Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Prediction of Hospitalization and Mortality in Patients with Chronic Obstructive Pulmonary Disease with the New Global Initiative for Chronic Obstructive Lung Disease 2023 Group Classification: A Prospective Cohort and a Retrospective Analysis
Authors Cheng W , Zhou A, Zeng Y , Lin L, Song Q, Liu C, Zhou Z, Peng Y , Yang M, Yang L, Chen Y, Cai S, Chen P
Received 5 July 2023
Accepted for publication 17 October 2023
Published 26 October 2023 Volume 2023:18 Pages 2341—2352
DOI https://doi.org/10.2147/COPD.S429104
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Wei Cheng,1 Aiyuan Zhou,2 Yuqin Zeng,1 Ling Lin,1 Qing Song,1 Cong Liu,1 Zijing Zhou,1 Yating Peng,1 Min Yang,1 Lizhen Yang,1 Yan Chen,1 Shan Cai,1 Ping Chen1
1Department of Pulmonary and Critical Care Medicine, the Second Xiangya Hospital; Research Unit of Respiratory Disease; Diagnosis and Treatment Center of Respiratory Disease, Central South University, Changsha, Hunan, 410011, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Xiangya Hospital, Central South University, Changsha, Hunan, 410008, People’s Republic of China
Correspondence: Ping Chen, Department of Pulmonary and Critical Care Medicine, the Second Xiangya Hospital; Research Unit of Respiratory Disease; Diagnosis and Treatment Center of Respiratory Disease, Central South University, Changsha, Hunan, 410011, People’s Republic of China, Tel +86-13873115563, Fax +86 731-85295848, Email [email protected]
Background: The revised Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2023 group ABE classification has undergone major modifications, which can simplify clinical assessment and optimize treatment recommendations for Chronic Obstructive Pulmonary Disease (COPD). However, the predictive value of the new grouping classification for prognosis is worth further exploration. We aimed to compare the prediction of hospitalization and mortality between this new GOLD group 2023 ABE classification and the earlier 2017 ABCD classification in a Chinese COPD cohort.
Methods: Data from 2,499 outpatients with COPD, who first registered in the RealDTC study of Second Xiangya Hospital from December 2016 to December 2019, were collected prospectively and assessed retrospectively. Patients were followed up on all-cause mortality until October 2022 or death.
Results: : Of the 2,499 patients with COPD, the risk of hospitalization during the first-year follow-up was higher in group E than in groups A and B. The mortality was higher in group E than in groups A and B, and group B was higher than group A. No differences were seen in the area under the curve (AUC) of 2017 vs 2023 GOLD grouping to predict hospitalization. The time-dependent AUC and concordance index for predicting mortality is slightly higher in the GOLD 2017 ABCD than in the 2023 ABE groups. The new GOLD 12-subgroup (1A– 4E) classification combining the GOLD 1– 4 staging and grouping performed similarly discriminate predictive power for mortality to the GOLD 2017 16-subgroup (A1– 4D) classification.
Conclusion: The risk of hospitalization during the first-year follow-up was higher in group E than in groups A and B. The all-cause mortality increased gradually from GOLD group A to E. The GOLD 2023 classification based on ABE groups did not predict mortality better than the earlier 2017 ABCD classifications.
Keywords: COPD, mortality, hospitalization, GOLD 2023
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a common, preventable, and treatable disease that is defined as a heterogeneous lung condition characterized by chronic respiratory symptoms and persistent airflow obstruction.1 The prevalence of COPD among people no less than 40 years is 13.7% in China.2 COPD, causing 3.23 million deaths in 2019, is now one of the top three causes of death worldwide.3,4
The Global Strategy for Diagnosis, Management, and Prevention of COPD program was launched first in 1997. The first report of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) was issued in 2001. GOLD 2017 ABCD groups were classified by symptoms and exacerbation risk and are no longer grouped based on the level of airflow limitation.5 Although GOLD groups were intended to guide pharmaceutical treatment, clinicians use the prognostic value of these groups to classify risk at the individual level.6 Han et al7 found that the GOLD 2017 ABCD classification had a good predictive ability for hospitalization and all-cause mortality. The HUNT sudy demonstrated that the GOLD 2017 ABCD groups were not associated with COPD all-cause mortality.8 Based on the above studies, the risk of all-cause mortality between GOLD 2017 ABCD groups is still controversial. Besides these, a classification was proposed based on a composite of COPD disease severity and GOLD 2017 ABCD group resulting in 16 subgroups (1A to 4D).9 Studies exhibited that the classification of the GOLD 2017 16 subgroups (1A to 4D) predicted mortality more accurately than the GOLD grouping in 2007, 2011, and 2017.7,9
China is a developing country with a lower education level in the elderly, low knowledge of disease, and low awareness of symptoms in COPD patients.10 Tobacco smoking and biomass smoking are the main risk factors for COPD in China.11 Previous studies presented that patients in group C account for less than 5% according to the GOLD 2017 ABCD groups.12–14 The latest GOLD 2023 report released in November 2022 combines groups C and D as group E based on high exacerbation risk. The revised new GOLD 2023 group is divided into the ABE groups, which can simplify clinical evaluation and optimize treatment recommendations.15 However, less is known about the prognostic value of this new grouping classification for COPD. Hence, we intend to compare the prediction of hospitalization and mortality between the GOLD 2023 group ABE classification and the GOLD 2017 group ABCD classification in a Chinese COPD cohort.
Materials and Methods
Study Participants
The observational study was made of a cohort of 2,499 outpatients with COPD who first visited the Department of Pulmonary and Critical Care Medicine and registered in the RealDTC study (ChiCTR-POC-17010431, database: http://120.77.177.175:9007/a/login) of Second Xiangya Hospital, Changsha, Hunan province. Participants were included between December 1, 2016 and December 31, 2019. Data were collected prospectively and assessed retrospectively. COPD was defined with the forced expiratory volume in 1 second divided by the forced vital capacity (FEV1/FVC) after bronchodilation less than 0.70 according to the GOLD 2017 recommendations.5 Patients no less than 40 years old who agreed to participate in the study were included. Patients previously diagnosed with bronchial asthma, bronchiectasis, interstitial lung disease, carcinoma, severe heart diseases, chronic liver diseases, or chronic kidney diseases were excluded (based on personal medical records). Patients who had an acute exacerbation of COPD within 1 month before the visit to the outpatient clinic (based on personal medical records) were also excluded. This study was approved by the local Ethics Committee of Second Xiangya Hospital of Central South University. We confirmed that all the research meets ethical guidelines and adheres to the legal requirements of China. This study was registered in the Chinese Clinical Trial Registry (ChiCTR-POC-17010431). All participants had signed the informed consent form when they first visited the outpatient clinic.
Variables and Definitions
Age, sex, body mass index (BMI), smoking status, pulmonary function tests, COPD assessment test (CAT) score, Modified Medical Research Council (mMRC) dyspnea scale, number of acute exacerbations (AE) in the past year, the severity of AE (moderate or severe), and inhaled drug prescription were collected at baseline. Smoker was defined as current or past smoking history of over 10 packs per year. Patients who had sustained abstinence for more than 6 months were former smokers.16 Never-smokers were defined as those who had a lifetime exposure to no more than 100 cigarettes or no more than 10 packs per year.17 Moderate exacerbation was defined as those requiring a prescription for oral corticosteroid and/or antibiotic. Severe exacerbation (hospitalization) required hospital management including emergency visits or admissions.5
FEV1 and FVC were measured by spirometry and predicted values were calculated according to the European Respiratory Society guidelines.18 COPD disease severity was classified using the GOLD guidelines and was divided into 1–4 stages.5 The CAT was an eight-item questionnaire that evaluated the health status of COPD patients.19 The mMRC was used to measure the severity of dyspnea, which is an important symptom in many COPD patients.20 GOLD 2017 ABCD groups were defined as follows: group A (less than two moderate AEs which do not lead to hospitalization, and mMRC less than 2 or CAT less than 10); group B (less than two moderate AEs which do not lead to hospitalization, and mMRC no less than 2 or CAT no less than 10); group C (no less than two moderate AEs or no less than one hospitalization, and mMRC less than 2 or CAT less than 10); group D (no less than two moderate AEs or no less than one hospitalization, and mMRC no less than 2 or CAT no less than 10).5 According to the GOLD 2017 classification, we used a classification based on a composite of COPD disease severity and the GOLD 2017 ABCD group resulting in 16 subgroups (1A to 4D).9 Now, GOLD 2023 proposed a further developed ABCD joint assessment tool, which identifies the clinical relevance of the exacerbations of the disease and is independent of the patient’s symptom level. GOLD 2023 ABE groups were defined as follows: group A (less than two moderate AEs which do not lead to hospitalization, and mMRC less than 2 or CAT less than 10); group B (less than two moderate AEs which do not lead to hospitalization, and mMRC no less than 2 or CAT no less than 10); group E (no less than two moderate AEs or no less than one hospitalization).15 According to the above 16 subgroups classifying from 1A to 4D, we used a classification based on a composite of COPD disease severity and GOLD 2023 ABE group, resulting in 12 subgroups (1A to 4E).
Patients were followed up every 6 months after their first registration in the outpatient healthcare database. The number of exacerbations during 6 months, the severity of exacerbation (moderate or severe), and survival status were collected per follow-up. All-cause mortality was defined as the outcome. Overall survival was defined as the length of time from the first visit to the outpatient clinic to death or last contact. These were obtained from personal follow-up. The study endpoint is death or the last routine follow-up before October 31, 2022.
Sample Size Estimation
The sample size was calculated by using PASS 15.0 in the section of Cox regression. We used the mortality as 6.70%, the hazard ratio of the GOLD 2017 group as 1.18 obtained from the pre-experiment, set the interval type as two-sided, and entered the power as 0.80 and the alpha (two-sided) as 0.05. Finally, the sample we acquired was 2,428.
Statistical Analysis
The univariable analysis of hospitalization rate and mortality was performed using Chi-square analysis. Multivariable logistic regression analyses were used for the analysis of hospitalization risk. Multivariable Cox regression analyses were used for mortality analysis. The odds ratio (OR), hazard ratio (HR), and 95% confidence intervals (CI) were estimated using regression analysis. Area under the receiver operating characteristic (ROC) curve (AUC) was used to assess the predictive power of different GOLD groups for AE and hospitalization. A method developed by J. A. Hanley and B. J. McNeil was used to compare the above ROC curves derived from the same subjects.21 The net reclassification index (NRI), integrated discrimination improvement (IDI), Concordance index (C-index), and/or area under the time-dependent ROC curve (time-dependent AUC) were used to evaluate the discriminative ability of death between different GOLD group classifications. Changes in NRI and IDI were used to compare the accuracy of mortality prediction between the 2017 and 2023 GOLD group classifications. A higher C-index or time-dependent AUC value represents a higher ability to predict death. All the analyses were performed with the statistical software packages R 4.1.2 (http://www.R-project.org, The R Foundation) and Free Statistics software version 1.7.1. A P-value less than 0.05 was considered to be statistically significant.
Results
A total of 2,499 participants were included for analysis. Of the 2,499 COPD patients, 87.0% were male, with a mean age of 63.2±8.2 years, and a mean FEV1% predicted of 53.1±20.8%. According to the classification of GOLD 2017 ABCD groups, group A accounts for 8.4%, group B accounts for 44.8%, group C accounts for 2.9%, and group D accounts for 43.9%. According to the new classification of GOLD 2023 ABE groups, group A accounts for 8.4%, group B accounts for 44.8%, and group E accounts for 46.8%. The distribution of 16 subgroups (1A to 4D) and 12 subgroups (1A to 4E), based on a composite of COPD disease severity and GOLD group, were summarized in Supplement Table 1. As shown in Table 1, there were significant differences in age, BMI, smoking status, FEV1% predicted, COPD severity, CAT, mMRC, the incidence of AE, and severe AE frequency in the past year between ABE groups.
 |
Table 1 Comparison of Demographic and Clinical Characteristics at Baseline, and Clinical Outcome During Follow-Up in COPD Patients in the New GOLD 2023 Group |
Overall, the mortality during follow-up was 7.0%. The median follow-up time was 38.0 months (range=4–68 months) in this study. During follow-up, there were significant differences in the percentage of AE/severe AE frequency in first-year follow-up and survival time between ABE groups (Table 1). The rate of hospitalization during the first-year follow-up was higher in group D than in groups A and B. However, there was no significant difference between ABC groups in the rate of hospitalization (Figure 1A). The rate of hospitalization during the first-year follow-up was higher in group E than in groups A and B (Figure 1B). In Table 2, after adjusting for sex, age, BMI, smoking status, and GOLD severity, COPD patients in group E (HR=1.57, 95% CI=1.07~2.30, P=0.02) had a higher risk of hospitalization than group A according to the new classification of GOLD 2023 ABE groups, and patients in group D (HR=1.60, 95% CI=1.09~2.35, P=0.015) had a higher risk of hospitalization than group A according to the classification of GOLD 2017 ABCD groups. However, there was no difference in the risk of hospitalization between groups C and A in COPD patients (P=0.606).
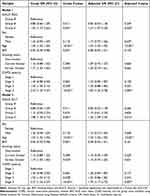 |
Table 2 Multiple Logistic Regression for Hospitalization of GOLD 2023 and GOLD 2017 Group in COPD |
As presented in Figure 1C, the mortality was higher in group D than in groups A and B, and higher in group B than group A. The mortality was higher in group E than in groups A and B, and group B was higher than group A (Figure 1D). The multiple Cox regression showed that patients in group E (HR=6.29, 95% CI=1.53~25.79, P=0.011) and group B (HR=5.34, 95% CI=1.30~21.94, P=0.02) had a higher risk of mortality than group A according to the classification of GOLD 2023 ABE groups. According to the classification of GOLD 2017 ABCD groups, patients in group D (HR=6.54, 95% CI=1.59~26.87, P=0.009) and group B (HR=5.42, 95% CI=1.32~22.25, P=0.019) had a higher risk of mortality than group A. But there was no difference in the risk of mortality between group C and group A in COPD patients (Table 3).
 |
Table 3 Multiple Cox Regression for Mortality of GOLD 2023 and GOLD 2017 Group in COPD |
In Figure 2, both AUCs for AE during the first-year follow-up were 0.59 (95% CI=0.57–0.61) for the GOLD 2023 and GOLD 2017 groups (P=0.062). Both AUCs for severe AE (hospitalization) during the first-year follow-up were 0.59 (95% CI=0.57–0.61) for the GOLD 2023 and GOLD 2017 groups (P=0.070). As presented in Figure 3A and B, the value of time-dependent AUC and C-index predicting 3-/4-/5-year all-cause mortality is slightly higher in the GOLD 2017 group ABCD classification than the GOLD 2023 group ABE classification. The changes in NRI and IDI were used to compare the accuracy of mortality between the classification of GOLD 2023 ABE groups and the classification of GOLD 2017 ABCD groups. As shown in Table 4, the changes in NRI and IDI were less than zero between these two classifications. But there is no difference in the time-dependent AUC and C-index predicting all-cause mortality between the classification of GOLD 2017 A1–D4 16 subgroups and GOLD 2023 A1–E4 12 subgroups (Figure 3C and D). The similarity trend was also found in the NRI and IDI analysis between 16 subgroups (A1–D4) and 12 (A1–E4) subgroups.
 |
Table 4 NRI and IDI Analysis Between GOLD 2023 ABE Group and GOLD 2017 ABCD Group in Mortality Prediction in COPD |
Discussion
Our study revealed that the new GOLD 2023 classification based on ABE groups did not predict mortality better than the earlier 2017 ABCD classifications. The predictive ability of the new 12-subgroup (1A–4E) classification for mortality is similar to that of the earlier GOLD 2017 16-subgroup (A1–4D) classification.
We found that the risk of first-year follow-up hospitalization in Group D (high exacerbation risk) was significantly higher than that in groups A and B (low exacerbation risk). Previous studies have also demonstrated patients with high exacerbation risk (severe or frequent AE) in the past had a higher risk of exacerbation and/or hospitalization,22–24 which may explain the above results. In addition, there was no obvious difference in the hospitalization risk between groups C and A in COPD patients. One of the possible explanations for this result may be related to group C patients with lower symptoms usually leading to a lower risk of exacerbation.25 Moreover, the CHAIN cohort study presented that the patients of group C have poor stability, and more than 70% of them are converted to groups A and B during first-year follow-up, which may provide another explanation.26 The new GOLD 2023 report released in November 2022 combines groups C and D as group E based on high exacerbation risk. The risk of hospitalization during first-year follow-up was higher in group E than in groups A and B, and we think the explanation of the above result was similar to the interpretation in group D described above. The predicted ability of severe exacerbation to result in hospitalization during the first-year follow-up was equal for the GOLD 2023 group ABE classification and GOLD 2017 group ABCD classification, with AUC values around 0.6 which was similar to the results reported in the previous study.8 These above results showed that the GOLD 2023 group classification combines groups C and D as group E did not reduce the predictive ability of GOLD group classification for the risk of hospitalization, but further simplified the clinical assessment.
Two large cohort studies exhibited that the order of mortality risk in GOLD 2017 ABCD grouping from low to high was group A, group C, group B and group D.9,14 The survival analysis of the GOLD 2017 group classification presented a higher mortality risk in group D than in groups B and A, and a higher mortality risk in group B than group A, consistent with the above previous studies. Besides this, we found no significant difference between group C and ABD groups of mortality in COPD patients. The HUNT study presented that the mortality was generally similar in groups C and D, which was similar to the result of our study.27 Furthermore, a cohort study presented that the majority of patients in group C were converted to groups A and B during follow-up, which may lead to similar mortality between groups C and A or group.26,28 In our adjusted Cox regression, the risk of death in groups E and B was significantly higher than in group A. Such trends in mortality risk were consistent with previous studies that reported that COPD patients with higher symptom burden or high exacerbation risk were associated with higher mortality.23,24,29 In this cohort, only 2.88% of COPD patients were classified into group C, and several previous studies also found that the proportion of patients in group C was less than 5%.12–14 The GOLD 2023 combines groups C and D as group E based on high exacerbation risk.15 This real-world cohort study revealed that the all-cause mortality increased gradually from GOLD group A to E. We believe that, compared with the 2017 GOLD group ABCD classification, the number of 2023 GOLD group ABE classification is reduced, the clinical classification will be easier to operate, and the prognosis evaluation between different groups will be easier to understand.
The time-dependent AUC value of all-cause mortality in GOLD 2023 groups ABE classification and GOLD 2017 group ABCD classification were between 0.54–0.60 which was similar to the results reported in the previous study.8,9 Furthermore, the C-index, time-dependent ROC curve, and IDI and NRI analysis both found that the GOLD 2017 group ABCD classification had slightly better mortality discrimination than the GOLD 2023 group ABE classification. However, a previous study revealed that the predictive ability of the GOLD 2023 group ABE classification for mortality is similar to that of the GOLD 2017 group ABCD classification.30 This study included 782 patients with FEV1% predicted of less than 60% who visited the Czech Respiratory Care Center from February 2013 to December 2016. The different populations of COPD patients and the relatively small sample in this study may lead to different research results from ours. Additionally, we found that, when using a composite of GOLD stages and GOLD groups, the discriminate predictive power of the GOLD 2023 A1–4E 12-subgroup classification is similar for mortality to the earlier GOLD 2017 A1–4D 16-subgroup classification. From the above results, we temporarily found no evidence that the GOLD 2023 group ABE classification had better predictive power for mortality than the GOLD 2017 group ABCD classification. Furthermore, the AUC values for exacerbation and mortality in the GOLD group classification were around 0.60, which did not provide sufficient predictive accuracy for individual risk assessment.
Our study also has several limitations. All patients were from one center, but our subjects included COPD patients in stages 1–4, and the study population was collected in eight provinces and 36 cities in China, which helped to improve the representation of COPD patients to some extent. Moreover, only 2.88% of COPD patients were classified into group C in this real-world cohort, which may be related to the characteristics of the population with COPD in China, and several previous studies also found that the proportion of patients in group C was less than 5%.12–14
Conclusion
The risk of hospitalization during first-year follow-up was higher in group E than in groups A and B. The all-cause mortality increased gradually from GOLD group A to E. The predicted ability of severe exacerbation to result in hospitalization during the first-year follow-up was equal for the GOLD 2023 group ABE classification and the GOLD 2017 group ABCD classification. The GOLD 2023 classification based on ABE groups did not predict mortality better than the earlier 2017 ABCD classifications. The new 12-subgroup (1A–4E) classification combining the GOLD 1–4 staging and grouping performed similarly discriminate predictive power for mortality to the earlier GOLD 2017 16-subgroup (A1–4D) classification.
Abbreviations
GOLD, Global Initiative for Chronic Obstructive Lung Disease; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second divided; FVC, forced vital capacity; BMI, body mass index; CAT, COPD assessment test; mMRC, modified Medical Research Council; AE, acute exacerbation; OR, odds ratio; HR, hazard ratio; CI, confidence intervals; ROC, receiver operating characteristic; AUC, area under the receiver operating characteristic curve; NRI, net reclassification index; IDI, integrated discrimination improvement; C-index, concordance index.
Data Sharing Statement
The data that support the findings of this study are available upon reasonable request from the first author.
Ethics Approval and Informed Consent
All procedures followed were in accordance with the Helsinki Declaration. This study was approved by the local Ethics Committee of Second Xiangya Hospital of Central South University. All participants had signed the informed consent form.
Acknowledgments
We gratefully thank research assistant Zhiwen Wang, research assistant Guolin Zhong, Dr. Chujuan Tang, Dr. Xiao Liu, and Dr. Xueshan Li of Second Xiangya Hospital for their contribution to the data collection and regular follow-up of patients.
Author Contributions
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the National Natural Science Foundation of China (NSFC, Grants 81970044), (NSFC, Grants 82270045), and (NSFC, Grants 82001490).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Celli B, Fabbri L, Criner G, et al. Definition and nomenclature of chronic obstructive pulmonary disease: time for its revision. Am J Respir Crit Care Med. 2022;206(11):1317–1325. doi:10.1164/rccm.202204-0671PP
2. Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717. doi:10.1016/s0140-6736(18)30841-9
3. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/s0140-6736(20)30925-9
4. Feigin V. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388(10053):1545–1602. doi:10.1016/s0140-6736(16)31678-6
5. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–582. doi:10.1164/rccm.201701-0218PP
6. Soriano JB, Lamprecht B, Ramírez AS, et al. Mortality prediction in chronic obstructive pulmonary disease comparing the GOLD 2007 and 2011 staging systems: a pooled analysis of individual patient data. Lancet Respir Med. 2015;3(6):443–450. doi:10.1016/s2213-2600(15)00157-5
7. Han MZ, Hsiue TR, Tsai SH, Huang TH, Liao XM, Chen CZ. Validation of the GOLD 2017 and new 16 subgroups (1A-4D) classifications in predicting exacerbation and mortality in COPD patients. Int J Chron Obstruct Pulmon Dis. 2018;13:3425–3433. doi:10.2147/copd.S179048
8. Bhatta L, Leivseth L, Mai XM, et al. GOLD classifications, COPD hospitalization, and all-cause mortality in chronic obstructive pulmonary disease: the HUNT study. Int J Chron Obstruct Pulmon Dis. 2020;15:225–233. doi:10.2147/copd.S228958
9. Gedebjerg A, Szépligeti SK, Wackerhausen LH, et al. Prediction of mortality in patients with chronic obstructive pulmonary disease with the new global initiative for chronic obstructive lung disease 2017 classification: a cohort study. Lancet Respir Med. 2018;6(3):204–212. doi:10.1016/s2213-2600(18)30002-x
10. Fan J, Wang N, Fang LW, et al. 2014年中国40岁及以上人群慢性阻塞性肺疾病知识知晓率及其影响因素 [Awareness of knowledge about chronic obstructive pulmonary disease and related factors in residents aged 40 years and older in China, 2014]. Zhonghua Liu Xing Bing Xue Za Zhi. 2018;39(5):586–592. Chinese. doi:10.3760/cma.j.issn.0254-6450.2018.05.009
11. Fang X, Wang X, Bai C. COPD in China: the burden and importance of proper management. Chest. 2011;139(4):920–929. doi:10.1378/chest.10-1393
12. Jian W, Zeng H, Zhang X, et al. Clinical characteristics of patients with chronic obstructive pulmonary disease assessed using GOLD 2016 and GOLD 2018 classifications: a cross-sectional study in China. J Thorac Dis. 2021;13(10):5701–5716. doi:10.21037/jtd-21-255
13. Zeng Y, Cai S, Chen Y, et al. Current status of the treatment of COPD in China: a multicenter prospective observational study. Int J Chron Obstruct Pulmon Dis. 2020;15:3227–3237. doi:10.2147/copd.S274024
14. Criner RN, Labaki WW, Regan EA, et al. Mortality and exacerbations by global initiative for chronic obstructive lung disease groups ABCD: 2011 versus 2017 in the COPDGene® cohort. Chronic Obstr Pulm Dis. 2019;6(1):64–73. doi:10.15326/jcopdf.6.1.2018.0130
15. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 report). Available from: https://goldcopd.org/?s=GOLD+2023.
16. Liu C, Cheng W, Zeng Y, et al. Different characteristics of ex-smokers and current smokers with COPD: a cross-sectional study in China. Int J Chron Obstruct Pulmon Dis. 2020;15:1613–1619. doi:10.2147/copd.S255028
17. Wheaton AG, Liu Y, Croft JB, et al. Chronic obstructive pulmonary disease and smoking status - United States, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(24):533–538. doi:10.15585/mmwr.mm6824a1
18. Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. report working party standardization of lung function tests, European community for steel and coal. official statement of the European respiratory Society. Eur Respir J Suppl. 1993;16(Suppl 16):5–40. doi:10.1183/09041950.005s1693
19. Pulido Herrero E, Villanueva Etxebarria A, Aramburu Ojembarrena A, et al. Influence of the COPD Assessment Test respiratory item score on the decision to hospitalize patients with disease exacerbation in a hospital emergency department. Emergencias. 2022;34(2):95–102.
20. Hsieh MJ, Chen NH, Cheng SL, et al. Comparing clinical outcomes of tiotropium/olodaterol, umeclidinium/vilanterol, and indacaterol/glycopyrronium fixed-dose combination therapy in patients with chronic obstructive pulmonary disease in Taiwan: a multicenter cohort study. Int J Chron Obstruct Pulmon Dis. 2022;17:967–976. doi:10.2147/copd.S353799
21. Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148(3):839–843. doi:10.1148/radiology.148.3.6878708
22. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883
23. Chenna PR, Mannino DM. Outcomes of severe COPD exacerbations requiring hospitalization. Semin Respir Crit Care Med. 2010;31(3):286–294. doi:10.1055/s-0030-1254069
24. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
25. Lee SD, Huang MS, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600–608. doi:10.1016/j.rmed.2013.12.014
26. Soriano JB, Hahsler M, Soriano C, et al. Temporal transitions in COPD severity stages within the GOLD 2017 classification system. Respir Med. 2018;142:81–85. doi:10.1016/j.rmed.2018.07.019
27. Leivseth L, Brumpton BM, Nilsen TI, Mai XM, Johnsen R, Langhammer A. GOLD classifications and mortality in chronic obstructive pulmonary disease: the HUNT Study, Norway. Thorax. 2013;68(10):914–921. doi:10.1136/thoraxjnl-2013-203270
28. Bernabeu-Mora R, Sánchez-Martínez MP, Montilla-Herrador J, Oliveira-Sousa SL, Gacto-Sánchez M, Medina-Mirapeix F. 2011 GOLD stages of COPD: transitions, predictor factors and comparison with 2017 GOLD stages. Int J Chron Obstruct Pulmon Dis. 2020;15:1519–1527. doi:10.2147/copd.S254434
29. Casanova C, Marin JM, Martinez-Gonzalez C, et al. Differential effect of modified medical research council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest. 2015;148(1):159–168. doi:10.1378/chest.14-2449
30. Brat K, Svoboda M, Zatloukal J, et al. Prognostic properties of the GOLD 2023 classification system. Int J Chron Obstruct Pulmon Dis. 2023;18:661–667. doi:10.2147/copd.S410372
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



