Back to Journals » Cancer Management and Research » Volume 11
Predictive And Prognostic Value Of Hepatic Steatosis In Conversion Therapy For Colorectal Liver-limited Metastases: A Propensity Score Matching Analysis
Authors Jian M , Chang W , Ren L, Liu T, Chen Y, Wei Y , Lin Q, Xu J, Qin X
Received 28 March 2019
Accepted for publication 1 September 2019
Published 11 September 2019 Volume 2019:11 Pages 8315—8326
DOI https://doi.org/10.2147/CMAR.S210185
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bilikere Dwarakanath
Mi Jian,1,* Wenju Chang,1,2,* Li Ren,1,2,* Tianyu Liu,1,* Yijiao Chen,1 Ye Wei,1,2 Qi Lin,1,2 Jianmin Xu,1,2 Xinyu Qin1,2
1Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai 200030, People’s Republic of China; 2Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive, Zhongshan Hospital, Fudan University, Shanghai 200030, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinyu Qin; Jianmin Xu
Department of General Surgery, Zhongshan Hospital, Fudan University, No. 180 Fenglin Road, Shanghai 200030, People’s Republic of China
Tel +86 21 6404 1990 Ext 3449
Fax +86 21 6403 8038
Email [email protected]; [email protected]
Purpose: To evaluate the role of hepatic steatosis (HS) in patients with synchronous colorectal liver-limited metastases (CLLMs) undergoing conversion therapy.
Patients and methods: From March 2013 to March 2017, a total of 406 patients with initially unresectable CLLMs accepted conversion therapy in multidisciplinary team (MDT). Before the implementation of conversion therapy, all patients underwent CT scan to assess the presence of hepatic steatosis and divided into the HS group (n = 124) and the non-HS group (n = 282). After using propensity score matching (PSM) to eliminate the potential confounding bias of the two groups, the conversion hepatectomy rate and long-term oncological survival in two groups were compared.
Results: After 1:1 PSM, no significant difference was observed at baseline between patients in the HS group (n = 119) and the non-HS group (n = 119). Patients in the HS group had higher conversion hepatectomy rate from MDT evaluation (31.1% vs 18.5%, P = 0.029) and actual hepatectomy rate (30.2% vs 18.5%, P = 0.030), when compared with patients in the non-HS group, respectively. In addition, the HS group achieved better progression-free survival (PFS, P = 0.047) and overall survival (OS, P = 0.035) than that of the non-HS group. Multivariate logistic analysis confirmed that pretreatment HS was an independent predictor for conversion hepatectomy rate (OR, 2.393; 95% CI, 1.463–4.315, P = 0.001), and multivariate Cox analysis revealed that HS was an independent prognostic factor for PFS (HR, 0.493, 95% CI 0.281–0.866, P = 0.014) and OS (HR, 0.559, 95% CI 0.398–0.785, P = 0.001).
Conclusion: For CLLM patients who underwent conversion therapy, hepatic steatosis could be an effective predictor for conversion hepatectomy rate and an independent prognostic factor for PFS and OS.
Keywords: colorectal cancer, liver metastases, conversion therapy, hepatic steatosis, objective response rate, survival
Introduction
Liver metastasis is the leading cause of death in patients with colorectal cancer (CRC).1 Approximately 25% of patients present with liver metastases at the time of the first diagnosis, and up to 50% will further develop recurrence in the liver during their disease course.2,3 The latest developments of systemic chemotherapies with or without molecular targeted agents have dramatically improved the tumor response rates and prognostic outcomes in patients with colorectal liver-limited metastases (CLLMs).2,4–6 However, surgical resection remains the only potentially curative therapeutic option for CLLMs.7 Highly effective chemotherapy regimens, termed as conversion therapy, help achieve radical resection for initially unresectable CLLMs. Conversion hepatectomy after oxaliplatin- or irinotecan-based systemic chemotherapy with molecular targets for initially unresectable liver metastasis has been achieved in approximately 12–54% of patients.8,9 The latest ESMO guidelines recommend that all CRC patients with potential resectable liver metastases undergo conversion therapy, and secondary resection is a chance of cure for patients with effective conversion.10 Although conversion hepatectomy achieved survival rates similar to those of patients who underwent liver resection initially, the high cost with obvious toxicity events was mentioned by previous studies.2 Therefore, how to identify patients who are more likely to benefit from conversion therapy for CLLMs is extremely important.
As demonstrated by Folprecht and colleagues, there is a strong correlation between response rates and hepatectomy rate among patients who have CLLMs.11 In the metastatic setting, tumor microenvironment of the target organ is a key factor modulating tumor cell invasion and chemotherapeutic response.12,13 Hepatic steatosis (HS) alters the component diversity of liver microenvironment, and it may affect metastases foci formation and chemotherapeutic response in patients with CLLMs. HS is reported to be 10–30% of the general population in various countries, and very common in China.14–16 Studies have demonstrated that HS may be a negative prognostic factor for the onset and progression of CLLMs, and HS was associated with lower incidence of liver metastases and improved prognosis in patients with CLLMs.17,18 However, for initially unresectable CLLM patients undergoing conversion therapy, the role of HS in efficacy of conversion therapy and prognostic outcome is still unclear.
The aim of this retrospective study was to analyze the effect of hepatic steatosis on conversion hepatectomy rate and survival in patients undergoing conversion therapy for unresectable CLLMs.
Materials And Methods
Patient Selection And Evaluations
Between March 2013 and March 2017, a total of 430 patients were diagnosed with resectable primary tumor and unresectable synchronous liver-limited metastases and accepted regularly conversion therapy under multidisciplinary team (MDT) at Zhongshan Hospital, Fudan University. The possibility of resection for tumor or metastases was assessed by a local MDT including more than three liver surgeons, one radiologist and one pathologist. Other criteria for eligibility were Eastern Cooperative Oncology Group performance status (ECOG score) of 0 to 1, life expectancy > 3 months, and adequate hematologic, hepatic, and renal function. Twenty-four patients with a past history of other cancers or splenectomy, or without pre-treatment non-enhanced computed tomography (CT) scan were excluded. A total of 406 patients were enrolled in analysis in this study.
Prior to conversion therapy, all patients were assessed by physical examination, routine hematology, carcinoembryonic antigen (CEA) levels and biochemistry analyses. CT scan or magnetic resonance image of the abdomen was done to define the extent of liver disease. Positron emission tomography CT (PET/CT) was done to rule out any extrahepatic metastasis.
Hepatic Steatosis Evaluation
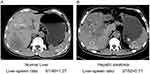
Accompanying non-enhanced CT scan was performed with the enhanced CT scan. Non-enhanced CT was performed with a tube potential of 120 kVp. Tube current was adjusted by automatic exposure control with a noise index of 10 in a slice thickness of 5 mm. All CT images were reviewed by using a picture archiving and communication system workstation (Digital Imaging and Communications in Medicine, DICOM, USA). The mean CT attenuation values (in Hounsfield Units [HU]) of the liver and the spleen were obtained using standard region of interest method (Figure 1). After measurement of the CT attenuation of the liver and spleen, liver–spleen ratio (liver attenuation (HU)/spleen attenuation (HU)) was calculated. Those patients with a liver–spleen ratio lower than 1.1 were diagnosed as HS, as previously reported.17,19 Since chemotherapy may have an effect on HS, we investigated the presence of HS in non-enhanced CT image prior to conversion therapy.
First-Line Chemotherapy Regiments And Decision Of Conversion
FOLFOX (oxaliplatin 85 mg/m2 and levofolinate calcium 200 mg/m2, followed by 5-FU, as a 400 mg/m2 intravenous bolus and 2,400 mg/m2 continuous infusion during 46 hrs) was administered every 2 weeks. CapeOX (oxaliplatin 130 mg/m2 over 2 hrs on day 1 plus oral capecitabine 1,000 mg/m2 twice daily on days 1–14) was administered every 3 weeks. FOLFIRI (150 mg/m2 irinotecan, and 200 mg/m2 levofolinate calcium, followed by 5-FU, as a 400 mg/m2 intravenous bolus and 2,400 mg/m2 continuous infusion during 46 hrs) was administered every 2 weeks. The choice of addition of cetuximab (400 mg/m2 initial infusion every 2 weeks) or bevacizumab (5mg/kg initial infusion every 2 weeks) depended on the doctors’ discretion; however, cetuximab antibodies were used only for CRC with wild-type Ras gene. Subjective symptoms, physical examination results, performance status and all adverse reactions were recorded before each treatment cycle. Treatment courses were repeated every 2 weeks for a total of four courses unless there was evidence of progressive disease.
Assessment Of Response And Adverse Events
Tumor response was measured after every 4 cycles by contrast-CT scan and magnetic resonance imaging (MRI) examination and the Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1) was applied to grade the best response in each case.20 All cases were discussed for eligibility for surgery by a multidisciplinary team including medical oncologists, radiologists and surgical oncologists. Patients with resectable disease were offered liver surgery within 2–4 weeks of the last treatment cycle. Patients who showed progression or no response were started on secondary chemotherapy. Adverse events were categorized according to the National Cancer Institute Common Toxicity Criteria, version 3.0.
Long-Term Prognosis
Overall survival (OS) was defined as the time interval between the starting date of conversion therapy to patient’s death or to last follow-up. Progression-free survival (PFS) was defined as the time interval between the starting date of conversion therapy to diagnosis of progression disease (PD) or to last follow-up. Patients who had not died at the time analysis were censored.
Statistical Analysis
The computer program "Statistical Package for The Social Sciences" version 19.0 for Windows (SPSS, Inc, Chicago, IL, USA) was used for statistical analysis, while propensity score matching (PSM) was conducted by R (https://www.r-project.org/). The propensity score model of the presence of HS was constructed via stepwise variable selection into a multivariable logistic regression model. Candidate variables included all variables significantly associated with hepatectomy rate from MDT via univariable analysis, with a threshold of P < 0.20 required for initial inclusion and P < 0.10 required to remain in the model.21 The categorical parameters were compared using two-sided Pearson’s χ2 test, Fisher’s exact test, as appropriate. Summary statistics on time-to-event variables were calculated according to the Kaplan–Meier method. Difference in prognosis was assessed by Cox regression analysis. All comparisons were two-tailed, and P < 0.05 was considered statistically significant.
Results
Baseline Characteristics In The HS Group And The Non-HS Group Before And After PSM
The clinical and pathological characteristics of the patients with HS and without HS are listed in Table 1. Before PSM, the mean age of the HS group (n = 124) was 57.5 years, while the mean age of the non-HS group (n = 282) was 57.7 years (P = 0.853). And the percentage of patients with BMI > 25 kg/m2 (36.3% vs 22.7%, P = 0.046), maximum size of CLLMs > 5cm (20.2% vs 32.6%, P = 0.038), number of CLLMs > 3 (46.0% vs 58.9%, P = 0.016), and bilobar distribution of CLLMs (50.8% vs 66.0%, P = 0.004) were statistically different in both groups. Because of the significant differences between the two groups, the application of PSM to make generate comparable groups is indispensable. After matching, the characteristics and conversion regiments were balanced between the HS group (n = 119) and the non-HS group (n = 119). The percentage of patients with BMI > 25 kg/m2 (34.5% vs 32.8%, P = 0.883), maximum size of CLLMs > 5cm (18.5% vs 18.5%, P = 1.000), number of CLLMs > 3 (46.2% vs 46.2%, P = 1.000), and bilobar distribution of CLLMs (51.3% vs 53.8%, P = 0.719) were comparable in both groups.
 |
Table 1 Baseline Variables In The HS Group And The non-HS Group |
In terms of conversion regiments, the percentage of patients undergoing systemic therapy first was comparable in both groups (31.9% vs 30.3%, P = 0.860). Furthermore, no significant differences were observed in the percentage of patients using doublet chemotherapy (FOLFOX or FOLFIRI) in first-line regiments (84.9% vs 86.6%, P = 0.860), targeted therapy (34.5% vs 34.5%, P = 1.000) or using transarterial chemoembolization (TACE, 14.3% vs 16.8%, P = 0.735). Overall, only 39 patients were treated with cetuximab in first-line regiments, although 186 patients were verified as Ras wild-type. Notably, for patients with progressive liver metastases, TACE was considered as a local treatment option. After PSM, a total of 37 patients (15.5%) underwent TACE treatment during conversion therapy period.
Responses Of Conversion Therapy Before And After PSM
The oncologic outcomes of conversion therapy of the two groups are listed in Table 2. Before PSM, objective response rate (ORR) of the HS group (n = 124) was improved than that of the non-HS group (n = 282) (36.3% vs 23.8%, P = 0.020), and the disease control rates (DCR) were comparable in two groups (74.2% vs 69.5%, P = 0.404). Following conversion therapy for every 6–8 circles, the possibility of hepatectomy was evaluated by multidisciplinary team (MDT). A total of 79 of 406 patients (19.5%) were determined to be eligible for radical hepatectomy during the implementation of conversion therapy. And the conversion hepatectomy rate of the HS group was significantly higher than that of the non-HS group (29.8% vs 14.9%, P < 0.001). Actually, three patients (one in the HS group, and two in the non-HS group) refused further surgical intervention. In another one patient in the HS group, radical resection could not be obtained at exploration. Ultimately, 35 patients in the HS group and 40 patients (28.2% vs 14.1%, P < 0.001) in the non-HS group achieved an actually radical resection.
 |
Table 2 Response And Safety Events Of Conversion Therapy In The HS Group And The non-HS Group |
After PSM, both ORR (44.5% vs 32.8%, P = 0.088) and DCR (76.5% vs 73.9%, P = 0.742) were comparable between the HS group (n = 119) and the non-HS group (n = 119). Notably, conversion hepatectomy rate (31.1% vs 18.5%, P = 0.029) and actual hepatectomy rate (29.4% vs 18.5%, P = 0.038) of the HS group were still significantly higher than that of the non-HS group.
Furthermore, after PSM, among 57 patients who underwent radical hepatectomy, forty-eight patients (20.6% of all patients) had staged resection of primary tumor and liver metastases, and 8 patients (3.4% of all patients) had simultaneous resection of primary tumor and liver metastases. There were no serious complications during the perioperative period except for mild abnormalities of liver function in 11 patients. A total of 39 patients (39/57, 68.4%) experienced relapse during follow-up; the recurrence primarily involved the hepatic remnant (29/39, 74.4%). Other sites of recurrence included lung (12/39, 23.1%) and abdomen/peritoneum (5/39, 12.8%; Table 3).
 |
Table 3 Long-term Outcomes After Hepatectomy By Hepatic Steatosis Before And After Propensity Score Matching |
For safety events, the observed toxicity was mostly mild in both groups, and no deaths were attributable to conversion therapy. Grades 3 and 4 toxicities are also listed in Table 2, with no significant differences between the two groups either before or after PSM. For patients who underwent hepatectomy, our study revealed no serious liver toxicities from conversion therapy.
Predictors For Conversion Hepatectomy
In univariate analysis, we indicated that pretreatment HS (odds ratio [OR] 2.170; 95% confidential interval [CI], 1.322–3.561; P = 0.014), maximum size of CLLMs (OR, 0.388; 95% CI, 0.192–0.786; P = 0.009), TACE (OR, 0.314; 95% CI, 0.132–0.744; P = 0.032), and targeted therapy (OR, 2.550; 95% CI, 1.574–4.131; P < 0.001) were predictors of conversion hepatectomy from MDT (Table 4). Those variables with P < 0.05 were further analyzed in multivariate logistic regression model, and we confirmed that the following variables were independent predictors for conversion hepatectomy from MDT, including pretreatment HS (OR 2.393; 95% CI, 1.384–4.139; P = 0.012), TACE (OR, 0.320; 95% CI, 0.126–0.812; P = 0.024), and targeted therapy (OR, 2.512; 95% CI, 1.463–4.315; P = 0.001; Table 4).
 |
Table 4 Predictors Of Conversion Hepatectomy Before Propensity Score Matching |
Long-Term Survival Of The HS Group And The Non-HS Group Before And After PSM
The mean follow-up was 40.6 months for the HS group and 39.7 months for the non-HS group. The median observation times were 40 months for overall survival (OS) and 15 months for progression-free survival (PFS), respectively. Before PSM, patients in the HS group experienced better survival than that of the non-HS group in terms of OS (3-year survival, 63% vs 49%; median, 41.7 vs 33.8 months; hazard ratio [HR], 0.541; 95% CI, 0.386–0.759; P < 0.001; Figure 2A) and PFS (median, 8.9 vs 6.9 months; HR, 0.719; 95% CI, 0.572–0.903; P = 0.005; Figure 2B). After PSM, patients in the HS group experienced greater survival benefit in terms of OS (3-year survival, 59% vs 41%; median, 40.8 vs 30.6 months; HR, 0.662; 95% CI, 0.451–0.971; P = 0.035; Figure 2C) and PFS (median, 8.9 vs 5.9 months; HR, 0.761; 95% CI, 0.581–0.996; P = 0.047; Figure 2D).
Prognostic Factors For Long-Term Survival
We performed univariate and multivariate Cox regression analyses to estimate the clinical significance of prognostic factors that may affect PFS or OS of the patients. By univariate analysis, pretreatment HS (HR, 0.544, 95% CI 0.317–0.936; P = 0.028), targeted therapy (HR, 0.433, 95% CI 0.262–0.717; P = 0.001) and TACE (HR, 2.010, 95% CI 1.119–3.610; P = 0.020) were statistically significant for PFS (Table 5, Table S1), while pretreatment HS (HR, 0.541, 95% CI 0.386–0.759; P <0.001), targeted therapy (HR, 0.699, 95% CI 0.517–0.946; P = 0.020) and treatment sequence (HR, 1.439, 95% CI 1.032–2.005; P = 0.032) were statistically significant for OS (Table 5, Table S2). At last, the multivariate Cox proportional hazards regression analysis confirmed that pretreatment HS (HR, 0.493, 95% CI 0.281–0.866; P = 0.014) targeted therapy (HR, 0.460, 95% CI 0.270–0.786; P = 0.005) and TACE (HR, 2.142, 95% CI 1.157–3.965; P = 0.015) were independent prognostic factors for PFS (Table 5, Table S1). For OS, pretreatment HS (HR, 0.559, 95% CI 0.398–0.785; P = 0.001) and treatment sequence (HR, 1.470, 95% CI 1.054–2.050; P = 0.023) and targeted therapy (HR, 0.695, 95% CI, 0.512–0.943; P = 0.019) served as independent prognostic factors for OS (Table 5, Table S2).
 |
Table 5 Prognostic Factors For Long-term Survival Before Propensity Score Matching |
Discussion
This study is dedicated to identify benefit patients from conversion therapy for unresectable synchronous colorectal liver-limited metastases (CLLMs). Our results demonstrated that the presence of pretreatment hepatic steatosis (HS) in patients undergoing conversion therapy was associated with improved hepatectomy rate, PFS and OS, when compared with patients without HS. In addition, pretreatment HS can serve as a valuable predictor for conversion hepatectomy and PFS in clinical practice. To the best of our knowledge, this is the first study to investigate the effect of HS on the treatment outcome and survival of conversion therapy.
Here, we analyzed the clinical and pathological characteristics of 124 patients with HS and 282 patients without HS. The baseline characteristics were significantly unbalanced. Patients with HS tended to have smaller volume, smaller number and lower proportion of bilobar distribution in CLLMs and higher BMI, which was similar to the previous studies.3,22 A smaller volume or number of CLLMs could give rise to a higher residual volume of normal liver and implicated a higher possibility of conversion hepatectomy and a longer survival.8,9 As it is widely accepted that the unbalanced baseline characteristics could result in interference of outcome, we used PSM to eliminate bias. After matching the remarkable differences of clinicopathological characteristics between the two groups, including the maximum size, the number and the distribution of CLLMs and BMI were comparable. After balance the potential bias, we found the conversion hepatectomy rate, PFS and OS were still significantly improved in patients with HS.
In terms of the higher conversion hepatectomy rate, multiple reasons have been proposed, including targeted therapy, doublet or triplet chemotherapy, cycles of chemotherapy, local treatment, the lower rate of RAS mutations, and smaller size or fewer number of CLLMs, all of which were regarded as predictors of higher radical resection rate after conversion therapy.3,7,8,23 We further use multivariate analysis to indicate that possibility of hepatectomy after conversion therapy was predominantly predicted by three biologic features: the presence of pretreatment HS, targeted therapy and use of TACE.
For conversion hepatectomy rate, pretreatment HS has greater predictive value than alternative clinicopathologic factors, like the number, the size or the distribution of liver metastases, and Ras mutation status.2,23,24 Importantly, the predictive value of HS is independent of using targeted therapy in our study. Clinical study and animal experiments reported that HS can decrease malignant metastases growth in the liver and alleviate tumor load, which depend on lipid accumulation, reduced angiogenic activity and reduced number of cancer-associated fibroblasts in the liver parenchyma.17,25,26 In general, hepatic steatosis provides an unsuitable environment for survival or development of metastatic cells.
In addition, we identify targeted therapy as another independent predictor in multivariate analysis. This was similar to previous studies, in which adding cetuximab or bevacizumab to chemotherapy was associated with facilitated radical resections of CLLMs.3,22 And TACE was also identified as another independent predictor in multivariate analysis. In our study, TACE was used to facilitate the response and palliate symptoms when CLLMs seemed to progress, and it is similar to the previously reported results.27,28 However, our results demonstrate that the number, size or distribution of liver metastases were not independent predictors for conversion hepatectomy rate, which may partially be due to the limited sample size after PSM. In addition, we did not observe the effect of Ras mutation on the outcome of conversion therapy, which may result from a relatively low rate of use of targeted therapy.
As previous reports suggested, the role of HS in patients with CRC was controversial.18,29,30 For CRC patients undergoing radical resection, the presence of HS was associated with a lower incidence of relapse of CLLMs and a longer DFS and OS than patients without HS.18 While in the development of carcinogenesis, the presence of HS was positively associated with the incidence of colorectal adenoma or adenocarcinoma.29,30 These results suggest that the presence of HS may play a different role in different developmental stages of CRC. As we know, hepatic steatosis includes simple steatosis and steatohepatitis, one with only lipid accumulation and the other one with inflammatory infiltrate plus lipid deposition. Due to the important and complicated role of inflammatory cells in cancer development and metastasis, distinguishing between these two conditions may partially explain the controversy results in the previous studies.31–34 Here, we offered more detailed data to demonstrate the relationship between HS and progression of patients with unresectable CLLMs and showed that patients with HS may have a prognostic benefit in PFS and OS than those without HS. In addition, by multivariate analysis, we confirmed that pretreatment HS was an independent prognostic factor for PFS and OS. One possible explanation is that other factors like second-line chemotherapy or change of targeted therapy may be weighted more than pretreatment HS for prolonged OS.6,8,20,23
In accordance with previous reports, our results also showed that targeted therapy served as an independent prognostic factor for both PFS and OS, while TACE, as a regular means of local treatment, was an independent prognostic factor for PFS.8,23,35 Our results also showed that patients with unresectable CLLMs would benefit from systemic therapy first mode rather than primary lesion resected mode in the overall survival, which is consistent with previous reports.36 The addition of targeted agents, like cetuximab and bevacizumab, has been reported to be associated with a favorable PFS and OS in the previous studies.3,22 And, it is also worth noting that local treatments, like TACE, are associated with poorly controlled progression of CLLMs and shorter PFS in our study, because local treatment was always used when the shrinkage of tumors was not apparent.23,29
The current study possesses some limitations. This study followed a retrospective design, considering inevitably missed information about the history of alcohol consumption. Moreover, pretreatment HS and liver metastases were mainly diagnosed via abdominal ultrasonography, CT scan, or MRI and were not confirmed by biopsy, possibly leading to false-positive diagnoses, although the imaging results were conducted and evaluated by imaging specialists.
Conclusion
In conclusion, for patients with unresectable synchronous colorectal liver-limited metastases undergoing conversion therapy, pretreatment hepatic steatosis is associated with improved hepatectomy rate, PFS and OS. In addition, pretreatment hepatic steatosis could be an effective predictor for conversion hepatectomy rate and an independent prognostic factor for PFS and OS.
Ethical approval and informed consent
The study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Each subject provided their written informed consent.
Abbreviations
HS, hepatic steatosis; non-HS, none of hepatic steatosis; CLLMs, colorectal liver-limited metastases; MDT, multidisciplinary team; ORR, objective response rate; OS, overall survival; PSM, propensity score matching; CRC, colorectal cancer; CT, computed tomography; CEA, carcinoembryonic antigen; PET/CT, positron emission tomography CT; HU, Hounsfield Units; TACE, transarterial chemoembolization; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate.
Acknowledgments
The authors wish to express their gratitude to Minzhi Lv, PhD, for statistical support and advice. This study was supported by grants from the National Natural Science Foundation of China (81602035, 81272390), the Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive (17DZ2250600) and the Shanghai Municipal Health Commission: Shanghai Outstanding Youth Specialist Training Program (Q2017-059); Clinical Science and Technology Innovation Project of Shanghai (SHDC12016104); and Shanghai Science and Technology Committee Project (17411951300).
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Engstrand J, Nilsson H, Stromberg C, et al. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. doi:10.1186/s12885-017-3925-x
2. Nozawa H, Ishihara S, Kawai K, et al. Conversion to resection in patients receiving systemic chemotherapy for unresectable and/or metastatic colorectal cancer—predictive factors and prognosis. Clin Colorectal Cancer. 2018;17:e91–e97. doi:10.1016/j.clcc.2017.10.002
3. Ye L, Liu T, Ren L, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal Liver-Limited metastases. J Clin Oncol. 2013;31:1931–1938. doi:10.1200/JCO.2012.44.8308
4. Huiskens J, van Gulik TM, van Lienden KP, et al. Treatment strategies in colorectal cancer patients with initially unresectable liver-only metastases, a study protocol of the randomised phase 3 CAIRO5 study of the Dutch Colorectal Cancer Group (DCCG). BMC Cancer. 2015;15:365. doi:10.1186/s12885-015-1584-3
5. Jawed I, Wilkerson J, Prasad V, et al. Colorectal cancer survival gains and novel treatment regimens: A systematic review and analysis. JAMA Oncol. 2015;1:787–795. doi:10.1001/jamaoncol.2015.1790
6. Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23:2038–2048. doi:10.1200/JCO.2005.00.349
7. Passot G, Soubrane O, Giuliante F, et al. Recent advances in chemotherapy and surgery for colorectal liver metastases. Liver Cancer. 2016;6:72–79. doi:10.1159/000449349
8. Shui L, Wu YS, Lin H, et al. Triplet chemotherapy (FOLFOXIRI) plus bevacizumab versus doublet chemotherapy (FOLFOX/FOLFIRI) plus bevacizumab in conversion therapy for metastatic colorectal cancer: A Meta-Analysis. Cell Physiol Biochem. 2018;48:1870–1881. doi:10.1159/000492508
9. Tomasello G, Petrelli F, Ghidini M, et al. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol. 2017;3:e170278. doi:10.1001/jamaoncol.2017.0278
10. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386–1422. doi:10.1093/annonc/mdw235
11. Folprecht G. Liver metastases in colorectal cancer. Am Soc Clin Oncol Educ Book. 2016;35:e186–e192. doi:10.1200/EDBK_159185
12. Konda B, Shum H, Rajdev L. Anti-angiogenic agents in metastatic colorectal cancer. World J Gastrointest Oncol. 2015;7:71–86. doi:10.4251/wjgo.v7.i7.71
13. Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. HEPATOLOGY. 2011;53:1192–1205. doi:10.1002/hep.24108
14. Rinella ME, Sanyal AJ. Management of NAFLD: A stage-based approach. Nat Rev Gastro Hepat. 2016;13:196–205. doi:10.1038/nrgastro.2016.3
15. Malhotra N, Beaton MD. Management of non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:2962–2967. doi:10.4254/wjh.v7.i30.2962
16. Fan JG. Epidemiology of alcoholic and nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol. 2013;28(Suppl 1):11–17. doi:10.1111/jgh.12036
17. Murono K, Kitayama J, Tsuno NH, et al. Hepatic steatosis is associated with lower incidence of liver metastasis from colorectal cancer. Int J Colorectal Dis. 2013;28:1065–1072. doi:10.1007/s00384-013-1656-2
18. Parkin E, O’Reilly DA, Adam R, et al. The effect of hepatic steatosis on survival following resection of colorectal liver metastases in patients without preoperative chemotherapy. HPB (Oxford). 2013;15(6):463–472. doi:10.1111/hpb.12007
19. Zhou X, Li Y, Zhang X, et al. Independent markers of nonalcoholic fatty liver disease in a gentrifying population-based Chinese cohort. Diabetes Metab Res Rev. 2019;35(5):e3156. doi:10.1002/dmrr.3156
20. Ma B, AD K, Leung L, et al. Identifying an early indicator of drug efficacy in patients with metastatic colorectal cancer-a prospective evaluation of circulating tumor cells, 18F-fluorodeoxyglucose positron-emission tomography and the RECIST criteria. Ann Oncol. 2017;28:1576–1581. doi:10.1093/annonc/mdx149
21. Rajyaguru DJ, Borgert AJ, Smith AL, et al. Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in non-surgically managed patients: analysis of the national cancer database. J Clin Oncol. 2018;36(6):600–608. doi:10.1200/JCO.2017.75.3228
22. Ruan WC, Che YP, Ding L, et al. Efficacy and toxicity of addition of bevacizumab to chemotherapy in patients with metastatic colorectal cancer. Comb Chem High Throughput Screen. 2018;21:718–724. doi:10.2174/1386207322666190119162352
23. Wang L, Sun Y, Zhao B, et al. Chemotherapy plus targeted drugs in conversion therapy for potentially resectable colorectal liver metastases: A meta-analysis. Oncotarget. 2016;7:55732–55740. doi:10.18632/oncotarget.9675
24. Marino D, Leone F, D’Avanzo F, et al. Potentially resectable metastatic colorectal cancer: an individualized approach to conversion therapy. Crit Rev Oncol Hematol. 2014;92:218–226. doi:10.1016/j.critrevonc.2014.05.010
25. Nakamura M, Suetsugu A, Hasegawa K, et al. Choline-deficient-diet-induced fatty liver is a metastasis-resistant microenvironment. Anticancer Res. 2017;37:3429–3434. doi:10.21873/anticanres.11710
26. Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68:280–295. doi:10.1016/j.jhep.2017.11.014
27. Gruber-Rouh T, Naguib NNN, Eichler K, et al. Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer. 2014;134(5):1225–1231. doi:10.1002/ijc.28443
28. Levy J, Zuckerman J, Garfinkle R, et al. Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB (Oxford). 2018;20(10):905–915. doi:10.1016/j.hpb.2018.04.001
29. Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017.
30. Ze EY, Kim BJ, Jun DH, et al. The fatty liver index: A simple and accurate predictor of colorectal adenoma in an average-risk population. Dis Colon Rectum. 2018;61:36–42. doi:10.1097/DCR.0000000000000973
31. Fonseca GM, de Mello ES, Faraj SF, et al. Prognostic significance of poorly differentiated clusters and tumor budding in colorectal liver metastases. J Surg Oncol. 2018;117(7):1364–1375. doi:10.1002/jso.25017
32. Shaler CR, Tun-Abraham ME, Skaro AI, et al. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol Immunother. 2017;66(12):1563–1575. doi:10.1007/s00262-017-2050-7
33. Cho Y, Lim SK, Joo SK, et al. Nonalcoholic steatohepatitis is associated with a higher risk of advanced colorectal neoplasm. Liver Int. 2019. doi:10.1111/liv.14163.
34. Halama N, Braun M. Kahlert C, et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin Cancer Res. 2011;17(4):678–689. doi:10.1158/1078-0432.CCR-10-2173
35. Wei N, Zhang B, Wang Y, et al. Transarterial chemoembolization with raltitrexed‑based or floxuridine‑based chemotherapy for unresectable colorectal cancer liver metastasis. Clin Transl Oncol. 2019;21:443–450. doi:10.1007/s12094-018-1942-0
36. Yoshino1 T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO–ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29:44–70. doi:10.1093/annonc/mdx807
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.