Back to Journals » Nature and Science of Sleep » Volume 13
Prediction of Epiglottic Collapse in Obstructive Sleep Apnea Patients: Epiglottic Length
Authors Kuo IC, Hsin LJ, Lee LA , Fang TJ, Tsai MS , Lee YC, Shen SC, Li HY
Received 30 August 2021
Accepted for publication 22 October 2021
Published 3 November 2021 Volume 2021:13 Pages 1985—1992
DOI https://doi.org/10.2147/NSS.S336019
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Pandi Ratnas
I-Chun Kuo,1,2 Li-Jen Hsin,1,2 Li-Ang Lee,1,2 Tuan-Jen Fang,1,2 Ming-Shao Tsai,2,3 Yi-Chan Lee,2,4 Shih-Chieh Shen,2,5 Hsueh-Yu Li1,2
1Departments of Otolaryngology-Head & Neck Surgery, Chang Gung Memorial Hospital, Linkou Main Branch, Tao-yuan City, Taiwan; 2College of Medicine, Chang Gung University, Tao-Yuan, Taiwan; 3Departments of Otolaryngology-Head & Neck Surgery, Chang Gung Memorial Hospital, Chiayi Branch, Chiayi City, Taiwan; 4Departments of Otolaryngology-Head & Neck Surgery, Chang Gung Memorial Hospital, Keelung Branch, Keelung City, Taiwan; 5Departments of Otolaryngology-Head & Neck Surgery, New Taipei City Municipal Tucheng Hospital, New Taipei City, Taiwan
Correspondence: Hsueh-Yu Li
Departments of Otolaryngology-Head & Neck Surgery, Chang Gung Memorial Hospital, Linkou Main Branch, Tao-yuan City, Taiwan
Tel +886-3-328-1200 ext.3966
Fax +886-3-397-9361
Email [email protected]
Objective: This study aims to explore the factors that contribute to epiglottic collapse (EC) in patients with obstructive sleep apnea (OSA).
Methods: This study enrolled 35 patients (34 males; median age, 39 years; median apnea–hypopnea index (AHI), 55.4 events/h; median body mass index (BMI), 26.9 kg/m2). EC (epiglottis attaching onto the posterior pharyngeal wall) was diagnosed by drug-induced sleep computed tomography (DI-SCT). Three dimensions were assessed for comparison between the EC and non-EC (NEC) groups that included anatomical measurement: epiglottic length and angle, endoscopic classification of epiglottis obstructing the glottis (Type I, none; Type II, partial; and Type III, total), and dynamic hyoid movement during DI-SCT (Δ hyoid = √(x2 + y2), maximal displacement of hyoid in x and y axes during sleep breathing cycle).
Results: EC was found in 12 patients (34%). No difference in age, gender, AHI, and BMI between the two groups was noted. The anatomical measurement revealed that epiglottis length was significantly different between the EC and NEC groups (21.2 vs 15.8 mm; p < 0.001), with a cutoff value of 16.6 mm (sensitivity, 100%; specificity, 65.2%). The EC group patients showed larger hyoid movement than the NEC group patients (Δ hyoid, 4.8 vs 3.0 mm; p = 0.027). By contrast, epiglottic angle and endoscopic classification revealed an insignificant difference between the two groups.
Conclusion: Epiglottis is a potential collapse site among multilevel obstruction in moderate to severe OSA patients. Epiglottic length is highly sensitive in predicting EC, with the cutoff value of 16.6 mm. Hyoid movement plays a role in contributing to EC in OSA patients.
Keywords: obstructive sleep apnea, epiglottic collapse, drug-induced sleep computed tomography, epiglottic length, hyoid bone movement
Introduction
Obstructive sleep apnea (OSA) is defined as repeated episodes of obstructive apnea and hypopnea during sleep and featured by daytime sleepiness and altered cardiopulmonary function.1,2 OSA is a multifactorial disease with individual phenotypes (anatomy, muscle tone, arousal, and loop) wherein the upper airway is obstructed during sleep.3 Airway obstruction in OSA patients is commonly multilevel and includes the velopharynx, oropharyngeal lateral wall, tongue, and epiglottis (VOTE) classification.4 Among the VOTE classification, velopharyngeal obstruction is the most common obstruction type. By contrast, epiglottic collapse (EC) is the least common obstruction type and is usually ignored in the OSA surgical plan.5 Although epiglottic collapse is usually ignored, the incidence of epiglottic collapse may be much higher. Clinically, EC may not be detected by awake examination.6
EC identification is important because it may cause treatment failure in both continuous positive airway pressure and uvulopalatopharyngoplasty.7,8 Kim et al9 demonstrated an OSA patient with epiglottic collapse that became worsened as CPAP was applied in a video presentation. Sung et al6 showed that OSA patients with epiglottic collapse have unfavorable clinical characteristics to CPAP compliance. Obstruction level assessment in OSA patients can be implemented during wakefulness or drug-induced sleep. Previous studies by awake examination focused on the retropalatal and retroglossal obstruction without exploring the existence of EC. After a wide use of drug-induced sleep endoscopy (DISE) for presurgical assessment, EC was found in approximately 36.4% of moderate to severe OSA patients.10–12 The necessity of sedation to simulate sleep status is a drawback of DISE that limits its general use. Thus, renewing traditional measures to enhance the awareness of EC before DISE is crucial and practical.
This study aims to explore the measurements (anatomical, endoscopic, and dynamic) that can predict and contribute to the mechanism of EC in patients with OSA.
Materials and Methods
This study was approved by The Institutional Review Board of Linkou-Chang Gung Memorial Hospital, Taoyuan, Taiwan (101-3547A3), which was performed according to the principles outlined in the Declaration of Helsinki. All participants in this study provided written informed consent, and all procedures were conducted under current regulations. Inclusion criteria were designed as: (1) chief complaint of snoring and/or daytime sleepiness; (2) moderate to severe OSA (hypopnea index (AHI) > 15); and (3) age between 18 and 60 years. Exclusion criteria were: (1) allergy to propofol or seizure history; (2) pregnant or lactating women; (3) poor general condition for surgery (eg, stroke, coronary heart disease, bleeding tendency, chronic obstructive pulmonary disease, uncontrolled asthma, neuromuscular disease, or pathological obesity); (4) previously received palate or tongue surgery; and (5) working abroad or incapability of regular follow-up.
The dynamic computed tomography (CT) scan used the Aquilion One system (320-detector row, Toshiba, Japan) to obtain images from the patient’s orbital floor to the hyoid bone for both awake (predrug) and drug-induced sleep status. Light sedation (BIS 70–75) was achieved with propofol. The radiation dose was 1.37 mSv for 10 s in a span of two to three respiration cycles.13 Images were collected as midsagittal and three-dimensional for reconstruction and postprocessing. All awake CT images were reviewed and measured by one single experienced radiologist who was blind to the results of drug-induced sleep CT (DI-SCT) and endoscopic findings.
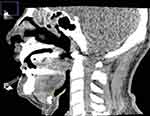
The primary outcome was aimed to understand the relationship between epiglottis anatomical features and its collapsibility. Epiglottic length (from the free edge to the base of the epiglottis) and epiglottic angle (the angle between the long axis of the epiglottis and the vertical plane) were measured in the midsagittal view of the CT scan during awake status with fixed head position before drug-induced sleep by ImageJ software (Figure 1).14
Through the sagittal view of dynamic DI-SCT, epiglottic movement and interaction between epiglottis and tongue base were clearly observed; primary (solitary) epiglottic collapse could be differentiated from secondary epiglottic collapse caused by tongue base compression. In this study, only primary epiglottic collapse was defined as EC. Collected images from DI-SCT were categorized into two groups on the basis of collapsibility of epiglottis: EC (anteroposterior swinging of the epiglottis with touching the posterior pharyngeal wall) and non-EC (NEC, no anteroposterior swinging of the epiglottis, or epiglottic swinging without touching posterior pharyngeal wall; Figure 2). The collapsibility of epiglottis under DI-SCT was correlated to its awake anatomical measurement.
Besides the mobility of epiglottis in breathing cycles during sleep, the hyoid bone also demonstrated obvious positional displacement toward the posteroinferior direction from DI-SCT that contributes to airway obstruction. Thus, the maximum displacement of the hyoid bone in the x and y axes during the sleep breathing cycle was measured by ImageJ software, and its delta hyoid bone (Δ hyoid = √(x2 + y2)) was calculated to obtain the quantitative data of hyoid bone movement in patients who underwent DI-SCT.
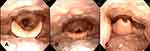
Digital image archives of the larynx via transnasal fiberoptic flexible endoscopy were also collected from patients under awake status in the sitting position. They were classified into three types according to the visibility of the glottis: type I (the whole glottis is visible); type II (the glottis is partially obscured by epiglottis); and type III (the glottis is obscured by the epiglottis; Figure 3). It is probable that visualization of the vocal fold varies to some extent according to the position of endoscopy. In this study, one single experienced physician (Li) performed all the endoscopy with standardized procedure to improve inter- and intra-reliability.
Statistical analyses were performed using RStudio software (RStudio Team, 2015 RStudio: Integrated Development for R. RStudio, Inc., Boston, MA, USA). All p values were two-sided, and statistical significance was accepted at p < 0.05. Descriptive data were expressed with median and range. The chi-square test was used to compare counted data among groups, and the Mann–Whitney U-test was used in testing the difference between continuous variables of different groups in this study. The statistical significance was accepted at p < 0.05.
Results
Patient Demographics
The prospective study used DI-SCT to both screen epiglottic collapse from sagittal view and measure corresponding epiglottic anatomy. Owing to the budget and duration of the research project, the sample size of study population was small. This study enrolled 35 patients (34 males) with a median age of 39 (range, 24–67) years, a median body mass index (BMI) of 26.9 (range, 19.6–32.8) kg/m2, and a median AHI of 55.4 (range, 15.0–122.1) events/h. Six (17%) of the subjects had moderate OSA, whereas 29 (83%) had severe OSA. All patients completed the DI-SCT examination with no event of airway compromise. EC and NEC were found in 12 (34%) and 23 (66%) patients, respectively. Moreover, no difference in age, gender, AHI, and BMI was noted between the EC and NEC groups (Table 1).
 |
Table 1 Demographics |
Endoscopic Score
According to the endoscopic classification, the patients’ distribution of types I, II, and III in the NEC group (n = 23) was 10, 12, and 1, respectively. By contrast, the patients’ distribution of types I, II, and III in the EC group (n = 12) was 1, 9, and 2, respectively. Although no statistical significance (p = 0.08, chi-square test) in EC was noted on the basis of endoscopic classification between the two groups, the percentage of EC revealed an increasing tendency along with increasing severity from endoscopic findings (type I, 9%; type II, 43%; and type III, 67%).
Anatomical Measurement of the Epiglottis
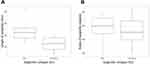
For epiglottic length, a significant difference was noted between the EC and NEC groups (21.2 ± 3.9 vs 15.8 ± 2.9 mm; p < 0.001). However, statistical insignificance in epiglottic angles was noted between the two groups (38.6 ± 9.4 vs 36.2 ± 9.3; p = 0.41; Figure 4). Individuals with epiglottic length more than a cutoff value of 16.6 mm were prone to EC (sensitivity, 100%; specificity, 65.2%; Youden index, 0.65; area under the ROC curve, 0.88; p < 0.0001; Figure 5). The relationship between BMI and epiglottic angle was analyzed and showed no correlation (p = 0.100).
 |
Figure 5 The cutoff value of the length of the epiglottis is 16.6 mm (sensitivity, 100%; specificity, 65.22%; Youden index: 0.65; area under the ROC curve: 0.88; p < 0.0001). |
Hyoid Movement During DI-SCT
The mean Δ hyoid of the EC and NEC groups was 4.8 ± 3.7 and 3.0 ± 3.7 mm, respectively. A comparison of the mean Δ hyoid between the EC and NEC groups showed a significant difference (p = 0.027; Figure 6).
 |
Figure 6 A significantly higher hyoid bone movement is seen in patients with epiglottic collapse than non-epiglottic collapse in drug-induced sleep computed tomography (4.8 vs 3.0 mm; p = 0.027). |
Discussion
Although some studies have already dealt with the anatomic, endoscopic, and hyoid position of epiglottic collapse individually, this study is the first to screen EC through multidimensional assessment including anatomical measurement, endoscopic manifestation, and dynamic hyoid movement in OSA patients. This study is the first to examine potential determining factors of EC through multidimensional assessment including anatomical measurement, endoscopic manifestation, and dynamic hyoid movement in OSA patients. The results showed that epiglottic length is highly sensitive in predicting EC, with the cutoff value of 16.6 mm. Increase of epiglottic length along with increasing severity of glottic concealment, and posterior and inferior hyoid movement, contributes to EC.
Endoscopy has been used to identify the obstruction level for the surgical plan in OSA patients. Muller’s maneuver was widely used in patient selection for uvulopalatoplasty.15 However, the results showed its incapability to identify tongue and EC in OSA patients.16 DISE is currently utilized to assess dynamic obstruction of the upper airway and accordingly determine a surgical plan for patients with OSA. Nevertheless, the medical cost is expensive, and patients must be sedated.12 Based on this understanding, a fiberoptic nasopharyngoscopy was performed in a sitting position at outpatient settings to observe the epiglottis and its concealment of glottis determining its predictivity for EC during sleep. No significant difference in EC among the three endoscopic types was found. Despite the increasing trend (type I, 9%; type II, 43%; and type III, 67%), the statistical insignificance implies that there is still uncertainty in the use of this classification, and we should not merely use awake endoscopic examination to predict EC. Additionally, the lack of statistical significance may be due to the small sample size population in the three groups.
This study took anatomical measurements of the epiglottis (length and angle) to examine its relationship to EC in fixed head position and awake status. The longer length of the epiglottis was observed to be prone to collapse during DI-SCT (p < 0.001), whereas the angle of epiglottis did not show any difference in the two epiglottis groups. After reviewing articles on laryngeal measurement, the link between epiglottic length and EC was speculated to possibly be related to the glottic inlet and vocal fold length. According to one study focused on the Taiwanese population, the maximum vocal fold length was 15.0 mm in men.17 Another study demonstrated that the vocal fold length was 15.3 mm in Taiwanese males under general anesthesia.18 This study found that the cutoff value in epiglottic length for EC is 16.6 mm, with sensitivity of 100% and specificity of 65.22%. The cutoff value of epiglottic length is larger than the vocal fold length and supports the inference that when the length of the epiglottis is longer than the vocal fold and laryngeal inlet, then the epiglottis is unlikely to resist inspiration-induced suction power during sleep and this consequently leads to EC. Epiglottic length can also be measured by routine image examination (eg, cephalometry), and this largely extends its clinical use when DISE is not available. This cutoff value of 16.6 mm in epiglottic length is helpful to patient selection for the necessity to treat epiglottis in multilevel obstruction, and this also rationalizes partial horizontal epiglottidectomy for EC. Although the cutoff value of 16.6 mm in epiglottic length may be helpful to patient selection for the necessity to treat epiglottis in multilevel obstruction, it does not mean we need to cut epiglottis of more than 16.6 mm in every patient. In the study, patients with EC and non-EC did not show significant difference in AHI, which means EC may not be a determining factor in sleep apnea, and the relationship between EC and sleep apnea or desaturation is complex and influenced by multiple factors. It is noteworthy that a longer epiglottis is the primer of epiglottis collapse but will not necessarily cause apnea and desaturation because there is still airspace between the lateral aspect of the epiglottis and the lateral pharyngeal wall. In addition, epiglottic width should also be considered since the two combined are more likely to cause total obstruction of the laryngeal airway.
It is worthy of attention that an omega-shaped epiglottis has narrowing in epiglottic width and its collapse may present as folding. With anterior-posterior collapse, it will not block the larynx completely due to the remnant airspace in the lateral aspect. Aerodynamic change of the upper airway is another concern. Epiglottic collapse may cause opening at the retropalatal and retroglossal airway since airflow is stopped entering the larynx and is transformed into air-splint in these structures. Once solitary resection of the epiglottis is performed, velopharynx and tongue base may revert to collapse, and that warrants follow-up. The impact on epiglottic collapse to AHI could be complex due to the composition of AHI involving both anatomical structure and respiratory physiology, and is likely to be variable in individual OSA patients. However, epiglottic collapse is part of multilevel obstruction in OSA patients. Partial epiglottectomy improves residual apnea–hypopnea index in patients with epiglottis collapse.19 Therefore, screening of epiglottic collapse is still important before treating OSA patients.
The size of the tongue has a positive correlation to BMI; the fat distribution of the tongue is uneven, as the posterior tongue has greater fat content than the anterior tongue.20 BMI was analyzed to influence the epiglottic angle and showed no correlation (p = 0.100). Nevertheless, previous studies revealed that the tongue fat, tongue base collapse, epiglottis, and BMI have a complicated correlation.6,9,21,22
The hyoid bone position has been inferred in the EC mechanism. Genta et al23 documented the hyoid bone position, and the base of the tongue in severe OSA patients is markedly different from the non-severe OSA patients from CT scan under wakeful status. Genta et al23 also found that airway collapsibility is associated with hyoid position by identifying its correlation with pharyngeal critical closing pressure. This study measured the dynamic change of hyoid position in both posterior (x axis) and inferior (y axis) dimensions from DI-SCT. The results showed that the inferior–posterior complex hyoid movement is significantly different between the EC and NEC groups (4.8 ± 3.7 and 3.0 ± 3.7 mm; p = 0.027). This suggests that hyoid movement in OSA patients plays a role in contributing to EC and rationalizes hyoid suspension for EC in OSA patients as in previous studies.24,25
The core value of DI-SCT is the demonstration of dynamic change of the upper airway through sagittal view of CT scan in simulated sleep status. Its advantages include observation of sequential obstruction of multilevel airway, differentiation of primary epiglottic collapse from secondary epiglottic collapse compressed by tongue base, and precise measure of anatomical structure. Because of high medical expense and radiation exposure, DI-SCT is limited to clinical research instead of routine implementation. However, the results obtained from DI-SCT here can be suitable to lateral cephalometry, and that broadens it clinical application.
The present study has certain limitations. First, EC defined from midline sagittal view in DI-SCT only reflects the anteroposterior collapse of epiglottis without taking epiglottic width into account. Potentially, a wider epiglottis may cover and block the epiglottis even more. Second, there is no clarification in the relationship of desaturation with epiglottic collapse. More data are needed regarding the simultaneous desaturation during epiglottic collapse in future study. Third, postoperative DI-SCT after epiglottectomy was not performed to prove whether EC still plays an important role. Further, the use of multilevel surgery impeded the knowing of the exact contribution in the reduction of AHI from epiglottectomy.26 Finally, the impact of position change to EC was not investigated. Potentially, EC may ameliorate during change of position, and positional therapy is also a treatment option for EC.27
Conclusion
EC is not uncommon in moderate to severe OSA patients and can be screened by the measurement of epiglottic length in image examination (CT scan or cephalometry); the clinical physician needs to be aware of the potential connection between long epiglottis and epiglottic collapse, and confirm this by DISE for decision-making of treatment modality. High hyoid mobility in breathing cycles during sleep contributes to EC in OSA patients.
Highlight
- OSA is commonly presented with multilevel obstructions, and identification of epiglottic collapse may improve the surgical success rate.
- The length instead of the epiglottis angle is correlated to its collapsibility during DI-SCT. The epiglottis length of more than 1.66 cm is indicative of its collapse.
- Epiglottic length can also be measured by routine image examination (eg, cephalometry without sedation) and largely extends its clinical application.
Acknowledgments
The authors would like to express our gratitude to Ms Yen-Ting Chiang (MSc) for her assistance in data collection. This project was supported by grants from the Ministry of Science and Technology, Taiwan, ROC (NSC 103-2314-B-182A-062) and the Linkou-Chang Gung Memorial Hospital, Taoyuan, Taiwan (grants 3B1861 and 3B1862).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Strollo PJ
2. Deeb R, Smeds MR, Bath J, et al. Snoring and carotid artery disease: a new risk factor emerges. Laryngoscope. 2019;129(1):265–268. doi:10.1002/lary.27314
3. Ayas NT, Owens RL, Kheirandish-Gozal L. Update in sleep medicine 2014. Am J Respir Crit Care Med. 2015;192(4):415–420. doi:10.1164/rccm.201503-0647UP
4. Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol. 2011;268(8):123–1236. doi:10.1007/s00405-011-1633-8
5. Amos JM, Durr ML, Nardone HC, Baldassari CM, Duggins A, Ishman SL. Systematic review of drug-induced sleep endoscopy scoring systems. Otolaryngol Head Neck Surg. 2018;158(2):240–248. doi:10.1177/0194599817737966
6. Sung CM, Tan SN, Shin MS, et al. The site of airway collapse in sleep apnea, its associations with disease severity and obesity, and implications for mechanical interventions. Am J Resp Crit Care Med. 2021;204:103–106. doi:10.1164/rccm.202011-4266LE
7. Catalfumo FJ, Golz A, Westerman ST, Gilbert LM, Joachims HZ, Goldenberg D. The epiglottis and obstructive sleep apnoea syndrome. J Laryngol Otol. 1998;112(10):940–943. doi:10.1017/S0022215100142136
8. Dedhia RC, Rosen CA, Soose RJ. What is the role of the larynx in adult obstructive sleep apnea? Laryngoscope. 2014;124(4):1029–1034. doi:10.1002/lary.24494
9. Kim HY, Sung CM, Jang HB, Kim HC, Lim SC, Yang HC. Patients with epiglottic collapse showed less severe obstructive sleep apnea and good response to treatment other than continuous positive airway pressure: a case-control study of 224 patients. J Clin Sleep Med. 2021;17:413–419. doi:10.5664/jcsm.8904
10. Fernández-Julián E, García-Pérez MÁ, García-Callejo J, Ferrer F, Martí F, Marco J. Surgical planning after sleep versus awake techniques in patients with obstructive sleep apnea. Laryngoscope. 2014;124(8):1970–1974. doi:10.1002/lary.24577
11. Torre C, Camacho M, Liu SY, Huon LK, Capasso R. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope. 2016;126(2):515–523. doi:10.1002/lary.25589
12. Certal VF, Pratas R, Guimarães L, et al. Awake examination versus DISE for surgical decision making in patients with OSA: a systematic review. Laryngoscope. 2016;126(3):768–774. doi:10.1002/lary.25722
13. Li HY, Lo YL, Wang CJ, et al. Dynamic drug-induced sleep computed tomography in adults with obstructive sleep apnea. Sci Rep. 2016;6(1):35849. doi:10.1038/srep35849
14. Schneider C, Rasband W, Eliceiri K. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671–675. doi:10.1038/nmeth.2089
15. Sher AE, Thorpy MJ, Shprintzen RJ, Spielman AJ, Burack B, McGregor PA. Predictive value of Müller maneuver in selection of patients for uvulopalatopharyngoplasty. Laryngoscope. 1985;95(12):1483–1487. doi:10.1288/00005537-198512000-00009
16. Cavaliere M, Russo F, Iemma M. Awake versus drug-induced sleep endoscopy: evaluation of airway obstruction in obstructive sleep apnea/hypopnoea syndrome. Laryngoscope. 2013;123(9):2315–2318. doi:10.1002/lary.23881
17. Kuo CJ, Kuo J, Hsiao SW, Lee CL, Lee JC, Ke BH. Automatic and quantitative measurement of laryngeal video stroboscopic images. Proc Inst Mech Eng H. 2017;231(1):48–57. doi:10.1177/0954411916679200
18. Su MC, Yeh TH, Tan CT, Lin CD, Linne OC, Lee SY. Measurement of adult vocal fold length. J Laryngol Otol. 2002;116(6):447–449. doi:10.1258/0022215021911257
19. Jeong SH, Man Sung C, Lim SC, Yang HC. Partial epiglottectomy improves residual apnea-hypopnea index in patients with epiglottis collapse. J Clin Sleep Med. 2020;16(9):1607–1610. doi:10.5664/jcsm.8640
20. Nashi N, Kang S, Barkdull GC, Lucas J, Davidson TM. Lingual fat at autopsy. Laryngoscope. 2007;117(8):1467–1473. doi:10.1097/MLG.0b013e318068b566
21. Wang SH, Keenan BT, Wiemken A, et al. Effect of weight loss on upper airway anatomy and the apnea-hypopnea index. The importance of tongue fat. Am J Respir Crit Care Med. 2020;201:718–727. doi:10.1164/rccm.201903-0692OC
22. Woo HJ, Lim JH, Ahn JC, et al. Characteristics of obstructive sleep apnea patients with a low body mass index: emphasis on the obstruction site determined by drug-induced sleep endoscopy. Clin Exp Otorhinolaryngol. 2020;13:415–421. doi:10.21053/ceo.2019.00794
23. Genta PR, Schorr F, Eckert DJ, et al. Upper airway collapsibility is associated with obesity and hyoid position. Sleep. 2014;37(10):1673–1678. doi:10.5665/sleep.4078
24. Stuck BA, Neff W, Hörmann K, et al. Anatomic changes after hyoid suspension for obstructive sleep apnea: an MRI study. Otolaryngol Head Neck Surg. 2005;133(3):397–402. doi:10.1016/j.otohns.2005.06.002
25. Riley RW, Powell NB, Guilleminault C. Inferior sagittal osteotomy of the mandible with hyoid myotomy-suspension: a new procedure for obstructive sleep apnea. Otolaryngol Head Neck Surg. 1986;94(5):589–593. doi:10.1177/019459988609400610
26. Lee CH, Kim DK, Kim SY, Rhee CS, Won TB. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position: a DISE study. Laryngoscope. 2015;125(1):248–254. doi:10.1002/lary.24825
27. Chiu FH, Chang Y, Liao WW, et al. Post-operative sleep endoscopy with target-controlled infusion after palatopharyngoplasty for obstructive sleep apnea: anatomical and polysomnographic outcomes. Nat Sci Sleep. 2021;13:1181–1193. doi:10.2147/NSS.S311702
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.