Back to Journals » International Journal of General Medicine » Volume 16
Prediction Score for Clinical Outcome of Chinese Patients with Cerebral Venous Thrombosis
Authors Li M, Zhang B, Xie J, Meng R, Ji X
Received 16 June 2023
Accepted for publication 4 September 2023
Published 11 September 2023 Volume 2023:16 Pages 4099—4107
DOI https://doi.org/10.2147/IJGM.S426238
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Min Li,1,2,* Binlong Zhang,3,* Jiangbo Xie,4 Ran Meng,1,2 Xunming Ji2,5
1Department of Neurology, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Beijing Institute for Brain Disorders, Capital Medical University, Beijing, People’s Republic of China; 3Department of Acupuncture, Moxibustion, and Neurology, Guang’anmen Hospital, Chinese Academy of Chinese Medical Sciences, Beijing, People’s Republic of China; 4Department of Neurology, Weifang Traditional Chinese Hospital, Weifang, Shandong, People’s Republic of China; 5Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ran Meng, Department of Neurology, Xuanwu Hospital, Capital Medical University, 45 Changchun Road, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-13693080599, Email [email protected] Xunming Ji, Department of Neurosurgery, Xuanwu Hospital, Capital Medical University, 45 Changchun Road, Xicheng District, Beijing, 100053, People’s Republic of China, Tel +86-13911077166, Email [email protected]
Background: Although numerous prognostic markers for cerebral venous thrombosis (CVT) have been reported, inconsistencies exist in their predictive values, leading to contradictory forecasts. This study was designed to develop a comprehensive clinical outcome prediction score for Chinese patients with CVT, integrating key prognostic markers to furnish an overall prognosis.
Methods: Participants were selected from the CCC cohort, a multicenter study encompassing 26 tertiary hospitals across mainland China. Between January 2021 and May 2022, 170 patients with CVT were prospectively recruited. Potential prognostic markers were extracted from the CCC database and subsequently analyzed.
Results: Age, diastolic blood pressure (DBP), neutrophil-to-lymphocyte ratio (NLR), and neuron-specific enolase (NSE) emerged as significant prognostic markers for CVT after a multivariate logistic analysis. Specific cut-off values were identified: Age > 27.5 years, DBP > 79.5 mmHg, NLR > 6.6, and NSE > 16.5 ng/mL. The points assigned were: one each for age and NSE level, two for DBP, and three for NLR, based on the adjusted odds ratio. A positive correlation was found between the baseline CVT outcome score and the mRS at six months’ follow-up. A CVT outcome score of 3.5 served as an effective cut-off value for predicting CVT clinical outcomes. Further analysis revealed that patients with CVT outcome scores > 3 exhibited significantly higher mRS scores than those with scores ≤ 3.
Conclusion: This study led to the development of the CVT outcome score, consisting of age, DBP, NLR, and NSE level, specifically for Chinese patients with CVT. The baseline CVT outcome score positively correlated with the mRS score at the six-month follow-up. A CVT outcome score of > 3 serves as a reliable indicator to identify patients at a higher risk of unfavorable clinical outcomes. These patients may benefit from additional care and early interventions to avert potential deterioration.
Keywords: clinical outcome, prognosis, cerebral venous thrombosis, prediction score, validation
Introduction
Cerebral venous thrombosis (CVT) accounts for 0.5–1% of all stroke cases, exhibiting an incidence rate of 1.3 to 1.6 per 100,000 population.1,2 With the recent advancements in neuroimaging, the diagnosis of CVT has achieved greater accuracy despite the diverse clinical manifestations observed in patients.2–4 Nevertheless, reports indicate a significant proportion of CVT patients experience poor clinical outcomes and elevated mortality rates.5,6 For instance, a multicenter study by Ferro et al5 found that 13.4% of CVT patients across 21 Western countries encountered poor clinical outcomes, with 8.3% succumbing to the condition. Similar findings were reported by Wasay et al6 for nine Asian countries and in Germany where 56.1% of severe CVT patients had unfavorable outcomes and 34.2% died.7 These observations underscore the urgent need for a predictive method for the clinical outcomes of CVT.
Prior research, including our previous studies, has shown that the neutrophil-to-lymphocyte ratio (NLR), neuron-specific enolase (NSE), and diastolic blood pressure (DBP) can be significant predictors of CVT outcomes.8–10 Although other studies has identified a range of prognostic markers for CVT,5,11,12 there is still uncertainty as to whether these markers might yield contradictory results. Consequently, the development of a CVT outcome score based on the identified prognostic markers may offer enhanced predictive capabilities for the clinical outcome of CVT.
Previously reported CVT outcome scores, such as those by Ferro et al6 in 2009 and Bushnaq et al13 in 2018, present significant discrepancies between their versions. While Ferro et al6 focused on CVT patients in Portugal, France, and Brazil, Bushnaq et al13 recruited patients from New Mexico in the United States. It is posited that different nations and ethnic populations may have distinct prognostic markers, leading to unique CVT outcome scores. In alignment with this notion, the current study aims to establish a CVT outcome score specifically tailored for Chinese patients with CVT.
Methods
Study Sample
The study sample was derived from the CCC cohort (NCT03919305), a multicenter study conducted across 26 tertiary hospitals in mainland China.14 Patients diagnosed with CVT were prospectively enrolled between January 2021 and May 2022. The diagnosis of CVT was established on the basis of clinical symptoms and neuroimaging findings, as delineated previously.10
Data Extraction
Potential prognostic indicators, including age, sex, level of consciousness (LOC),15 new-onset epilepsy,16 mental disorders,6 malignancy,17 central nervous system (CNS) infections,5 deep venous system thrombosis,18 utilization of oral contraceptive pills (OCPs),19 DBP,10 papilledema,16 number of sinuses involved,16 occurrence of parenchymal lesions,20 C-reactive protein (CRP),12 fasting blood glucose,11 NLR,8 NSE,9 platelet count (PLT),16 and serum sodium level16 were extracted from the CCC database for further analysis.
Outcome Measurement
CVT outcome was assessed using the modified Rankin scale (mRS) at six months post-onset. In alignment with prior studies, an mRS of ≤ 1 was characterized as a good outcome, and an mRS of > 1 as a poor outcome.8,12 The binarized mRS outcomes were employed for additional analyses.
Statistical Analysis
Statistical evaluations were conducted employing SPSS version 19.0 (SPSS Inc., Chicago, IL, USA). Two-sample t-tests were utilized to examine differences in continuous variables between the groups, while the chi-square test was applied for categorical variables. The maximum value of the Youden index (sum of sensitivity and specificity minus one) in the receiver operating characteristic (ROC) curve21 was used to identify cut-off values. Univariate logistic regression analysis, followed by multivariate logistic regression modeling, was performed to identify prognostic markers in Chinese patients with CVT. The identified prognostic markers were implemented to construct CVT outcome scores, with one point designated to the lowest OR and multiple points allocated based on the multiplicities of the lowest OR.22 The efficacy of the CVT risk score was gauged through classification accuracy, as ascertained by the area under the ROC curve (AUC),23 the distribution of the mRS score according to the CVT risk score, and a two-sample t-test. Statistical significance was determined as a two-sided p value of < 0.05.
Results
A total of 170 patients with CVT, comprising 75 males and 95 females, were studied with a mean age of 35.71 (standard error of mean [SEM] = 1.03). The most frequent occurrences were papilledema (55.88%), new-onset epilepsy (32.35%), and decreased LOC (14.12%). Among the patients, 95 (55.88%) had involvement in three or more sinuses, and 60 (35.29%) had parenchymal lesions. The specific data on the reported prognostic markers are delineated in Table 1.
 |
Table 1 The Detailed Data of Reported Prognostic Markers in Chinese CVT Patients |
The patients were subsequently classified into groups with good outcomes (n = 129) and poor outcomes (n = 26) based on the mRS scores at a six-month follow-up. Factors including age (P = 0.0331), DBP (P = 0.0436), NLR (P = 0.0006), NSE (P = 0.0001), the number of patients with decreased LOC (P = 0.0415), new-onset epilepsy (P = 0.0339), and parenchymal lesions (P = 0.0005) were found to be significantly different between the two groups (Table 1).
Univariate logistic regression analysis revealed age (P = 0.036), decreased LOC (P = 0.048), new-onset epilepsy (P = 0.038), DBP (P = 0.047), parenchymal lesions (P = 0.001), CRP level (P = 0.002), NLR (P = 0.012), and NSE level (P = 0.008) as significant prognostic markers in Chinese patients with CVT (Table 2).
 |
Table 2 Univariate Logistic Regression Between Reported Prognostic Markers at Baseline and the Clinical Outcome at the 6-Month Follow-Up in Chinese CVT Patients |
Cut-off values for age (27.5 years), DBP (79.5 mmHg), CRP (30 mg/L), NLR (6.6), and NSE (16.5 ng/mL) were ascertained using the Youden index’s maximum value (Figure 1). These continuous variables were transformed into binary variables for further multivariate modeling using the cut-off values. AUC analysis confirmed that age (AUC = 0.6263, P = 0.0426), DBP (AUC = 0.6434, P = 0.0291), NLR (AUC = 0.7667, P = 0.0027), and NSE (AUC = 0.6545, P = 0.0346) independently predicted CVT’s clinical outcomes with high specificity and sensitivity. Nevertheless, CRP’s predictive value (AUC = 0.5875, P = 0.2441) exhibited low sensitivity and specificity.
 |
Figure 1 Analysis of the ROC curve to determine cut-off values. The ROC analysis was utilized to identify the cut-off values for age (A), DBP (B), CRP (C), NLR (D), and NSE (E). |
A multivariate model was constructed including age, decreased LOC, new-onset epilepsy, DBP, parenchymal lesions, CRP, NLR, and NSE levels (Table 3). The significant variables for predicting the clinical outcome of Chinese CVT patients were identified as age > 27.5 years (P = 0.036), DBP > 79.5 mmHg (P = 0.003), NLR > 6.6 (P < 0.001), and NSE > 16.5 ng/mL (P = 0.015).
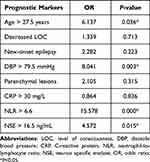 |
Table 3 Multivariate Logistic Regression Between Identified Prognostic Markers at Baseline and the Clinical Outcome at the 6-Month Follow-Up in Chinese CVT Patients |
A CVT outcome score was established using four prognostic markers (age, DBP, NLR, and NSE) identified in the multivariate logistic analysis. Points were assigned as follows: one point for NSE > 16.5 ng/mL and age > 27.5 years; two points for DBP > 79.5 mmHg; and three points for NLR > 6.6 (Table 4). There was a positive correlation between the CVT outcome score at baseline and the mRS at six months follow-up (P < 0.001, Figure 2A). ROC analysis indicated that the CVT outcome score robustly predicted CVT’s clinical outcome (AUC = 0.8785, P = 0.0001, Figure 2B). Additionally, the Youden index identified 3.5 as the cut-off value for the CVT outcome score, so patients were categorized into CVT outcome scores of > 3 and ≤ 3. A two-sample t-test revealed that the mRS score in patients with a CVT outcome score of > 3 was substantially higher than that in patients with a CVT outcome score of ≤ 3 (Figure 2C).
 |
Table 4 Determination of CVT Outcome Score for Chinese Patients |
Discussion
In the present study, both univariate logistic analysis and multivariate logistic analysis were utilized to identify age, DBP, NLR, and NSE as prognostic indicators for CVT. A CVT outcome score, encompassing the aforementioned four variables, was constructed to assess the clinical outcomes in Chinese patients diagnosed with CVT.
Previous findings from two multicenter CVT cohorts, ISCVT and VENOST, have shown that older age correlates with adverse CVT outcomes.5,24 In our investigation, the cut-off age was established at 27.5 years, contrasting with 37 years in the ISCVT study, and 50 years in the VENOST study.5,24 This discrepancy is likely attributed to the unique study populations, as neither ISCVT nor VENOST included patients with CVT from clinical centers within mainland China.
NSE, recognized as a biomarker for brain injury released upon neuronal cell membrane damage,25 was identified in our previous work as an effective predictor for CVT severity and outcome.9 The prognostic role of NSE was further validated in this study through multivariate logistic analysis. Furthermore, a DBP reading greater than 79.5 mmHg emerged as a significant prognostic factor for CVT following multivariate logistic analysis. An elevated DBP is associated with increased intracranial pressure, which may consequently induce heightened inflammatory reactions, oxidative stress, disruptions to the blood-brain barrier, and even cerebral herniation.26,27
Inflammation is recognized as playing a pivotal role in CVT. The NLR serves as a straightforward parameter to gauge inflammatory status and is often deemed more robust than isolated changes in neutrophils or lymphocytes.28,29 Earlier studies revealed a correlation between NLR and adverse clinical outcomes in CVT.8,12 The findings of the current study reinforced the significance of NLR in forecasting the clinical outcomes of CVT. Additionally, SII, defined as PLT × NLR, emerges as a novel inflammatory indicator for evaluating host immune and inflammatory conditions. The SII has previously been reported to act as a prognostic marker for CVT.30 In this particular investigation, we established that NLR, rather than PLT, anticipated the clinical outcomes of patients suffering from CVT. This observation has been proposed to be integral to the prognostic efficacy of SII in CVT patients.
The baseline CVT outcome score was found to be positively associated with the mRS score at six months post-onset. Furthermore, the CVT outcome score efficiently predicted the clinical outcome of CVT, pinpointing a cut-off value of 3.5. Since the CVT outcome score is an integer, a score exceeding 3 signifies a poor clinical outcome, whereas a score of 3 or less is indicative of a favorable clinical result. Endovascular therapy is currently considered when patients exhibit deterioration despite anticoagulation measures.2 Research has shown that CVT patients at a higher risk of undesirable clinical outcomes may benefit from early endovascular therapy, thereby preventing thrombus formation.31 The CVT outcome score could serve as a vital tool in identifying CVT patients at risk for poor clinical outcomes, thereby enabling timely implementation of endovascular therapy.
The scope of this study was limited by a relatively modest sample size. However, we aim to corroborate the CVT outcome score using a more extensive cohort in future research.
Conclusions
This study successfully developed a CVT outcome score that encompasses age, DBP, NLR, and NSE for the assessment of Chinese patients. The correlation analysis revealed that the baseline CVT outcome score was positively associated with the mRS score at a follow-up period of six months. This finding demonstrates that a CVT outcome score greater than 3 may serve as an effective marker to identify patients with CVT who are at an elevated risk for poor clinical outcomes. Consequently, patients exhibiting CVT outcome scores above 3 should receive prioritized attention, including additional care and early intervention measures, to mitigate the risk of clinical deterioration. However, the relatively small number of samples obtained is a limitation of this study. Therefore, the results should be interpreted more cautiously.
Abbreviations
AUC, area under the ROC curve; CNS, central nervous system; CRP, C-reactive protein; CVT, cerebral venous thrombosis; DBP, diastolic blood pressure; LOC, level of consciousness; mRS, modified Rankin scale; NLR, neutrophil-to-lymphocyte ratio; NSE, neuron-specific enolase; OCPs, oral contraceptive pills; PLT, platelet count; ROC, receiver operating characteristic; SEM, standard error of the mean.
Data Sharing Statement
Data and materials supporting the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval
This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and was approved by the Ethics Committee of Xuanwu Hospital (2019[006]).
Consent to Publish
All participants provided signed consent for publication.
Funding
This study was supported by the Beijing Natural Science Foundation (No. 7212047). The funding agencies had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclosure
Min Li and Binlong Zhang are co-first authors for this study. All authors declare that they have no competing interests in this work.
References
1. Idiculla PS, Gurala D, Palanisamy M, Vijayakumar R, Dhandapani S, Nagarajan E. Cerebral venous thrombosis: a comprehensive review. Eur Neurol. 2020;83(4):369–379. doi:10.1159/000509802
2. Silvis SM, De Sousa DA, Ferro JM, Coutinho JM. Cerebral venous thrombosis. Nat Rev Neurol. 2017;13(9):555–565. doi:10.1038/nrneurol.2017.104
3. Dmytriw AA, Song JSA, Yu E, Poon CS. Cerebral venous thrombosis: state of the art diagnosis and management. Neuroradiology. 2018;60(7):669–685. doi:10.1007/s00234-018-2032-2
4. Wang G, Yang X, Duan J, et al. Cerebral venous thrombosis: MR black-blood thrombus imaging with enhanced blood signal suppression. Am J Neurorad. 2019;40(10):1725–1730. doi:10.3174/ajnr.A6212
5. Ferro JM, Canhão P, Stam J, Bousser M-G, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the international study on cerebral vein and dural sinus thrombosis (ISCVT). Stroke. 2004;35(3):664–670. doi:10.1161/01.STR.0000117571.76197.26
6. Ferro JM, Bacelar-Nicolau H, Rodrigues T, et al. Risk score to predict the outcome of patients with cerebral vein and dural sinus thrombosis. Cerebrovasc Dis. 2009;28(1):39–44. doi:10.1159/000215942
7. Kowoll CM, Kaminski J, Weiß V, et al. Severe cerebral venous and sinus thrombosis: clinical course, imaging correlates, and prognosis. Neurocrit Care. 2016;25(3):392–399. doi:10.1007/s12028-016-0256-8
8. Wang L, Duan J, Bian T, et al. Inflammation is correlated with severity and outcome of cerebral venous thrombosis. J Neuroinflammation. 2018;15(1):329. doi:10.1186/s12974-018-1369-0
9. Hu Y, Meng R, Zhang X, et al. Serum neuron specific enolase may be a marker to predict the severity and outcome of cerebral venous thrombosis. J Neurol. 2018;265(1):46–51. doi:10.1007/s00415-017-8659-9
10. Li M, Pan L, Gao X, Hou J, Meng R, Ji X. Low diastolic blood pressure predicts good clinical outcome in patients with cerebral venous thrombosis. Front Neurol. 2021;2021:1573.
11. Wu Y, Zhou L, Yao M, et al. Elevated fasting blood glucose is predictive of the severity and poor outcome in nondiabetic patients with cerebral venous thrombosis. J Neurol Sci. 2020;417:117017. doi:10.1016/j.jns.2020.117017
12. Aguiar de Sousa D, Pereira‐Santos MC, Serra‐Caetano A, et al. Blood biomarkers associated with inflammation predict poor prognosis in cerebral venous thrombosis: a multicenter prospective observational study. Eur J Neurol. 2021;28(1):202–208. doi:10.1111/ene.14526
13. Bushnaq SA, Qeadan F, Thacker T, Abbas M, Carlson AP. High-risk features of delayed clinical progression in cerebral venous thrombosis: a proposed prediction score for early intervention. Interv Neurol. 2018;7(6):297–307. doi:10.1159/000487960
14. Li M, Gao X, Yang Q, et al. Protocol of a multicenter cohort observational study of cerebral venous thrombosis in china mainland. Cerebrovasc Dis. 2022;2022:1–7.
15. Barboza MA, Chiquete E, Arauz A, et al. A practical score for prediction of outcome after cerebral venous thrombosis. Front Neurol. 2018;9:882. doi:10.3389/fneur.2018.00882
16. Buyck P-J, Zuurbier SM, Garcia-Esperon C, et al. Diagnostic accuracy of noncontrast CT imaging markers in cerebral venous thrombosis. Neurology. 2019;92(8):e841–e851. doi:10.1212/WNL.0000000000006959
17. Silvis S, Hiltunen S, Lindgren E, et al. Cancer and risk of cerebral venous thrombosis: a case–control study. J Thromb Haemost. 2018;16(1):90–95. doi:10.1111/jth.13903
18. Girot M, Ferro JM, Canhão P, et al. Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke. 2007;38(2):337–342. doi:10.1161/01.STR.0000254579.16319.35
19. Özdemir HH, Varol S, Akıl E, Acar A, Demir CF. Evaluation of cerebral venous thrombosis secondary to oral contraceptive use in adolescents. Neurol Sci. 2015;36(1):149–153. doi:10.1007/s10072-014-1914-2
20. Appenzeller S, Zeller CB, Annichino-Bizzachi JM, et al. Cerebral venous thrombosis: influence of risk factors and imaging findings on prognosis. Clin Neurol Neurosurg. 2005;107(5):371–378. doi:10.1016/j.clineuro.2004.10.004
21. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–35. doi:10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3
22. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi:10.1148/radiology.143.1.7063747
23. Zuurbier SM, Hiltunen S, Tatlisumak T, et al. Admission hyperglycemia and clinical outcome in cerebral venous thrombosis. Stroke. 2016;47(2):390–396. doi:10.1161/STROKEAHA.115.011177
24. Duman T, Uluduz D, Midi I, et al. A multicenter study of 1144 patients with cerebral venous thrombosis: the VENOST study. J Stroke Cerebrovasc Dis. 2017;26(8):1848–1857. doi:10.1016/j.jstrokecerebrovasdis.2017.04.020
25. Haque A, Polcyn R, Matzelle D, Banik NL. New insights into the role of neuron-specific enolase in neuro-inflammation, neurodegeneration, and neuroprotection. Brain Sci. 2018;8(2):33. doi:10.3390/brainsci8020033
26. Tiwari HS, Misra UK, Kalita J, Mishra A, Shukla S. Oxidative stress and glutamate excitotoxicity contribute to apoptosis in cerebral venous sinus thrombosis. Neurochem Int. 2016;100:91–96. doi:10.1016/j.neuint.2016.09.003
27. Rashad S, Niizuma K, Sato-Maeda M, et al. Early BBB breakdown and subacute inflammasome activation and pyroptosis as a result of cerebral venous thrombosis. Brain Res. 2018;1699:54–68. doi:10.1016/j.brainres.2018.06.029
28. Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi:10.1186/s13104-016-2335-5
29. Akboga YE, Bektas H, Anlar O. Usefulness of platelet to lymphocyte and neutrophil to lymphocyte ratios in predicting the presence of cerebral venous sinus thrombosis and in-hospital major adverse cerebral events. J Neurol Sci. 2017;380:226–229. doi:10.1016/j.jns.2017.07.036
30. Li S, Liu K, Gao Y, et al. Prognostic value of systemic immune–inflammation index in acute/subacute patients with cerebral venous sinus thrombosis. Stroke Vasc Neurol. 2020;5(4):368–373. doi:10.1136/svn-2020-000362
31. Bushnaq S, Thacker T, Abbas M, Qeadan F, Carlson A. O-035 Risk factors for delayed deterioration after cerebral venous thrombosis: a model to identify patients for early aggressive endovascular therapy. J Neurointerv Surg. 2017;9:A20–A21.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

