age and gender, should be considered. Thus, due to the impact of MS reduction and its complications in the lives of diabetic people, the findings of this study have the potential to contribute to clinical practice in a multi-professional, simple and economical way and can be used in the definition of therapeutic strategies and early recognition of predictors of both the increase and decline of MS. Concerning practical applications, elderly with low muscle performance should be encouraged to practice exercise programs to improve their physical condition, including muscle strength and hypertrophy.
Considering the growing population of the elderly and how diabetes can affect their quality of life, the screening factors that influence muscle weakness can assist in the preservation of maximum functional capacity and protect against disease complications, besides reduction of care expenses and bringing back their overall health. Concerning practical applications, the older people with type 2 diabetes mellitus with low MS may be offered guidance for reducing potential consequences of this response or increasing benefits through resistance training by changing the prescription according to their individual values of MS.
This study has some limitations. Because this was a cross-sectional study, it was not possible to establish a cause-and-effect relationship between the clinical variables and outcomes. Moreover, the sample size did not allow the inclusion of more variables in the multivariate linear regression model. Another relevant factor concerns sample selection. Since these were individuals with DM2, most of the participants presented dyslipidemia, obesity, inflammation, and higher risk of developing METS. Thus, it was possible to identify only a few differences for the anthropometric, metabolic, and inflammatory markers analyzed. In addition, the use of other means substituted for DXA is suggested, provided they are validated, for measuring LBM.
Conclusion
In summary, the main factors of AMS variation in the elderly are related to obesity and sedentary lifestyle. WHtR and PLR predicted a decrease, whereas male gender and LBM predicted an increase in AMS. These markers are easy to use as screening measures of AMS variation in elderly people with DM2. Hence, our findings may benefit professional practice in PHC, by suggesting the use of these markers for early identification and management of the variables related to MS decline in the elderly.
Acknowledgments
We would like to offer thanks to all people who accepted to participate in this study. We also thank the Foundation for Research Support of the Federal District (FAP-DF) and National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), and the University of Brasilia which have supported this research.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lublóy Á. Medical crowdfunding in a healthcare system with universal coverage: an exploratory study. BMC Public Health. 2020;20(1):1672. doi:10.1186/s12889-020-09693-3
2. Li Z, Zhang Z, Ren Y, et al. Aging and age‐related diseases: from mechanisms to therapeutic strategies. Biogerontology. 2021;22(2):165–187. doi:10.1007/s10522-021-09910-5
3. Malta DC, Duncan BB, Schmidt MI, et al. Prevalence of diabetes mellitus as determined by glycated hemoglobin in the Brazilian adult population, National Health Survey. Rev Bras Epidemiol. 2019;22(suppl 2):E190006–SUPL. doi:10.1590/1980-549720190006.supl.2
4. Brazilian Institute of Geography and Statistics.. National health survey: 2019: perception of health status, lifestyles, chronic diseases and oral health: Brazil and major regions. IBGE, Work and Income Coordination; 2020. Available from: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101764.pdf.
5. Teo ZL, Tham YC, Yu M, et al. Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology. 2021;128:1580–1591. doi:10.1016/j.ophtha.2021.04.027
6. Lima LR, Stival MM, Funghetto SS, et al. Lower quality of life, lower limb pain with neuropathic characteristics, female sex, and ineffective metabolic control are predictors of depressive symptoms in patients with type 2 diabetes mellitus treated in primary care. Int J Diabetes Dev Ctries. 2019;39(3):463–470. doi:10.1007/s13410-018-0667-5
7. Gurney JK, Stanley J, York S, Rosenbaum D, Sarfati D. Risk of lower limb amputation in a national prevalent cohort of patients with diabetes. Diabetologia. 2018;61(3):626–635. doi:10.1007/s00125-017-4488-8
8. International Diabetes Federation. Diabetes Atlas.
9. American Diabetes Association. 2. classification and diagnosis of diabetes: standards of medical care in diabetes—2021. Diabet Care. 2021. doi:10.2337/dc21-S002
10. Shou J, Chen PJ, Xiao WH. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol Metab Syndr. 2020;12(1):14. doi:10.1186/s13098-020-0523-x
11. Mesinovic J, Zengin A, De Courten B, Ebeling PR, Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabet Metab Syndr Obes Targets Ther. 2019;12:1057–1072. doi:10.2147/DMSO.S186600
12. Carvalho DHT, Scholes S, Santos JLF, de Oliveira C, Alexandre T. Does abdominal obesity accelerate muscle strength decline in older adults? Evidence from the English longitudinal study of ageing. J Gerontol Ser A. 2019;74(7):1105–1111. doi:10.1093/gerona/gly178
13. Manda CM, Hokimoto T, Okura T, Isoda H, Shimano H, Wagatsuma Y. Handgrip strength predicts new prediabetes cases among adults: a prospective cohort study. Prevent Med Rep. 2020;17. doi:10.1016/j.pmedr.2020.101056
14. Koo BK. The differential association between muscle strength and diabetes mellitus according to the presence or absence of obesity. J Obes Metab Syndr. 2019;28(1):46–52. doi:10.7570/jomes.2019.28.1.46
15. Lombardo M, Padua E, Campoli F, et al. Relative handgrip strength is inversely associated with the presence of type 2 diabetes in overweight elderly women with varying nutritional status. Acta Diabetol. 2021;58(1):25–32. doi:10.1007/s00592-020-01588-4
16. Paiva FTF, Stival MM, Lima LR, et al. Predictive factors for reduced functional mobility in elderly diabetics and non-diabetics. Int J Diabetes Dev Ctries. 2021;41(2):314–321. doi:10.1007/s13410-020-00873-w
17. Takahashi F, Hashimoto Y, Kaji A, et al. Sarcopenia is associated with a risk of mortality in people with type 2 diabetes mellitus. Front Endocrinol. 2021;12:1510. doi:10.3389/fendo.2021.783363
18. Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes. Diabet Care. 2007;30(6):1507–1512. doi:10.2337/dc06-2537
19. Nie G, Hou S, Zhang M, Peng W. High TG/HDL ratio suggests a higher risk of metabolic syndrome among an elderly Chinese population: a cross-sectional study. BMJ Open. 2021;11(3):e041519. doi:10.1136/bmjopen-2020-041519
20. Lin YA, Chen YJ, Tsao YC, et al. Relationship between obesity indices and hypertension among middle-aged and elderly populations in Taiwan: a community-based, cross-sectional study. BMJ Open. 2019;9(10):e031660. doi:10.1136/bmjopen-2019-031660
21. Leite MM, Dutra MT, da Costa MVG, et al. Comparative evaluation of inflammatory parameters and substitute insulin resistance indices in elderly women with and without type 2 diabetes mellitus. Exp Gerontol. 2021;150:111389. doi:10.1016/j.exger.2021.111389
22. Ramdas Nayak VK, Nayak KR, Vidyasagar S, R P. Predictive performance of traditional and novel lipid combined anthropometric indices to identify prediabetes. Diabet Metab Syndr Clin Res Rev. 2020;14(5):1265–1272. doi:10.1016/j.dsx.2020.06.045
23. Wang N, Chen M, Fang D. Relationship between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia occurrence rate in community-dwelling Chinese adults. Lipids Health Dis. 2020;19(1):248. doi:10.1186/s12944-020-01422-4
24. Chen W, Chen K, Xu Z, et al. Neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio predict mortality in patients with diabetic foot ulcers undergoing amputations. Diabet Metab Syndr Obes Targets Ther. 2021;14:821–829. doi:10.2147/DMSO.S284583
25. Atak B, Aktas G, Duman TT, Erkus E, Kocak MZ, Savli H. Diabetes control could through platelet-to-lymphocyte ratio in hemograms. Rev Assoc Med Bras. 2019;65(1):38–42. doi:10.1590/1806-9282.65.1.38
26. Zamboni M, Rubele S, Rossi AP. Sarcopenia and obesity. Curr Opin Clin Nutr Metab Care. 2019;22(1):13–19. doi:10.1097/MCO.0000000000000519
27. Crescioli C. Targeting age-dependent functional and metabolic decline of human skeletal muscle: the geroprotective role of exercise, myokine IL-6, and vitamin D. Int J Mol Sci. 2020;21(3):1010. doi:10.3390/ijms21031010
28. Chua KY, Lim WS, Lin X, Yuan JM, Koh WP. Handgrip strength and Timed Up-and-Go (TUG) test are predictors of short-term mortality among elderly in a population-based cohort in Singapore. J Nutr Health Aging. 2020;24(4):371–378. doi:10.1007/s12603-020-1337-0
29. Melo DM, Barbosa AJG. O uso do Mini-Exame do Estado Mental em pesquisas com idosos no Brasil: uma revisão sistemática. Cien Saude Colet. 2015;20(12):3865–3876. doi:10.1590/1413-812320152012.06032015
30. Moreno CR, Santos JLF, Lebrão ML, Ulhôa MA, Duarte YA. Problemas de sono em idosos estão associados a sexo feminino, dor e incontinência urinária. Rev Bras Epidemiol. 2018;21(suppl 2):e180018. doi:10.1590/1980-549720180018.supl.2
31. Oliveira DV, Oliveira VB, Caruzo GA, et al. The level of physical activity as an intervening factor in the cognitive state of primary care older adults. Cien Saude Colet. 2019;24(11):4163–4170. doi:10.1590/1413-812320182411.29762017
32. Vb Malachias M. Brazilian guideline of arterial hypertension: presentation. Arq Bras Cardiol. 2016;107(3 Suppl 3):1. doi:10.5935/abc.20160140
33. Brazilian Diabetes Society. Brazilian Diabetes Society Guidelines-2019–2020; 2019.
34. Lohman TG. Advances in Body Composition Assessment: Current Issues in Exercise Science Series.
35. Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72(3):694–701. doi:10.1093/ajcn/72.3.694
36. Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated Interleukin-6 and C-Reactive protein levels with mortality in the elderly. Am J Med. 1999;106(5):506–512. doi:10.1016/S0002-9343(99)00066-2
37. Abdel-Moneim A, Mahmoud B, Sultan EA, Mahmoud R. Relationship of leukocytes, platelet indices and adipocytokines in metabolic syndrome patients. Diabet Metab Syndr Clin Res Rev. 2019;13(1):874–880. doi:10.1016/j.dsx.2018.12.016
38. Lipschitz DA. Screening for nutritional status in the elderly. Prim Care Clin off Pract. 1994;21(1):55–67. doi:10.1016/S0095-4543(21)00452-8
39. Nguyen Minh Q, Nguyen Vo MH. Anthropometric indexes for predicting high blood pressure in Vietnamese adults: a cross-sectional study. Integr Blood Press Control. 2020;13:181–186. doi:10.2147/IBPC.S281996
40. Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M Screening and treatment of subclinical hypothyroidism or hyperthyroidism; 2012.
41. Demirbas N, Kutlu R. Importance of measured body fat, visceral adiposity index, and lipid accumulation product index in predicting cardiometabolic risk factors. Metab Syndr Relat Disord. 2021;19(3):174–179. doi:10.1089/met.2020.0098
42. Bilgin S, Aktas G, Zahid Kocak M, et al. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2020;23(5):923–927. doi:10.1080/13685538.2019.1632283
43. Bağcı A, Aksoy F, Baş HA. Systemic immune-inflammation index may predict the development of contrast-induced nephropathy in patients with ST-segment elevation myocardial infarction. Angiology. 2022;73(3):218–224. doi:10.1177/00033197211030053
44. Byeon JY, Lee MK, Yu MS, et al. Lower relative handgrip strength is significantly associated with a higher prevalence of the metabolic syndrome in adults. Metab Syndr Relat Disord. 2019;17(5):280–288. doi:10.1089/met.2018.0111
45. Costa MVG, de Freitas ÁCC, Leite MM, et al. Factors associated with eating habits and sedentary life style in the obese elderly. Estud. interdiscipl. envelhec. 2019;24(3):81-100.
46. Soh Y, Won CW. Sex differences in association between body composition and frailty or physical performance in community-dwelling older adults. Medicine. 2021;100(4):e24400. doi:10.1097/MD.0000000000024400
47. Ponti F, Santoro A, Mercatelli D, et al. Aging and imaging assessment of body composition: from fat to facts. Front Endocrinol. 2020;10:861. doi:10.3389/fendo.2019.00861
48. Wu R, De Vito G, Delahunt E, Ditroilo M. Age-related changes in motor function (I). Mechanical and neuromuscular factors. Int J Sports Med. 2020. doi:10.1055/a-1144-3408
49. Levi N, Papismadov N, Solomonov I, Sagi I, Krizhanovsky V. The ECM path of senescence in aging: components and modifiers. FEBS J. 2020;287(13):2636–2646. doi:10.1111/febs.15282
50. Hiol AN, von Hurst PR, Conlon CA, Mugridge O, Beck KL. Body composition associations with muscle strength in older adults living in Auckland, New Zealand. PLoS One. 2021;16(5):e0250439. doi:10.1371/journal.pone.0250439
51. Borges VS, Lima-Costa MFF, Andrade FBD. A nationwide study on prevalence and factors associated with dynapenia in older adults: ELSI-Brazil. Cad Saude Publica. 2020;36(4):e00107319. doi:10.1590/0102-311x00107319
52. Ferreira AP, Szwarcwald CL, Damacena GN. Prevalência e fatores associados da obesidade na população brasileira: estudo com dados aferidos da Pesquisa Nacional de Saúde, 2013. Rev Bras Epidemiol. 2019;22. doi:10.1590/1980-549720190024
53. Wu H, Ballantyne CM. Metabolic inflammation and insulin resistance in obesity. Circ Res. 2020;126(11):1549–1564. doi:10.1161/CIRCRESAHA.119.315896
54. Lee J, Park J. Abdominal fat ratio estimation equation by abdominal type in elderly women. Fash Text. 2021;8(1):16. doi:10.1186/s40691-020-00241-6
55. Assumpção D, Ferraz R, Borim FSA, Neri AL, Francisco PMSB. Pontos de corte da circunferência da cintura e da razão cintura/estatura para excesso de peso: estudo transversal com idosos de sete cidades brasileiras, 2008–2009 *. Epidemiol e Serviços Saúde. 2020;29(4):e2019502. doi:10.5123/S1679-49742020000300003
56. Morato Stival M, Ramos de Lima L, Cristina Rodrigues da Silva I, et al. Impact of metabolic syndrome components in high-risk cardiovascular disease development in older adults. Clin Interv Aging. 2020;15:1691–1700. doi:10.2147/CIA.S252589
57. Ribeiro IA, Lima LR, Volpe CRG, Funghetto SS, Rehem TCMSB, Stival MM. Frailty syndrome in the elderly in elderly with chronic diseases in primary care. Rev da Esc Enferm da USP. 2019;53. doi:10.1590/s1980-220x2018002603449
58. Anguita-Ruiz A, Bustos-Aibar M, Plaza-Díaz J, et al. Omics approaches in adipose tissue and skeletal muscle addressing the role of extracellular matrix in obesity and metabolic dysfunction. Int J Mol Sci. 2021;22(5):2756. doi:10.3390/ijms22052756
59. Ai Y, Xu R, Liu L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13(1):93. doi:10.1186/s13098-021-00707-7
60. Galicia-Garcia U, Benito-Vicente A, Jebari S, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. doi:10.3390/ijms21176275
61. Varbo A, Freiberg JJ, Nordestgaard BG. Remnant cholesterol and myocardial infarction in normal weight, overweight, and obese individuals from the Copenhagen General Population Study. Clin Chem. 2018;64(1):219–230. doi:10.1373/clinchem.2017.279463
62. Lee J. Associations between handgrip strength and disease-specific mortality including cancer, cardiovascular, and respiratory diseases in older adults: a meta-analysis. J Aging Phys Act. 2020;28(2):320–331. doi:10.1123/japa.2018-0348
63. Gubbels Bupp MR, Potluri T, Fink AL, Klein SL. The Confluence of Sex Hormones and Aging on Immunity. Front Immunol. 2018;9:1269. doi:10.3389/fimmu.2018.01269
64. Ferreira JP, Leal AMO, Vasilceac FA, et al. Decreased muscle strength is associated with proinflammatory cytokines but not testosterone levels in men with diabetes. Brazilian J Med Biol Res. 2018;51(9). doi:10.1590/1414-431x20187394
65. Shimobayashi M, Albert V, Woelnerhanssen B, et al. Insulin resistance causes inflammation in adipose tissue. J Clin Invest. 2018;128(4):1538–1550. doi:10.1172/JCI96139
66. Mochón-Benguigui S, Carneiro-Barrera A, Castillo MJ, Amaro-Gahete FJ. Role of physical activity and fitness on sleep in sedentary middle-aged adults: the FIT-AGEING study. Sci Rep. 2021;11(1):539. doi:10.1038/s41598-020-79355-2
67. Nakayama H, Yamada Y, Yamada K, et al. Distinct relevance of nightly sleep duration to metabolic, anthropometric, and lifestyle factors in patients with type 2 diabetes. Intern Med. 2021;60(5):681–688. doi:10.2169/internalmedicine.5078-20
68. Izquierdo M, Merchant RA, Morley JE, et al. International exercise recommendations in older adults (ICFSR): expert consensus guidelines. J Nutr Health Aging. 2021;25(7):824–853. doi:10.1007/s12603-021-1665-8
69. Cheval B, Maltagliati S, Sieber S, et al. Why are individuals with diabetes less active? The mediating role of physical, emotional, and cognitive factors. Ann Behav Med. 2021;55(9):904–917. doi:10.1093/abm/kaaa120
70. Chung TH, Kwon YJ, Shim JY, Lee YJ. Association between serum triglyceride to high-density lipoprotein cholesterol ratio and sarcopenia in elderly Korean males: the Korean National Health and Nutrition Examination Survey. Clin Chim Acta. 2016;463:165–168. doi:10.1016/j.cca.2016.10.032
71. Ling CHY, Meskers CGM, Maier AB. Can anthropometric measures be used as proxies for body composition and physical function in geriatric outpatients? Arch Gerontol Geriatr. 2021;94:104379. doi:10.1016/j.archger.2021.104379
72. Corrêa MM, Thumé E, De Oliveira ERA, Tomasi E. Performance of the waist-to-height ratio in identifying obesity and predicting non-communicable diseases in the elderly population: a systematic literature review. Arch Gerontol Geriatr. 2016;65:174–182. doi:10.1016/j.archger.2016.03.021
73. Gu Z, Li D, He H, et al. Body mass index, waist circumference, and waist-to-height ratio for prediction of multiple metabolic risk factors in Chinese elderly population. Sci Rep. 2018;8(1):385. doi:10.1038/s41598-017-18854-1
74. Costa MVG, Lima LR, Tcmsb R, Funghetto SS, Stival MM, Stival MM. Risco cardiovascular aumentado e o papel da síndrome metabólica em idosos hipertensos. Esc Anna Nery. 2021;25(1):1–8. doi:10.1590/2177-9465-ean-2020-0055
75. Richter-Stretton GL, Fenning AS, Vella RK. Skeletal muscle – a bystander or influencer of metabolic syndrome? Diabet Metab Syndr Clin Res Rev. 2020;14(5):867–875. doi:10.1016/j.dsx.2020.06.006
76. Hong S. Association of relative handgrip strength and metabolic syndrome in Korean older adults: Korea National Health and Nutrition Examination Survey VII-1. J Obes Metab Syndr. 2019;28(1):53–60. doi:10.7570/jomes.2019.28.1.53
77. Patel SM, Duchowny KA, Kiel DP, et al. Sarcopenia definition & outcomes consortium defined low grip strength in two cross‐sectional, population‐based cohorts. J Am Geriatr Soc. 2020;68(7):1438–1444. doi:10.1111/jgs.16419
78. Liaw FY, Huang CF, Chen WL, et al. Higher platelet-to-lymphocyte ratio increased the risk of sarcopenia in the community-dwelling older adults. Sci Rep. 2017;7(1):16609. doi:10.1038/s41598-017-16924-y
79. Walzik D, Joisten N, Zacher J, Zimmer P. Transferring clinically established immune inflammation markers into exercise physiology: focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur J Appl Physiol. 2021;121(7):1803–1814. doi:10.1007/s00421-021-04668-7
80. Koca TT. Does obesity cause chronic inflammation? The association between complete blood parameters with body mass index and fasting glucose. Pakistan J Med Sci. 2017;33(1):65. doi:10.12669/pjms.331.11532
81. Akboga MK, Canpolat U, Yuksel M, et al. Platelet to lymphocyte ratio as a novel indicator of inflammation is correlated with the severity of metabolic syndrome: a single center large-scale study. Platelets. 2016;27(2):178–183. doi:10.3109/09537104.2015.1064518
82. Nebuloni CC, Oliveira C, de Oliveira C, Alexandre TDS. Uncontrolled diabetes as an associated factor with dynapenia in adults aged 50 years or older: sex differences. J Gerontol Ser A. 2020;75(6):1191–1197. doi:10.1093/gerona/glz257
83. Colleluori G, Chen R, Napoli N, et al. Fat mass follows a U-shaped distribution based on estradiol levels in postmenopausal women. Front Endocrinol. 2018;9:315. doi:10.3389/fendo.2018.00315
84. Razmjou S, Abdulnour J, Bastard JP, et al. Body composition, cardiometabolic risk factors, physical activity, and inflammatory markers in premenopausal women after a 10-year follow-up: a MONET study. Menopause. 2018;25(1):89–97. doi:10.1097/GME.0000000000000951
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.