Back to Journals » International Journal of Nanomedicine » Volume 19
Prediction of Response to Chemoradiotherapy by Dynamic Changes of Circulating Exosome Levels in Patients with Esophageal Squamous Cell Carcinoma
Authors Zhang C, Guo Z, Jing Z
Received 12 October 2023
Accepted for publication 1 February 2024
Published 9 February 2024 Volume 2024:19 Pages 1351—1362
DOI https://doi.org/10.2147/IJN.S440684
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Chuanfeng Zhang, Zhen Guo, Zhao Jing
Department of Oncology, Zhejiang Hospital, Hangzhou, Zhejiang, 310013, People’s Republic of China
Correspondence: Zhao Jing, Department of Oncology, Zhejiang Hospital, No. 12 Lingyin Road, Hangzhou, Zhejiang, 310013, People’s Republic of China, Tel +86-571-87377308, Email [email protected]
Background: The exosomes‐based liquid biopsy represents a prospective biomarker for tumor screening, prognosis prediction, and tumor regression. This study aimed to isolate circulating exosomes (CEs) from plasma of the esophageal squamous cell carcinoma (ESCC) patients who received chemoradiotherapy through exosome detection method via the ultrafast-isolation system (EXODUS) and investigated the association between the dynamic changes of CE levels and therapeutic effect.
Methods: We isolated and quantitatively analyzed CEs from plasma of locally advanced ESCC patients received chemoradiotherapy at 2 time points: baseline (pre-chemoradiotherapy) and 2 months after the chemoradiotherapy (post-chemoradiotherapy). We isolated exosomes from plasma by EXODUS platform and confirmed them through nanoparticle tracking analysis (NTA), transmission electron microscope (TEM), and Western blot. The associations of CE level with clinicopathological characteristics, tumor regression, and progression-free survival (PFS) were analyzed.
Results: The average diameter of CEs was 107.4± 14.3 nm at baseline and 101.7± 17.1 nm at post-chemoradiotherapy. The mean exosome concentration significantly decreased after chemoradiotherapy (7.3× 1011 particles/mL vs 5.4× 1011 particles/mL, P < 0.001). The patients with stage III–IVA and tumor length ≥ 5cm had obviously higher baseline CE levels. Dynamic changes in CE levels were successfully applied for evaluation of chemoradiotherapy response and PFS. Furthermore, through multivariate Cox regression analysis, it was revealed that dynamic changes of CE levels were an independent predictor of PFS in locally advanced ESCC patients who received chemoradiotherapy.
Conclusion: Here, we demonstrated EXODUS platform isolated and enriched CEs from plasma of ESCC patients with high-purity and high-yield. The EXODUS platform can facilitate liquid biopsy based on exosomes translation to the clinic. Baseline CE levels can reflect ESCC tumor burden. The dynamic changes of CE levels during chemoradiotherapy allow the prediction of treatment effect and PFS of ESCC patients, requiring further investigations in larger patient cohorts.
Keywords: exosomes, EXODUS platform, locally advanced ESCC, chemoradiotherapy
Introduction
Esophageal cancer is now the sixth most deadly cancer.1 More than half of new cases with esophageal cancer are in China, and esophageal squamous cell carcinoma (ESCC) is the main pathological type.2 Surgery is firstly be considered when diagnosed with esophageal cancer, with a median overall survival time of 13.6–19.3 months.3 However, about 30% of new patients have the chance for surgery. The Radiation Therapy Oncology Group (RTOG) 85–01 trial showed that 5-fluorouracil plus cisplatin combined with radiotherapy significantly improved median survival and 5-year OS rate compared with radiotherapy alone for patients with locally advanced esophageal cancer.4 Concurrent chemoradiotherapy has become the standard treatment for patients with locally advanced esophageal cancer, but the local recurrence rate is over 50%.5,6
The accurate TNM staging system is widely used to guide treatment measures and evaluate prognosis in clinical practice.7 However, due to the tumor heterogeneity, patients with the same stage often exhibit different responses to therapy and outcome. Therefore, it is essential to seek out effective biomarkers which can predict the treatment response.8 Early detection of therapeutic effect or disease progression may offer the opportunity to adjust the treatment plan in time and improve the clinical outcome. Currently, there are no widely acknowledged biomarkers for ESCC early diagnosis, predicting for treatment effect and tumor recurrence.9 Traditional monitoring methods include imaging technology and endoscopic examination with biopsy.10 The invasion, low sensitivity and discomfort limit the clinical implication of these approaches. Hence, noninvasive and convenient biomarkers for monitoring response to treatment and progression of ESCC remain an urgent need.
Liquid biopsy can detect tumor-specific molecular biomarkers circulating in biological fluid sample with advantage of atraumatic, acute, and actual time analysis for screening and treatment monitoring.11,12 Exosomes are nano-sized (30–150nm) extracellular vesicles secreted by many cell types. Exosomes carry several types of bioactive substances, such as lipids, proteins, mRNAs, miRNAs, and so on.13 Recent studies have found that exosomes play key roles in tumor progression, metastasis, and drug resistance.14,15 Exosomes have been brought into sharp focus in liquid biopsy because of extensive existence in most biofluids and stability of cargoes.16 Exosome-based noninvasive monitoring platform may offer the most prospective biomarkers for tumor screening, prognosis evaluation, and treatment effect prediction. Most studies have focused on circulating exosomes (CEs) as latent biomarkers for tumor liquid biopsy. Zhao et al17 found that elevated levels of CE were associated with short survival time in ESCC patients. Liu et al18 revealed that upregulation of serum exosomal miR-766-3p levels was related to advanced TNM stage and poor prognosis in ESCC. The hemocircular exosomal miR-340-5p and miR-339-5p might be prospective biomarkers for prediction of radiotherapy effect in patients with ESCC.19,20 Although exosomes have enormous potentiality for application, the isolation and enrichment of exosomes from biological fluids are still the most obstacles that limit their wide application.21
The present commonly used methodologies for isolation and enrichment of exosome (ultracentrifugation, size-exclusion chromatography, immunoaffinity capture-based methods, and polymer-based precipitation kits) suffer from a lot of drawbacks including the inconvenience, long processing time, low reproducibility and purity.22 It is urgent to develop new strategies for separation and enrichment of exosomes with high purity and efficiency.
The exosome detection method via the ultrafast-isolation system (EXODUS) is an efficient exosome isolation platform that enables automated, label-free depuration of exosomes through negative pressure oscillation and the use of a double-coupled harmonic oscillator–enabled membrane vibration.23 EXODUS offers a novel ultrafiltration strategy to achieve clog-free and ultrafast purification, allowing for the handy and fast isolation of exosomes with improved processing speed, yield, and purity from various biofluids. The EXODUS platform is significantly better than current extraction methods for exosomes.
In this study, we evaluated the feasibility of EXODUS platform to isolate and enrich CEs from plasma samples of the ESCC patients before and after chemoradiotherapy. We further investigated the role of CE levels as a biomarker for monitoring therapeutic effect and prediction prognosis in prospective ESCC patient cohorts after chemoradiotherapy. To our knowledge, studies analyzing changes of CE levels in paired ESCC patient samples before and after chemoradiotherapy have never been reported. We demonstrated that the changes of CE levels could serve as a new biomarker for prediction of response to therapy.
Methods
Study Patients and Ethics Statements
Patients with histologically confirmed medically inoperable or clinically unresectable ESCC were enrolled, and the tumor staged as T2 to T4, N0/1, M0/1a (only supraclavicular/celiac lymph node metastasis and not any other distant metastasis) according to the 6th edition of the American Joint Committee on Cancer (AJCC). Other eligibility criteria included adults aged 18–70 years old; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; adequate renal, liver, and bone marrow reserve; and adequate nutritional intake. Ineligibility criteria included history of other tumors, tracheoesophageal fistula, metastatic disease, prior systemic treatment or thoracic radiation, active infection, and severe cardiovascular disease. Clinical and demographic information was obtained from each patient. The study complied with the Declaration of Helsinki. All the including patients signed the informed consent form. The study protocol was approved by the Medical Ethics Committee of Zhejiang hospital (2021–161k) and Hangzhou cancer hospital (HZCH-2020).
Treatments Administered and Response Evaluation
Radiotherapy was administered with intensity modulated radiotherapy (IMRT) technique to a total dose of 60 Gy in 30 fractions (5 days per week at 2 Gy/d) according to the treatment guideline of radiotherapy for Chinese Society of Clinical Oncology (CSCO) of esophageal carcinoma. Gross tumor volume was defined as the primary tumor and the involved regional lymph nodes. Clinical target volume included the gross tumor volume plus a 3-cm expansion superiorly and inferiorly along the length of the esophagus and a 1-cm expansion of supraclavicular and mediastinal lymph node regions. All patients received chemotherapy comprising intravenous paclitaxel (135 mg/m2, day 1) and cisplatin (25 mg/m2, days 1–3) or oxaliplatin (130 mg/m2, day 2) every 4 weeks for two cycles.
Clinical response was assessed according to Response Evaluation Criteria in Solid Tumors (RECIST 1.1) 2 months after the completion of chemoradiotherapy. Response was evaluated by esophagography, esophagoscopy with biopsy, and CT imaging. PET–CT imaging was optional. Tumor response assessed by CT scan was based on its vertical length, maximal transverse thickness, and maximum thickness of the esophageal wall. Patients were regularly followed up for 2 years every 3 months, followed by 3 years every 6 months, and then annually. Additional assessments were carried out if disease progression was suspected. Patients were considered responders if they had either a complete response (CR) or partial response (PR) and non-responders if they had stable disease (SD) or progressive disease (PD).
Preparation of Plasma
Peripheral blood specimens (5 mL) were obtained from all patients into heparinized tubes at the designated time points before (baseline) and after treatment (2 months). The whole-blood samples were centrifuged at 4000 rpm for 10 min at 4 °C to isolate the plasma. The plasma samples were stored in aliquots at −80 °C before use. Specimens were thawed just prior exosome isolation.
Exosomes Isolation by EXODUS Platform
Exosome isolation and purification were performed on plasma samples using the EXODUS platform. Detailed steps and operation instructions were described in previous literature.23 Briefly, 200 μL plasma sample was diluted to a final volume of 1 mL with phosphate buffer saline (PBS) and then loaded on the EXODUS device for exosome isolation and collection. Followed the operation instructions, the EXODUS device could be programmed to perform the automatic exosome isolation and purification procedure. Finally, the exosome solutions from the EXODUS device were collected and stored at −80°C for further analysis.
Nanoparticle Tracking Analysis of Plasma Exosomes
The size and concentration of plasma exosomes were determined through a nanoparticle tracking analysis (NTA) in a Nanosight NS300 (Malvern Instruments) following the manufacturer’s instructions. Each exosome sample was introduced to the instrument using a syringe-micropump system. For optimal analysis, exosome samples purified via EXODUS were diluted 50 to 100-fold in particle-free PBS to obtain approximately 50 particles in the field of view. Each sample was diluted in triplicate. Three captures with a duration of 30s for each diluted sample were acquired, and for statistical calculations the average was used. All NTA measurements were done with identical system settings for consistency.
Protein Quantification of Plasma-Derived Exosomes
The proteins from exosomes were lysed with cell lysis buffer (Takara) and protease inhibitors (SigmaFastTM, Sigma) on ice for 30 min. Exosome lysates were added per well of a 96-well plate suitable for absorbance measurement. The total protein content of exosomes was determined using the bicinchoninic acid (BCA) protein assay kit (Thermo Scientific).
Western Blot Analysis of Plasma Exosomes
The proteins were separated using a precast polyacrylamide mini-gels (Tri-glycine pH 8.3) with a Mini Trans-Blot module (Bio-Rad). Protein lysates were subsequently resolved in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membrane (PVDF). Membranes were blocked with 3% bovine serum albumin (BSA) for 1 h at room temperature, followed by primary antibody incubation overnight at 4 °C. The following antibodies were used for Western blot analysis: anti-CD9 (Cell Signaling Technology), anti-HSP90 (Santa Cruz), anti-Alix (Santa Cruz), and anti-Flot 1 (BD biosciences). Membranes were incubated with primary antibodies (1:1,000 dilution) overnight at 4 °C. After washing each membrane three times with Tris-buffered saline containing 0.05% Tween 20 for 10 min, they were incubated with HRP-conjugated anti-mouse IgG or HRP-conjugated anti-rabbit IgG (Cell Signaling Technology) as the secondary antibody (1:3,000) for 1 h at room temperature. The membranes were washed three times with Tris-buffered saline containing 0.05% Tween 20 for 10 min. Finally, the immunoreactive bands were visualized using an enhanced chemiluminescence (P&Q Science & Technology).
Transmission Electron Microscope Analysis of Plasma Exosomes
For transmission electron microscope (TEM), exosomes were fixed with 4% paraformaldehyde (PFA) and loaded on carbon-coated copper grids. Grids were washed with PBS and transferred to a 50 μL drop of 1% glutaraldehyde for 5 min. Afterward, the grids were washed thoroughly with PBS and then with 100 μL of distilled water. The grids were stained with uranyl acetate (2%) for 30s and washed again in PBS. After air drying, the samples were observed by a transmission electron microscope (Talos F200S, Thermo).
Statistical Analysis
SPSS version 27 and GraphPad Prism 9 software were used for graphical representation and statistical analyses. Summary statistics including means, medians, standard deviations [SD], ranges, and 95% confidence intervals (CI) were reported when appropriate. The error bars in the graphical data represented the means ± standard deviations. Correlations of the two quantification methods were evaluated by Pearson correlation coefficients. CE level differences between groups based on patients’ characteristics were assessed using one way analysis of variance (ANOVA) or t–tests. PFS was defined as the duration from the ESCC diagnosis to the time of disease progression or death from any cause. Those living patients without disease progression were reviewed during their last follow-up. The PFS was analyzed by the Kaplan–Meier method and compared by the Log rank test. Prediction factors were determined by univariate and multivariate analyses (Cox proportional hazard regression model). We used Cox proportional hazards regression models to estimate the effects of risk factors on the hazard ratios (HRs) accompanying 95% confidence intervals (CIs). All reported p values were two-sided with a significance level of 0.05.
Results
Patient Characteristics
Between January 1, 2021, and June 30, 2022, 40 patients with newly diagnosed ESCC were enrolled. Detailed demographic and clinical characteristics were listed in Table 1. The median age was 59.5 years (range, 43–70 years). Twenty-nine patients were men (72.5%), and 11 were women (27.5%). Twenty-three patients had stage I/II cancer (57.5%), and 17 patients had stage III/IVA cancer (42.5%). The location of the primary tumors included cervical/upper/middle/lower thoracic portions, and the respective numbers were as follows: 3/8/20/9 (7.5%/20.0%/50.0%/22.5%).
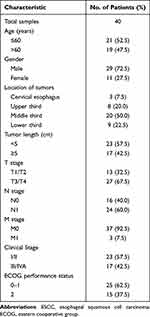 |
Table 1 Demographics and Clinicopathological Characteristics of ESCC Patients |
Efficacy
Five patients achieved CR and 22 patients achieved PR. Nine patients exhibited SD and the remaining 4 patients had PD. The overall response rate was 67.5%. The median follow-up time was 15.6 months. The responder patients had a significantly better survival compared with the non-responder patients, with a median PFS of 19.0 months vs 9.0 months (log-rank P < 0.01).
Characterization of Plasma-Derived CEs in ESCC Patients
The CEs isolated from plasma of ESCC patients by EXODUS were further confirmed by TEM observation, western blot verification of membrane proteins, and NTA detection of vesicle size and concentration. Western blot analysis demonstrated the presence of exosomal marker proteins Alix, Hsp90, Flot 1, and CD9 in exosomes from plasma of ESCC patients (Figure 1A). NTA results revealed that the average diameter of CEs was 107.4±14.3 nm at baseline and 101.7±17.1 nm at post-chemoradiotherapy (Figure 1B). The TEM analysis showed a typical round double lipid membrane morphology (Figure 1C). These results indicated EXODUS might provide a new platform to isolate and enrich exosomes from plasma of ESCC patients.
In terms of exosomes quantification, the relativity of BCA and NTA quantification methods were assessed. Forty exosome samples of ESCC patients were all assessed with BCA and NTA methods. The mean CEs concentration was 7.3×1011 particles/mL with standard deviation (SD) of 1.26×1011 particles/mL at baseline. Baseline mean CEs protein concentration was 346.6 ug/mL, with standard deviation (SD) of 133.2 ug/mL. There was significant correlation between BCA and NTA quantification methods (Pearson r = 0.748, P < 0.001, Figure 1D).
Association Between CE Levels and Characteristics of ESCC Patients
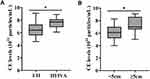
Furthermore, we explored the correlation between CE levels and characteristics of ESCC patients. Baseline CE levels in tumors with stage III–IVA were much higher than those staged as I–II (P = 0.027, Figure 2A, Supplementary Table S1). Meanwhile, baseline CE levels in tumors with length ≥5 cm were obviously higher than those in tumors with length <5 cm (P = 0.016, Figure 2B, Supplementary Table S1). There were no significant differences in baseline CE levels with respect to age, gender, tumor location, or ECOG performance status (P = 0.33, 0.78, 0.27, and 0.53, respectively). Therefore, we arrived at a conclusion that CE levels might be related to tumor burden in ESCC patients.
Predictive Role of CE Levels for Response to Chemoradiotherapy in ESCC Patients
The mean CE concentrations in plasma were 7.3×1011 particles/mL at baseline and 5.4×1011 particles/mL at post-chemoradiotherapy. Paired comparison of baseline and post-chemoradiotherapy levels showed that CE levels obviously decreased after chemoradiotherapy (P < 0.05, Figure 3A). There were no significant discrepancies in CE levels at baseline (P = 0.115) or post-chemoradiotherapy (P = 0.426) in ESCC patients with different responses to chemoradiotherapy. Among 40 patients treated with chemoradiotherapy, CE levels were dynamically measured and related to tumor regression to chemoradiotherapy according to RECIST criteria. The dynamic changes of CE levels for responders and non-responders at baseline and post-chemoradiotherapy were depicted in Figure 3B and C. Comparing CE levels at baseline and post-chemoradiotherapy, 9 of 14 (64.3%) patients those did not experienced a decline in CE levels after chemoradiotherapy were non-responders (SD or PD), whereas 84.6% (22/26) of those who experienced a decline in CE levels after chemoradiotherapy was responders (CR or PR) (P = 0.027; Supplementary Table S2). Logistic regression analysis revealed that changes in CE levels at baseline and post-chemoradiotherapy [ΔCE = (CE levels after chemoradiotherapy) – (CE levels before chemoradiotherapy)] were a predictor of tumor regression (OR = 8.73, 95% CI: 1.35–56.47; P = 0.023) with adjustment of clinicopathological characteristics (Table 2). Thus, we concluded that dynamic CE levels were potentially a promising biomarker for monitoring the treatment effect of patients with ESCC received chemoradiotherapy.
 |
Table 2 Logistic Regression Analysis for Parameters to Predict SD/PD in ESCC Patients Received Chemoradiotherapy |
Kaplan–Meier analysis demonstrated that a positive ΔCE (a rise in CE after therapy) was related to an obviously shorter PFS (P = 0.021; Figure 3D) than a negative ΔCE (a fall in CE after therapy). Multivariable Cox regression analysis showed that ΔCE was an independent prognostic factor of PFS in ESCC patients after concurrent chemoradiotherapy (HR = 2.36; 95% CI: 1.21–9.79; P = 0.041; Table 3). Therefore, ΔCE can predict prognosis in patients with ESCC received chemoradiotherapy.
 |
Table 3 Univariate and Multivariate Cox Proportional Hazards Analyses of Factors Associated with Shorter PFS in ESCC Patients Received Chemoradiotherapy |
Discussion
Radical chemoradiotherapy has become the standard treatment for unresectable locally advanced ESCC. The objective response rate was 67.5% in the present study, and the result closely approximated those observed in other studies.24,25 Even in curatively resected patients, the 5-year survival rate of ESCC is below 50% after surgery. Studies have demonstrated the importance of tumor screening and detection of treatment resistance on improved survival.26,27 Currently, the monitoring of ESCC was mainly through contrast CT scan and endoscopy/biopsy with invasive and discomfort. Several tumor markers in peripheral blood are routinely applied for ESCC monitoring, including squamous cell carcinoma antigen (SCCA), carcinoembryonic antigen (CEA), and cytokeratin 19 fragment (CYFRA21-1), but these biomarkers have been challenged by low specificity and sensitivity.28
Liquid biopsy has attracted amount of attention due to its non-invasive feature in cancer early diagnosis and predicting treatment effect in recent years. Tumor-secreted exosomes have emerged as potential liquid biopsy substrate for cancer diagnosis or prognosis because it may carry multiple tumor-specific genetic or epigenetic information.29,30 Exosome-based liquid biopsies have a few advantages over traditional methods. First, due to its non-invasive nature, serial samples of exosomes can be acquired at different time points during treatment for accurate evaluation of the therapeutic effect. Second, exosomes are very steady and can be stored for a long time. Third, the lipidic membrane of exosomes protects its contents from degradation in the circulation. The exosome and its contents have been a focus of new biomarkers for tumor in recent years.31 Recently, it was found that exosomal lncRNA UCA1 might be a potential biomarker for diagnosing esophageal cancer and its sensitivity and specificity were 86.7% and 70.2%, respectively.32 Fan et al validated circRNA has-circ-0001946 and has-circ-0043603 secreted by ESCC cells might be potential diagnostic biomarkers for ESCC. The sensitivity and specificity of has-circ-0001946 were 92% and 80%, 64% and 92% for has-circ-0043603, so joint detection might be more accurate in diagnosis.33 The detection of exosomes from plasma of patients would be preferred due to convenience, noninvasive, high sensitivity, and comfort.
Although exosomes have broad prospects for clinical application, the major obstacle in the clinical translation of exosomes is the lack of convenient, reliable and high-efficiency isolation methods. The current isolation methods have drawbacks such as complex operation, time consuming, poor yields, and low purity impeding downstream analysis of exosomes. Chen et al23 described an efficient exosome ultrafast-isolation system (EXODUS) allowing automated label-free purification of exosomes from different biofluids. Compared to traditional methods, EXODUS provides a better speed, low cost, high efficiency and stability tool in exosomes isolation and enrichment.
In this study, we used EXODUS platform to isolate and enrich CEs from the plasma of patients, with ESCC receiving chemoradiotherapy. Isolated exosomes were confirmed and characterized by NTA, immunoblotting and TEM. There are still no standard exosome concentration quantification methods.34,35 In this study, the exosome concentration was determined by both NTA and BCA assay. Correlation analyses indicated that there was good correlation between the two methods. Our experiments also revealed that the concentration of exosomes separated by EXODUS detected was significantly higher than that isolated by traditional methods reported in the previous literature.23,36 Therefore, we used EXODUS platform to isolate exosomes with high purity and high yield from the plasma of patients with ESCC. Overall, these data demonstrated that EXODUS platform could achieve high-purity and high-yield plasma exosome isolation for downstream exosome-based liquid biopsy applications.
Previous studies have indicated that exosome level is associated with advanced tumor stage.37,38 The present study revealed that CE levels were higher in ESCC patients who had stage III–IV and length ≥5cm disease than that of stage I–II and length <5cm disease. The results demonstrated that CE levels could reflect the advanced stage and heavy tumor burden in patients with ESCC.
We demonstrated that ΔCE acted as a biomarker of predicting therapeutic response and PFS after chemoradiotherapy. We also found that rising CE levels after chemoradiotherapy was related to shorter PFS, and larger cohorts with longer follow-up were required to confirm this observation. This research demonstrated that consecutive detection of CE levels through EXODUS platform during cancer treatment was practicable and could be a useful tool to predict response to treatment and outcome.
Emerging evidences have shown that tumor-derived exosomes (TEXs) play crucial roles in tumor progression, immune regulation, metastasis, and drug resistance.15,39 Plasma-derived exosomes are heterogeneous mixtures of exosomes originating from many different cell types, including tumor cells, immune cells, fibroblasts, and endothelial cells. Isolation of TEXs from bulk plasma exosomes is important to assess the roles of TEXs as potential biomarkers of tumor progression and outcome. However, the isolation of TEXs and discriminating them from non-TEXs are still difficult due to the lack of specific markers or effective identification methods. Determining antibodies selectively binding to proteins uniquely expressed on the tumor cell surface, and TEXs are necessary to specifically isolate the TEXs. Yang et al40 developed a novel immuno-biochip isolation method by targeting tumor associated protein biomarkers via surface-tethered antibodies. Mondal et al41 isolated the melanoma cell-derived exosomes from plasma of melanoma patients through immune affinity-based capture with chondroitin sulfate peptidoglycan 4 (CSPG4) antibody. There were several limitations of the present study. One drawback is the overall small sample size. A larger sample study is needed to verify this conclusion in the future. Secondly, there are a few disadvantages in the quantification methods used in our study. There is no “gold standard” method for exosome quantification, and NTA is currently considered the reliable method available for exosome quantification. However, the results of NTA could be influenced by some factors such as exosome aggregation, co-isolation of lipoprotein or chylomicrons, and so on.42 Thirdly, the follow-up time is too short, so we cannot analysis the correlation of CE levels with overall survival of ESCC patients. Fourthly, the exosomal-cargoes based molecular biomarkers as predictor for response to therapy have not been discussed in this study and will be explored in the further study.
Conclusion
We isolated high-purity and high-yield exosomes from plasma of ESCC patients through the EXODUS platform. We identified CE levels as a predictive biomarker for monitoring tumor burden and treatment effect to tailor therapeutic decisions. Due to the small number of samples, the results of this study should be regarded as hypothesis generating for future studies. The validation of the results on larger cohorts of locally advanced ESCC patients will be able to confirm the role of dynamic changes of CE levels in predicting response to chemoradiotherapy in clinical practice. Monitoring of dynamic CE changes during treatment may be useful for individualizing treatment of ESCC patients in the future.
Ethics Statement
The study protocol was approved by the Medical Ethics Committee of Zhejiang hospital (2021-161k) and Hangzhou cancer hospital (HZCH-2020). All patients provided written informed consent to participate in the study.
Acknowledgments
This study was supported by the Zhejiang Province Public Welfare Technology Application Research Project (grant numbers LGC21H160001) and the General Research Program of Zhejiang Provincial Health Department of China (grant numbers 2022KY472). The funders were not involved in the design or interpretation of the results.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Dj U, Eo T, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13(6):1010–1021. doi:10.1007/s12328-020-01237-x
2. Collaborators GBDOC, Nasrollahzadeh D, Safiri S. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastro Hepatol. 2020;5(6):582–597. doi:10.1016/S2468-1253(20)30007-8
3. Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50(1):12–20. doi:10.1007/s00595-019-01878-7
4. Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation therapy oncology group. JAMA. 1999;281(17):1623–1627. doi:10.1001/jama.281.17.1623
5. Hulshof M, Geijsen ED, Rozema T, et al. Randomized study on dose escalation in definitive chemoradiation for patients with locally advanced esophageal cancer (ARTDECO study). J Clin Oncol. 2021;39(25):2816–2824. doi:10.1200/JCO.20.03697
6. Xia X, Wu M, Gao Q, Sun X, Ge X. Consolidation chemotherapy rather than induction chemotherapy can prolong the survival rate of inoperable esophageal cancer patients who received concurrent chemoradiotherapy. Curr Oncol. 2022;29(9):6342–6349. doi:10.3390/curroncol29090499
7. Lord AC, D’Souza N, Shaw A, et al. MRI-diagnosed tumor deposits and EMVI status have superior prognostic accuracy to current clinical TNM staging in rectal cancer. Ann Surg. 2022;276(2):334–344. doi:10.1097/SLA.0000000000004499
8. Chen S, Wu Y, Wang S, Wu J, Wu X, Zheng Z. A risk model of gene signatures for predicting platinum response and survival in ovarian cancer. J Ovarian Res. 2022;15(1):39. doi:10.1186/s13048-022-00969-3
9. Reichenbach ZW, Murray MG, Saxena R, et al. Clinical and translational advances in esophageal squamous cell carcinoma. Adv Cancer Res. 2019;144:95–135.
10. Chaber-Ciopinska A, Kiprian D, Kawecki A, Kaminski MF. Surveillance of patients at high-risk of squamous cell esophageal cancer. Best Pract Res Clin Gastro. 2016;30(6):893–900. doi:10.1016/j.bpg.2016.10.003
11. Glennon KI, Maralani M, Abdian N, et al. Rational development of liquid biopsy analysis in renal cell carcinoma. Cancers. 2021;13(22):22. doi:10.3390/cancers13225825
12. Li W, Liu JB, Hou LK, et al. Liquid biopsy in lung cancer: significance in diagnostics, prediction, and treatment monitoring. Mol Cancer. 2022;21(1):25. doi:10.1186/s12943-022-01505-z
13. Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
14. Paskeh MDA, Entezari M, Mirzaei S, et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol. 2022;15(1):83. doi:10.1186/s13045-022-01305-4
15. Jing Z, Chen K, Gong L. The significance of exosomes in pathogenesis, diagnosis, and treatment of esophageal cancer. Int J Nanomed. 2021;16:6115–6127. doi:10.2147/IJN.S321555
16. Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. doi:10.1186/s12943-022-01509-9
17. Zhao A, Guo L, Xu J, et al. Identification and validation of circulating exosomes-based liquid biopsy for esophageal cancer. Cancer Med. 2019;8(7):3566–3574. doi:10.1002/cam4.2224
18. Liu S, Lin Z, Zheng Z, et al. Serum exosomal microRNA-766-3p expression is associated with poor prognosis of esophageal squamous cell carcinoma. Cancer Sci. 2020;111(10):3881–3892. doi:10.1111/cas.14550
19. Chen F, Xu B, Li J, et al. Hypoxic tumour cell-derived exosomal miR-340-5p promotes radioresistance of oesophageal squamous cell carcinoma via KLF10. J Exp Clin Cancer Res. 2021;40(1):38. doi:10.1186/s13046-021-01834-9
20. Luo A, Zhou X, Shi X, et al. Exosome-derived miR-339-5p mediates radiosensitivity by targeting Cdc25A in locally advanced esophageal squamous cell carcinoma. Oncogene. 2019;38(25):4990–5006. doi:10.1038/s41388-019-0771-0
21. Yu W, Hurley J, Roberts D, et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann Oncol. 2021;32(4):466–477. doi:10.1016/j.annonc.2021.01.074
22. Zhu L, Sun HT, Wang S, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13(1):152. doi:10.1186/s13045-020-00987-y
23. Chen Y, Zhu Q, Cheng L, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. 2021;18(2):212–218. doi:10.1038/s41592-020-01034-x
24. Yao B, Tan B, Wang C, et al. Comparison of definitive chemoradiotherapy in locally advanced esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23(7):2367–2372. doi:10.1245/s10434-016-5154-y
25. Hsieh JC, Chiang PC, Hung TM, et al. Definitive concurrent chemoradiotherapy with paclitaxel plus carboplatin is superior to cisplatin plus 5-fluorouracil in patients with inoperable esophageal squamous cell carcinoma using retrospective, real-world evidence. Cancer Med. 2021;10(23):8300–8309. doi:10.1002/cam4.4025
26. Kandiah K, Chedgy FJ, Subramaniam S, Thayalasekaran S, Kurup A, Bhandari P. Early squamous neoplasia of the esophagus: the endoscopic approach to diagnosis and management. Saudi J Gastroenterol. 2017;23(2):75–81. doi:10.4103/1319-3767.203366
27. Visaggi P, Barberio B, Ghisa M, et al. Modern diagnosis of early esophageal cancer: from blood biomarkers to advanced endoscopy and artificial intelligence. Cancers. 2021;13(13):3162. doi:10.3390/cancers13133162
28. Chu LY, Peng YH, Weng XF, Xie JJ, Xu YW. Blood-based biomarkers for early detection of esophageal squamous cell carcinoma. World J Gastroenterol. 2020;26(15):1708–1725. doi:10.3748/wjg.v26.i15.1708
29. Liang LJ, Yang Y, Wei WF, et al. Tumor-secreted exosomal Wnt2B activates fibroblasts to promote cervical cancer progression. Oncogenesis. 2021;10(3):30. doi:10.1038/s41389-021-00319-w
30. Zhu Y, Li F, Wan Y, et al. Cancer-secreted exosomal MiR-620 inhibits ESCC aerobic glycolysis via FOXM1/HER2 pathway and promotes metastasis. Front Oncol. 2022;12:756109. doi:10.3389/fonc.2022.756109
31. Bhat EA, Sajjad N, Thokar FM. Current advancement of exosomes as biomarkers for cancer diagnosis and forecasting. Cancer Treat Res Commun. 2021;28:100417. doi:10.1016/j.ctarc.2021.100417
32. Zhu Z, Wang H, Pang Y, Hu H, Zhang H, Wang W. Exosomal long non-coding RNA UCA1 functions as growth inhibitor in esophageal cancer. Aging. 2020;12(20):20523–20539. doi:10.18632/aging.103911
33. Fan L, Cao Q, Liu J, Zhang J, Li B. Circular RNA profiling and its potential for esophageal squamous cell cancer diagnosis and prognosis. Mol Cancer. 2019;18(1):16. doi:10.1186/s12943-018-0936-4
34. Thone MN, Kwon YJ. Extracellular blebs: artificially-induced extracellular vesicles for facile production and clinical translation. Methods. 2020;177:135–145. doi:10.1016/j.ymeth.2019.11.007
35. Maas SL, Broekman ML, de Vrij J. Tunable resistive pulse sensing for the characterization of extracellular vesicles. Methods Mol Biol. 2017;1545:21–33.
36. Liu Q, Xiang Y, Yuan S, et al. Plasma exosome levels in non-small-cell lung cancer: correlation with clinicopathological features and prognostic implications. Cancer Biomark. 2018;22(2):267–274. doi:10.3233/CBM-170955
37. Tengler L, Schutz J, Tiedtke M, et al. Plasma-derived small extracellular vesicles unleash the angiogenic potential in head and neck cancer patients. Mol Med. 2023;29(1):69. doi:10.1186/s10020-023-00659-w
38. Joncas FH, Lucien F, Rouleau M, et al. Plasma extracellular vesicles as phenotypic biomarkers in prostate cancer patients. Prostate. 2019;79(15):1767–1776. doi:10.1002/pros.23901
39. Khan NA, Asim M, Biswas KH, et al. Exosome nanovesicles as potential biomarkers and immune checkpoint signaling modulators in lung cancer microenvironment: recent advances and emerging concepts. J Exp Clin Cancer Res. 2023;42(1):221. doi:10.1186/s13046-023-02753-7
40. Yang Y, Kannisto E, Yu G, Reid ME, Patnaik SK, Wu Y. An immuno-biochip selectively captures tumor-derived exosomes and detects exosomal RNAs for cancer diagnosis. ACS Appl Mater Interfaces. 2018;10(50):43375–43386. doi:10.1021/acsami.8b13971
41. Mondal SK, Whiteside TL. Immunoaffinity-based isolation of melanoma cell-derived and t cell-derived exosomes from plasma of melanoma patients. Methods Mol Biol. 2021;2265:305–321.
42. Caradec J, Kharmate G, Hosseini-Beheshti E, Adomat H, Gleave M, Guns E. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem. 2014;47(13–14):1286–1292. doi:10.1016/j.clinbiochem.2014.06.011
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.