Back to Journals » Clinical and Experimental Gastroenterology » Volume 16
Prediction of High-Risk Varices in Patients with Compensated Advanced Chronic Liver Disease in Saudi Arabia
Authors Ismail M
Received 8 March 2023
Accepted for publication 11 July 2023
Published 19 July 2023 Volume 2023:16 Pages 117—127
DOI https://doi.org/10.2147/CEG.S410041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Santosh Shenoy
Mona Ismail1,2
1Division of Gastroenterology, Department of Internal Medicine, King Fahd Hospital of the University, Alkhobar, Saudi Arabia; 2College of Medicine, Imam Abdulrahman bin Faisal University, Dammam, Saudi Arabia
Correspondence: Mona Ismail, Tel/Fax +00966-3-8966741, Email [email protected]
Purpose: Liver stiffness and low platelet count can predict portal hypertension and high-risk varices (HRVs) in patients with cirrhosis. Thus, screening endoscopy may not be required for all patients with compensated advanced chronic liver disease (cACLD). However, data from Saudi Arabia are limited. This study aimed to validate the Baveno VI and expanded Baveno VI criteria for screening endoscopy and identify the risk factors associated with HRVs in patients with cACLD in Saudi Arabia.
Patients and Methods: We analyzed data from 215 patients with cACLD diagnosed on transient elastography (LSM > 10 kPa) and had paired platelet count and screening upper endoscopy performed within one year of diagnosis. HRVs or varices needing treatment (VNTs) were defined as medium-to-large esophageal varices (EVs), small EVs with red flags, or gastric varices. Sensitivity, specificity, and area under the receiver operating characteristic curve were calculated. Univariate and multivariate logistic regression analyses identified HRV risk factors.
Results: The Baveno VI criteria spared 50.7% of endoscopies, missing 3.7% of VNTs, while the expanded Baveno VI criteria spared 63.7% of endoscopies, missing 5.1% VNTs. An LSM < 20 kPa and platelet count > 150,000/μL were associated with HRV in 8.1% and 8.3%, respectively. While an LSM < 25 kPa and platelet count > 110,000/μL were associated with HRV in 9.7% and 9%, respectively. The Baveno VI criteria had sensitivity and specificity of 76% and 55%, while the expanded criteria had 67% and 69%, respectively. Baveno VI criteria performed better in hepatitis C virus patients than nonalcoholic fatty liver disease patients. Multivariate logistic regression analysis revealed platelet count and LSM as predictors of HRV.
Conclusion: The Baveno VI criteria effectively identified HRVs in cACLD patients from Saudi Arabia, reducing unnecessary endoscopies. Although the expanded criteria avoided more endoscopies, it led to a higher rate of missed HRVs.
Keywords: esophageal varices, elastography, spared endoscopies, cirrhosis, Saudi Arabia
Introduction
Acute variceal hemorrhage is common in patients with cirrhosis and is associated with a mortality rate of 20–40%.1,2 Esophageal varices (EVs) develop and progress at variable rates and are present in 30–40% of patients with compensated cirrhosis and 85% of patients with decompensated cirrhosis.3 Endoscopy is the gold standard investigation for screening patients with cirrhosis for varices needing treatment (VNTs) with nonselective beta-blockers or endoscopic variceal band ligation.4 However, endoscopy is invasive, expensive, and uncomfortable for patients and often requires sedation.5
In patients with chronic liver disease, both liver stiffness measurement (LSM) by transient elastography (TE) and hepatic venous pressure gradient predicted decompensation and complications with portal hypertension.6 The Baveno VI guidelines4 was developed to identify patients with compensated advanced chronic liver disease (cACLD) and VNTs. Based on the combination of LSM (< 20 kPa) on TE and platelet count (> 150,000/µL), the Baveno VI criteria stratify the risk of VNTs in patients with cACLD. As a result, screening endoscopies are avoided in 40–45% of patients, with an acceptable risk of missed high-risk varices (HRVs) of < 5%.
Although most studies have confirmed the validity of the Baveno VI criteria, the results have varied between studies. For example, in a retrospective analysis of 518 patients from centers in Europe and Canada, the ANTICIPATE study concluded that the Baveno VI criteria were useful for excluding VNTs.7 Furthermore, Augustin et al8 reanalyzed data from the ANTICIPATE study and found that almost half of the unnecessary endoscopies could have been avoided by expanding the Baveno criteria to include a platelet count > 110,000/µL and LSM < 25 kPa. Despite using the expanded Baveno VI criteria, the risk of missing VNTs was still 1.6%.8 By contrast, Bae et al9 found that using expanded Baveno VI criteria in a Korean population was associated with an unacceptably high rate of missed HRVs (6.8% vs 3.8% with the original Baveno VI criteria), suggesting limited generalizability of the previous study results. As a result, several systematic reviews and meta-analyses evaluated the diagnostic accuracy of TE and platelet counts for detecting VNTs. Cheng et al10 analyzed 44 studies that included 7294 patients with cirrhosis and found that the Baveno VI criteria had a high degree of diagnostic precision (area under the receiver operating characteristic curve [AUROC] = 0.83) for grade 1–3 EVs, sizeable EVs (ie, grade 2–3; AUROC = 0.84), and large EV (AUROC = 0.92). Despite the comprehensive analysis in the present study, its main limitation was the significant heterogeneity in the TE cutoff values among the included studies, which made it difficult to identify an optimal diagnostic threshold for EVs. In addition, many studies were susceptible to disease progression bias because of failure to account for the time interval between TE and EGD. The results of previous studies showed that the Baveno VI and expanded Baveno VI criteria were useful for risk stratification of patients with cACLD, which led international guidelines to adapt them.11–13
However, the usefulness of these criteria in Middle Eastern patients, particularly from Saudi Arabia, is scarce. For example, Alswat et al14 demonstrated the excellent performance of the EVendo score and the Baveno VI criteria in screening patients with cirrhosis for predicting gastroesophageal varices and VNT in cirrhotic patients in a real-world clinical setting in Saudi Arabia. However, they included a small sample size (103 patients) and patients with decompensated cirrhosis (41.8%). Moreover, acute variceal bleeding is a common cause of hospital admission in patients with liver cirrhosis, yet data from the Middle East and Saudi Arabia are limited.15,16
Therefore, this study aimed to validate the applicability and accuracy of the Baveno VI and expanded Baveno VI criteria in Middle Eastern patients with cACLD and the effectiveness of these criteria in avoiding unnecessary screening endoscopies for EVs in this population. As well as identify and evaluate the risk factors associated with the development of HRVs among patients with cACLD. This study will provide valuable insight into the usefulness of these criteria for other populations and their universal applicability to all populations.
Materials and Methods
We performed a retrospective study of all patients who presented to the Hepatology Clinics at the King Fahd Hospital of the University of Al-Khobar, Saudi Arabia, between December 2013 and December 2021. Our hospital is a referral center for chronic liver disease (including cirrhosis and its complications), with viral hepatitis and non-alcoholic fatty liver disease (NAFLD) being the most common causes of liver disease. We diagnosed cACLD as asymptomatic chronic liver disease and LSM ≥ 10 kPa.4 We included patients aged ≥ 18 years diagnosed with cACLD of any etiology based on TE (ie, LSM ≥ 10 kPa) and paired data available on the platelet count and TE results within 12 months of upper endoscopy. We excluded patients with decompensated cirrhosis (ie, ascites, hepatic encephalopathy, or previous variceal bleeding), previous EV band ligation, portal or splenic vein thrombosis, previous splenectomy, hepatocellular carcinoma, past or current propranolol therapy, or liver transplantation.
Clinical and Laboratory Parameters
We recorded the demographic characteristics of patients, including age, sex, body weight (kg), height (cm), body mass index (BMI; kg/m2), and laboratory parameters, including bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, and creatinine levels, international normalized ratio (INR), hepatitis B and C serology and liver disease etiology. We classified the etiology of liver disease as hepatitis B virus (HBV), hepatitis C virus (HCV) (before the initiation of direct-acting antivirals), NAFLD, and others. All patients were diagnosed with cirrhosis based on clinical, laboratory, liver ultrasonography, upper endoscopy findings, and liver stiffness by TE. We also calculated the Child-Turcotte-Pugh (CTP) class and Model for End-Stage Liver Disease (MELD) score. cACLD was defined as LSM ≥ 10 kPa and CTP class A or B.
Transient Elastography (TE)
TE was performed using FibroScan (502 Touch, Echosens, Paris, France) by an experienced practitioner (M.I.) according to the manufacturer’s recommendations. LSM was measured using the M probe, or if the M probe was unreliable due to obesity, the XL probe. If more than one LSM was available for a patient during the study period, the LSM with the lowest variability (interquartile range [IQR]/median) was selected for analysis. The results were considered unreliable if the number of valid attempts was < 10, the success rate was < 60%, or the IQR/median was > 30%.
Upper Endoscopy Examination
We reviewed the upper endoscopy results of all patients performed within 12 months of TE. The Baveno VI criteria were used to categorize the gastroesophageal varices as low-risk varices (LRVs; ie, grade ≤ 2) or HRVs (large esophageal varices [grade ≥ 3], or small varices with high-risk stigmata of bleeding [red wale signs or cherry-red spots], and gastric varices). All HRVs were considered VNTs. The assessor was not blinded to the results of the upper endoscopy.
Statistical Analyses
The primary study outcome was the presence of HRVs. Categorical data are presented as absolute numbers and percentages, while continuous data are presented as means and standard deviations (SDs) or medians and IQRs, as appropriate. The patients were categorized according to the presence of HRVs. Student’s t-test or Mann–Whitney U-test was used to examine the differences in continuous data, as appropriate. The chi-square test or Fisher’s exact test was used to compare the categorical data, as appropriate. The diagnostic accuracy, ie, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), of the Baveno VI criteria, the expanded Baveno VI criteria, LSM (< 15, < 20, and < 25 kPa), and platelets (> 150,000/µL and >110,000/µL) were calculated and expressed with 95% confidence intervals (CIs). We also calculated the number of endoscopies spared. We evaluated the accuracy of LSM and platelet count to identify patients with HRVs compared to the gold standard investigation (ie, upper endoscopy). We performed sub-group analyses according to the main etiologies of liver disease (HCV and NAFLD). Receiver operating characteristic (ROC) curves were constructed for the continuous predictors of HRV to detect their diagnostic performance. The area under the receiver operating characteristics (AUROCs) was calculated from regression models. The predictors of HRVs were identified using multivariate logistic regression modeling and ROC curves. Variables showing a P<0.10 at univariate analysis were included in a multivariable backward stepwise logistic regression. The analyses were performed using SPSS software (version 27.0; IBM Corp., Armonk, NY, USA). P < 0.05 was considered statistically significant.
Ethical Approval
King Fahad Hospital of the University is the hospital of Imam Abdulrahman Bin Faisal University. Hence, this study’s ethical approval was issued by the ethics committee of the institutional review board (IRB) at Imam Abdulrahman Bin Faisal University (IRB-2020-01-009). This research project was done in accordance with the principles stated in the Declaration of Helsinki. Written informed consent was waived due to the retrospective nature of the study. All collected data were anonymized and kept confidential to ensure patients’ privacy.
Results
Baseline Characteristics and Prevalence of HRVs
We identified 359 patients with cACLD, of whom 215 underwent TE and upper endoscopy within one year and fulfilled our inclusion criteria (Figure 1). The median (IQR) age of the patients was 56 (44–64) years, and the majority were males and Saudi nationals (52.1% and 82.3%, respectively). The main etiology of liver disease was HCV (38.4%), followed by NAFLD (35.3%) and HBV (17.2%). The median time interval between upper endoscopy and TE was 23 (range: 2–124) days. Most patients had CTP class A (84.2%), while 15.3% had class B cirrhosis.
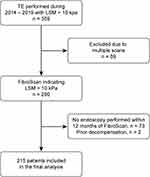 |
Figure 1 Study flow chart. |
Of the 215 patients who underwent screening upper endoscopy, 159 (73.95%) had varices, and 33 (15.3%) had HRVs. Patients with HRVs were mainly Saudi men (69.7%, p < 0.039) with cACLD due to HCV. As shown in Table 1, patients with HRVs had significantly higher bilirubin levels, INR, LSM value, and MELD score, and significantly lower platelet count and albumin level than those without HRVs.
 |
Table 1 Baseline Characteristics of Study Participants (n = 215) |
Diagnostic Performance of the Baveno VI and Expanded Baveno VI Criteria for the Prediction of HRVs
Next, we assessed the performance of the Baveno VI and expanded Baveno VI criteria for detecting HRVs in patients with cACLD (Table 2). Using the Baveno VI criteria, 109 of 215 (50.7%) patients with cACLD did not require screening upper endoscopy for HRVs. Of these 109 patients, 8 (7.3%) had HRVs and were misclassified, which would have led to a missed diagnosis of HRVs of 3.7% (Table 2). Similarly, using the expanded Baveno VI criteria, 137 of 215 (63.7%) patients did not require screening upper endoscopy. Of these 137 patients, 11 (8%) had HRVs, resulting in a misclassification rate for HRVs of 5.1% (Table 2). The performance outcomes of the predictors of HRVs according to different LSM value cutoffs and platelet counts are presented in Table 2.
 |
Table 2 Diagnostic Performances of Criteria for the Prediction of HRVs (n = 215) |
The sensitivity, specificity, PPV, NPV, positive likelihood ratio (LR+), and negative likelihood ratio (LR−) of the Baveno VI criteria for the diagnosis of HRVs in patients with cACLD were 76%, 55%, 24%, 93%, 1.70, and 0.44, respectively. The sensitivity, specificity, PPV, NPV, LR+, and LR− of the expanded Baveno VI criteria for diagnosing HRVs were 67%, 69%, 28%, 92%, 2.17, and 0.48, respectively. Furthermore, a cutoff platelet count of ≤ 110 × 103/µL was associated with the highest specificity (ie, 88%) (Table 2).
Diagnostic Performance of the Criteria for the Prediction of HRVs in Patients with HCV or NAFLD
We performed subgroup analysis on the main etiologies of liver disease (HCV and NAFLD) (Table 3); however, analyses of the other liver disease etiologies could not be performed, as the sample sizes were too small to ensure robust conclusions.
 |
Table 3 Diagnostic Performances of Criteria for the Prediction of HRVs in Patients with HCV (n = 83) and NAFLD (n = 73) |
In the HCV subgroup (n = 83), using Baveno VI criteria, avoided upper endoscopy in 37.3%, resulting in a missed HRV rate of 3.6% (Table 3). In the HCV subgroup, the sensitivity, specificity, LR+, and LR− of these criteria for the diagnosis of HRVs were 82%, 42%, 1.43, and 0.42, respectively. Additionally, the expanded Baveno VI criteria in the HCV subgroup increased the rate of spared endoscopies to 54.2% but also increased the rate of missed HRVs to 6.0%. Furthermore, the sensitivity, specificity, LR+, and LR− of the expanded criteria for diagnosing HRVs in patients with HCV were 71%, 61%, 1.79, and 0.48, respectively.
However, in patients with NAFLD (n = 76), the Baveno VI and expanded Baveno VI criteria avoided the upper endoscopy in 57.9% and 68.4% of patients, respectively, resulting in a rate of missed HRVs of 5.3% and 6.6%, respectively. For the patients with NAFLD, the Baveno VI and expanded Baveno VI criteria had sensitivities of 60% and 50%, respectively, and specificities of 61% and 71%, respectively.
AUROCs for the Detection of HRVs
The AUROCs for different diagnostic criteria for excluding the presence of HRVs are presented in Table 4 and Figure 2. The cutoff platelet counts of > 110 × 103/µL and > 150 × 103/µL showed AUROCs of 0.70 and 0.681, respectively, was acceptable for excluding the presence of HRVs. While the AUROC for Baveno VI, the expanded Baveno VI criteria and albumin were 0.656, 0.679 and 0.629, respectively.
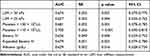 |
Table 4 AUCs for Predictors of High-Risk Varices (n = 215) |
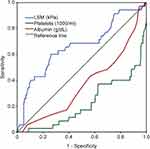 |
Figure 2 AUROC curves for each criterion (n = 215). Abbreviation: AUROC, area under the receiver operating characteristics. |
Predictors of the Development of HRVs
In univariate and multivariate logistic regression analyses, platelet count and LSM were independently associated with the presence of HRVs in both models (Table 5). In contrast, none of the other clinical characteristics, such as age, BMI, blood counts, CTP score, or etiology of liver disease, predicted the presence of HRVs (The clinical characteristics of missed compared with not missed patients with HRVs are seen in Supplementary Table 1).
 |
Table 5 Univariable and Multivariable Logistic Regression Models for Factors Associated with HRVs |
Discussion
It is important to validate non-invasive diagnostic tools for clinically significant portal hypertension, such as Baveno VI and expanded Baveno VI criteria, on various populations before they can be universally adopted into clinical practice. We aimed to identify patients with cACLD who can safely avoid screening upper endoscopies with low risk of HRV in a Saudi tertiary care university-based hospital. The present study identified a high prevalence of HRVs (15.3%) in patients with cACLD. The Baveno VI criteria performed better than the expanded Baveno VI criteria for excluding the presence of VNTs, with missed rates of VNTs of 3.7% and 5.1% for both criteria, respectively; however, the use of Baveno VI criteria avoided fewer endoscopies than the use of the expanded Baveno VI criteria (50.7% vs 63.7%, respectively). Similarly, the Baveno VI criteria were more useful for patients with HCV than those with NAFLD. Furthermore, platelet count and LSM predicted the presence of HRVs in logistic regression analyses. Thus, identifying patients with a low probability of having HRVs that require prophylactic therapy is essential to avoid unnecessary screening of upper endoscopies, thus reducing the associated time, cost, and procedural risks.
In the present study, gastroesophageal varices were present in 73.9% of patients (LRVs: 58.6% and HRVs: 15.3%). Furthermore, the prevalence of HRVs in our patients with cACLD was higher than that of Maurice et al17 in a Western cohort with cACLD (15.3% vs 5%, respectively). A possible explanation is that the prevalence of HRVs varies according to the underlying liver disease and its severity in different populations.
We validated the Baveno VI and the expanded Baveno VI criteria in our patients. Compared to the expanded Baveno VI criteria, the Baveno VI criteria misclassified fewer HRVs (3.7% vs 5.1%, respectively) and avoided fewer unnecessary endoscopies (50.7% vs 63.7%, respectively). Similarly, Bae et al9 reported that the use of Baveno VI criteria in an Asian population led to a lower missed rate for HRVs (3.8% vs 6.8%) and spared fewer endoscopies (27.6% vs.51.7%) compared to the use of the expanded Baveno VI criteria. Therefore, the expanded criteria increased the risk of missing HRVs to greater than the acceptable threshold of 5%.
The sensitivity and NPV of the Baveno VI criteria for diagnosing HRVs (76% and 93%, respectively) were superior to those of the expanded Baveno VI criteria (67% and 92%, respectively). However, the expanded Baveno VI criteria showed higher specificity than the Baveno VI criteria (69% and 55%, respectively). This is in contrast with a meta-analysis of 30 studies, including 8469 patients with cACLD, which showed pooled sensitivities of 97% and 90% for the Baveno VI and expanded Baveno VI criteria, respectively, for the detection of HRVs.18 Furthermore, the pooled specificities of the Baveno VI and expanded Baveno VI criteria were 32% and 51%, respectively. The main limitations of this meta-analysis included the inclusion of retrospective studies, which can introduce selection bias, miss patients with mild disease, and lead to interobserver variability in the interpretation of endoscopy (ie, presence of varices) and TE (ie, liver stiffness) findings.
We performed further analyses to investigate the diagnostic performance of both criteria for patients with specific etiologies of liver disease; the Baveno VI criteria showed a good performance in patients with HCV, with a missed rate of HRVs of 3.6%. In comparison, in patients with NAFLD, although the use of expanded Baveno VI criteria avoided a greater number of unnecessary endoscopies than the use of Baveno VI criteria (68.4% vs 57.9%, respectively), the use of both criteria was associated with a rate of missed VNTs (6.6% vs 5.3%, respectively) that exceeded the proposed cutoff value of < 5%. The latter finding is in contrast with the results of a large multicenter study of 790 patients with NAFLD-related compensated cirrhosis by Petta et al19 which showed that the expanded Baveno VI criteria were more effective at excluding VNTs, and avoided a greater number of unnecessary upper-endoscopies (58% and 33.8%, respectively), compared to the Baveno VI criteria, despite leading to a slight increase in the rate of missed VNTs (3.8% vs 0.9%, respectively). Thus, in patients with cACLD, the underlying etiology may affect the criteria accuracy based on LSM and platelet count.
Our regression analysis showed that high LSM and low platelet count predicted the presence of HRVs in patients with cACLD. These results agree with previous studies.20
The present study had some limitations. The main limitations were that the study included a hospital-based patient population and had a retrospective study design, which may be associated with an overestimated diagnostic accuracy and selection bias. For instance, patients with mild diseases may have been excluded if they did not undergo screening endoscopy. In addition, data were collected from a single tertiary center where endoscopies were performed by several experienced operators, thus limiting the generalizability of our results. Further studies must validate the Baveno VI and expanded Baveno VI criteria for different etiologies of cACLD. The low prevalence of VNTs in our study, although within the range reported in other studies, would have impacted the sensitivity of the criteria tested. Finally, subgroup analysis could only be performed for some etiologies because of the insufficient number of patients in those subgroups. However, the fundamental strengths of the present study include the validation of the Baveno VI and expanded Baveno VI criteria in an unexplored population. Second, our results are consistent with previous studies that reported different prevalences of VNTs. Finally, despite the retrospective nature of our study, our results support the validity of the Baveno VI criteria.
In conclusion, the Baveno VI criteria performed well in patients with cACLD from Saudi Arabia, effectively reducing the need for unnecessary screening endoscopies. However, the expanded Baveno VI criteria, while further decreasing the number of unnecessary endoscopies, also exhibited a higher rate of missed high-risk varices. Further studies that provide alternatives to the TE-based criteria, which requires fibroscan, are needed.
Acknowledgments
The author thanks Dr. Aiman El-Saed (at Infection Prevention and Control Department, King Abdulaziz Medical City, Riyadh, Saudi Arabia) for his help with statistical analysis.
Disclosure
The author reports no conflicts of interest in relation to this work.
References
1. North Italian Endoscopic Club for the Study and Treatment of Esophageal Varices. Prediction of the first variceal hemorrhage in patients with cirrhosis of the liver and esophageal varices. N Engl J Med. 1988;319(15):983–989. doi:10.1056/NEJM198810133191505
2. D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231. doi:10.1016/j.jhep.2005.10.013
3. Merli M, Nicolini G, Angeloni S, et al. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38(3):266–272. doi:10.1016/S0168-8278(02)00420-8
4. De Franchis R, Baveno V. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):
5. Ben-Menachem T, Decker GA, Early DS, et al. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76(4):707–718. doi:10.1016/j.gie.2012.03.252
6. Robic MA, Procopet B, Metivier S, et al. Liver stiffness accurately predicts portal hypertension related complications in patients with chronic liver disease: a prospective study. J Hepatol. 2011;55(5):1017–1024. doi:10.1016/j.jhep.2011.01.051
7. Abraldes JG, Bureau C, Stefanescu H, et al. Noninvasive tools and risk of clinically significant portal hypertension and varices in compensated cirrhosis: the “Anticipate” study. Hepatology. 2016;64(6):2173–2184. doi:10.1002/hep.28824
8. Augustin S, Pons M, Maurice JB, et al. Expanding the Baveno VI criteria for the screening of varices in patients with compensated advanced chronic liver disease. Hepatology. 2017;66(6):1980–1988. doi:10.1002/hep.29363
9. Bae J, Sinn DH, Kang W, et al. Validation of the Baveno VI and the expanded Baveno VI criteria to identify patients who could avoid screening endoscopy. Liver Int. 2018;38(8):1442–1448. doi:10.1111/liv.13732
10. Cheng F, Cao H, Liu J, et al. Meta-analysis of the accuracy of transient elastography in measuring liver stiffness to diagnose esophageal varices in cirrhosis. Medicine. 2018;97(28):e11368. doi:10.1097/MD.0000000000011368
11. Berzigotti A, Tsochatzis E, Boursier J, et al. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J Hepatol. 2021;75(3):659–689. doi:10.1016/j.jhep.2021.05.025
12. European Association for Study of Liver. Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH clinical practice guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63(1):237–264. doi:10.1016/j.jhep.2015.04.006
13. Singh S, Muir AJ, Dieterich DT, Falck-Ytter YT. American gastroenterological association institute technical review on the role of elastography in chronic liver diseases. Gastroenterology. 2017;152(6):1544–1577. doi:10.1053/j.gastro.2017.03.016
14. Alswat K, Alanazi M, Bashmail A, et al. Validation of the EVendo score for the prediction of varices in cirrhotic patients. Saudi J Gastroenterol. 2022;28(5):378–384. doi:10.4103/sjg.sjg_624_21
15. Fallatah HI, Al Nahdi H, Al Khatabi M, et al. Variceal hemorrhage: Saudi tertiary center experience of clinical presentations, complications and mortality. World J Hepatol. 2012;4(9):268–273. doi:10.4254/wjh.v4.i9.268
16. Gado AS, Ebeid BA, Abdelmohsen AM, et al. Clinical Outcome of Acute Upper Gastrointestinal Hemorrhage among Patients Admitted to a Government Hospital in Egypt. Saudi J Gastroenterol. 2012;18(1):34–39. doi:10.4103/1319-3767.91737
17. Maurice JB, Brodkin E, Arnold F, et al. Validation of the Baveno VI criteria to identify low risk cirrhotic patients not requiring endoscopic surveillance for varices. J Hepatol. 2016;65(5):899–905. doi:10.1016/j.jhep.2016.06.021
18. Stafylidou M, Paschos P, Katsoula A, et al. Performance of Baveno VI and expanded Baveno VI criteria for excluding high-risk varices in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2019;17(9):1744–1755.e11. doi:10.1016/j.cgh.2019.04.062
19. Petta S, Sebastiani G, Bugianesi E, et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69(4):878–885. doi:10.1016/j.jhep.2018.05.019
20. Ding NS, Nguyen T, Iser DM, et al. Liver stiffness plus platelet count can be used to exclude high-risk oesophageal varices. Liver Int. 2016;36(2):240–245. doi:10.1111/liv.12916
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
