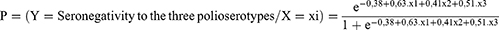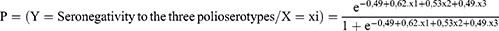Back to Journals » Pragmatic and Observational Research » Volume 14
Prediction Model with Validation for Polioseronegativity in Malnourished Children from Poliomyelitis Transmission High-Risk Area of the Democratic Republic of the Congo (DRC)
Authors Mwamba GN , Kabamba M , Hoff NA, Mukadi PK, Musene KK, Gerber SK, Halbrook M, Sinai C , Fuller T, Voorman A, Mawaw PM, Numbi OL, Wemakoy EO, Mechael PN, Tamfum JJM, Mapatano MA, Rimoin AW, Lusamba Dikassa PS
Received 25 September 2023
Accepted for publication 11 December 2023
Published 21 December 2023 Volume 2023:14 Pages 155—165
DOI https://doi.org/10.2147/POR.S437485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. David Price
Guillaume Ngoie Mwamba,1,2 Michel Kabamba,1,2 Nicole A Hoff,3 Patrick K Mukadi,4 Kamy Kaminye Musene,5 Sue K Gerber,6 Megan Halbrook,3 Cyrus Sinai,3 Trevon Fuller,3 Arie Voorman,6 Paul Makan Mawaw,7 Oscar Luboya Numbi,7 Emile Okitolonda Wemakoy,8 Patricia N Mechael,9 Jean Jacques Muyembe Tamfum,4 Mala Ali Mapatano,10,* Anne W Rimoin,3,* Paul-Samson Lusamba Dikassa8,*
1Department of Public Health, University of Kamina, Kamina, Haut-Lomami, Democratic Republic of the Congo; 2Expanded Programme on Immunization, Ministry of Health, Kinshasa, Democratic Republic of the Congo; 3Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, USA; 4National Institute of Biomedical Research (INRB), Ministry of Health, Kinshasa, Democratic Republic of the Congo; 5Health Research and Training Program, UCLA-DRC, Kinshasa, Democratic Republic of the Congo; 6Polio eradication program, The Bill and Melinda Gates Foundation, Seattle, WA, 98109, USA; 7Faculty of Medicine, University of Lubumbashi, Lubumbashi, Haut-Katanga, 1825, Democratic Republic of the Congo; 8Department of Epidemiology and Biostatistics, School of Public Health, University of Kinshasa, Kinshasa, Democratic Republic of the Congo; 9Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, 21205, USA; 10Department of Nutrition, School of Public Health, University of Kinshasa, Kinshasa, Democratic Republic of the Congo
*These authors contributed equally to this work
Correspondence: Michel Kabamba, Department of Public Health, Faculty of Medicine, University of Kamina, Kamina, Haut-Lomami, 279, Democratic Republic of the Congo, Tel +243978467432, Email [email protected]
Background: Malnutrition is identified as a risk factor for insufficient polio seroconversion in the context of a vaccine-derived poliovirus (VDPV) outbreak-prone region. In the Democratic Republic of Congo (DRC), underweight decreased from 31% (in 2001) to 26% (in 2018). Since 2004, VDPV serotype 2 outbreaks (cVDPV2) have been documented and were geographically limited around the Haut-Lomami and Tanganyika Provinces.
Methods: To develop and validate a predictive model for poliomyelitis vaccine response in malnourished infants, a cross-sectional household study was carried out in the Haut-Lomami and Tanganyika provinces. Healthy children aged 6 to 59 months (n=968) were enrolled from eight health zones (HZ) out of 27, in March 2018. We performed a bivariate and multivariate logistics analysis. Final models were selected using a stepwise Wald method, and variables were selected based on the criterion p < 0.05. The association between nutritional variables, explaining polio seronegativity for the three serotypes, was assessed using the receiver operating characteristic curve (ROC curve).
Results: Factors significantly associated with seronegativity to the three polio serotypes were underweight, non-administration of vitamin A, and the age group of 12 to 59 months. The sensitivity was 10.5%, and its specificity was 96.4% while the positive predictive values (PPV) and negative (PNV) were 62.7% and 65.3%, respectively. We found a convergence of the curves of the initial sample and two split samples. Based on the comparison of the overlapping confidence intervals of the ROC curve, we concluded that our prediction model is valid.
Conclusion: This study proposed the first tool which variables are easy to collect by any health worker in charge of vaccination or in charge of nutrition. It will bring on top, the collaboration between the Immunization and the Nutritional programs in DRC integration policy, and its replicability in other low- and middle-income countries with endemic poliovirus.
Keywords: prediction model, validation, child health, child nutrition, underweight, serotypes, polio serotype, seronegativity, poliomyelitis-neutralizing antibodies, malnutrition, DRC
Introduction
Malnutrition, including micronutrient insufficiency, like copper, iodine, vitamin A, zinc, iron, and selenium,1,2 is a major2 global health problem, not just in low- and middle-income countries (LMIC).3,4 Additionally, malnutrition can act as an aggravating factor for other illnesses.5 The food security report reveals that, in 2022, about 26.4 million people living in the DRC were in severe food insecurity. Root causes have been attributed to generalized poverty, conflicts and population displacement, insecurity, low agricultural production, material cost inflation, and the lack of basic infrastructure.6,7 In DRC, the national prevalence of underweight decreased from 31% to 26% in rural and urban areas from 2001 to 2018.8
Malnutrition is responsible, both directly and indirectly, for 54% of the 10.8 million deaths per year and contributes to half of infectious disease-related deaths among children under five LMICs. Previous studies have shown that several immune mechanisms may be defective in malnourished infants suffering from severe infections and constitute the vicious infection-malnutrition cycle.9
Poliomyelitis is an infectious disease of viral origin that can lead to paralysis. It is caused by one of three polio serotypes (types 1, type 2, and type 3) and mainly affects children less than five years old.10 Only one out of 200 polio infections experience paralytic symptoms or Acute Flaccid Paralysis (AFP) cases.11 Since August 2020, WHO certified the African Region free from all three types of wild poliovirus (WPV) when Nigeria became the last African country to interrupt wild poliovirus transmission.12 The DRC has been certified polio-free since 2012, however, since 2004, it has documented continued transmission13 circulating derived poliovirus type 2 (cVDPV2) and type 1 (cVDPV1) cases.14,15 Since May 2017, there has been an ongoing outbreak of VDPVs in the DRC. In 2021, 28 AFP cases were confirmed for cVDPV21, and in 2022, 377 cVDPV2 cases and 143 cVDPV1 cases were confirmed as well as 11 samples with cVDPV2 from environmental surveillance.16
In 2015, the Global Polio Eradication Initiative (GPEI) advocated for the introduction of an Inactivated Polio Vaccine (IPV) worldwide in all countries that had not yet introduced it. On 24 April 30th, 2016, the DRC along with every other country participated in the worldwide switch from the trivalent OPV (tOPV) to the bivalent OPV (bOPV), wherein type 2 was taken out of the tOPV. Multiple reasons are attributed to this switch including that PV2 was declared eradicated in September 2015 and studies suggest that bOPV led to significantly higher immunogenicity for type 1 and type 3 than tOPV.17,18
The 2017–2018 Multiple Indicator Cluster Surveys (MICS) revealed that only 35.0% of children 12–23 months were fully vaccinated in the DRC. However, there was provincial-level variation, and Haut Lomami and Tanganyika were estimated to have vaccine coverage estimates for children 12–23 months of 35.7% and 21.2%, respectively. The primary reasons for not being fully vaccinated included lack of knowledge among mothers and caregivers (29.0%), lack of time (18.4%), mistrust (16.1%), and challenges related to service delivery (28.7%). These include vaccinator unavailability, vaccine stock-outs, long waiting times, and financial barriers. This MICS revealed poor nutritional status. In the Haut-Lomami province, the Z scores were successively −1.2 for the weight/age ratio, −1.8 for the height/age ratio, −0.2 for the weight/height ratio. In Tanganyika province, the Z-score scores were successively −0.9 for weight-for-age, −1.6 for height-for-age, 0.1 for weight for size.19
Serological studies on polio vaccinations have been recommended by the WHO to assess and orient polio vaccination activities,20 evaluate the risk of poliovirus outbreaks, and identify immunity issues in the population. They have been performed in DRC,21 Nigeria,22 India,23 Pakistan,24 West Africa,25 and Madagascar26 and they contribute in poliomyelitis eradication efforts in these countries. Additionally, low immunogenicity has been identified in severe wasting cases, but this does not necessarily explain the reduced effectiveness of the vaccine against vaccine-preventable diseases, especially in countries where malnutrition is prevalent.27,28
To develop a predictive model for poliomyelitis vaccine response in malnourished children in VDPV outbreak-prone areas, the cross-sectional study was conducted in two DRC south-eastern provinces.
Methods
Study Population
This was a community-based, cross-sectional survey carried out in 2018 in four of the 16 Hz in Haut-Lomami Province: Butumba, Lwamba, Malemba-Nkulu, and Mukanga and four of the 11 Hz in Tanganyika Province: Ankoro, Kabalo, Kongolo, and Manono.29
Study procedures have been previously described by Halbrook, 202,026. Children were selected using a three-stage cluster sampling technique. During the first stage, eight health zones were selected based on the number of cVDPV2 registered and mass campaign responses organized. Within each health zone, five villages were selected by stratified random sampling using settlement feature layers derived from satellite imagery. During the second stage, we selected villages using a GIS point methodology with buffers. The clusters (health areas) were randomly selected using ArcGIS software based on two parameters: 1) not being in the same health area and 2) being separated by at least 500 meters. Households in which children aged 6 to 59 months reside formed the subgroups of these clusters. Overall, 327 households in Haut-Lomami and 641 in Tanganyika were investigated by simple random sampling. All households in the cluster were given the opportunity to be surveyed until the expected sample size was reached. Households that refused to participate were marked as “refused” in the tablet-based questionnaire.
Data Collection and Study Variables
Informed consent and a questionnaire were administered orally in the participants’ preferred language (French or Swahili) by trained interviewers. For selected households, we used the concept “healthy child” to mean a child who does not present the specific signs of serious illness (such as lethargy, unconsciousness, and convulsion),2 disability, cough or difficulty breathing, dehydration or persistent diarrhea, fever, edema of both feet, palmar pallor at the assessment time. Nine hundred and sixty-eight children fit this operational definition.
Consenting parents or guardians of children were administered a questionnaire designed to collect basic demographic data, as well as data on the participants’ work practices and health and child immunization data. A tablet-based questionnaire was used to collect data on current health status, anthropometric measures (height, weight), and other behaviors, including knowledge of vaccinations and utilization of the routine immunization system to receive vaccinations, which could be associated with the diseases.2 Height, weight, and mid-upper arm circumference (MUAC) were measured using a wooden infant-cum-stadiometer, SECA 874 digital weighing scales, and tricolor MUAC tapes, respectively. Standard methods were followed to take weight, height/length, and MUAC as recommended by the WHO. Additionally, a dried blood spot (DBS) sample was obtained through a finger prick. Questionnaires and collected DBS specimens were assigned linking barcode numbers to facilitate data analysis.
The following quantitative and qualitative variables were used: age, sex, marital status, tribe, level of education, religion, main occupation, nutritional status, and seroprevalence. Household density was estimated at six per household based on estimates from previous studies.4 We generated z-scores using ENA (Emergency Nutrition Assessment) software for SMART (Standardized Monitoring and Assessment of Relief and Transitions). Anthropometric index based on the WHO 2006 standards (stunting, wasting, and undernutrition) were evaluated using ENA software (July 2015 version Manufactured by Action Against Hunger Canada). Using WHO classification of nutritional status of infants and children. Underweight, wasting, and stunting were respectively defined as weight-for- age Z-score ≤-2, weight-for-height Z-score ≤-2, and height-for-age Z-score ≤-2 standard deviations of the WHO Child Growth Standards median.
Laboratory Analysis
Testing for neutralizing antibodies against poliovirus types 1(PV1), 2(PV2), and 3(PV3) was conducted at the US Centers for Disease Control and Prevention (CDC), Atlanta, GA. A modified poliovirus microneutralization assay was used to measure the ability of antibodies in serum or eluted from DBS punches to block the infectivity of poliovirus in an in vitro cell culture system. Following collection and in-country processing, the DBS were shipped at ambient temperature and stored at −20°C. The testing process has been previously described elsewhere. In brief, equivalent to approximately 60 ul of sera were collected from each card and processed for the low-volume poliovirus neutralization assay. A series of dilutions of DBS elute were combined with a fixed amount of virus before inoculation of poliovirus-susceptible cells. After five days of incubation, a luminescent cell viability reagent was added to detect live cells. The presence of live cells indicates protection from the virus cytopathic effect, which is the neutralization of virus infectivity. Neutralization titers are reported in a log2 format, with 2.5 log2 as the lower limit of detection and 10.5 log2 as the upper limit of detection. Neutralizing antibodies have been evaluated against PV1, PV2, and PV3, and titers ≥ 3.0 log2 are considered evidence of seroprotection.3,6
Statistical Analysis
Data from this study were analyzed using SPSS 23 and STATA 14.0 software. After calculating the prevalence of malnutrition, the different frequencies were compared using the Pearson Chi-square test, and a p-value <0.05 was considered statistically significant. Univariate analyses were expressed as frequency distributions and percentages as appropriate. In the bivariate analysis, the chi-square test was used to assess the link between nutritional status and seronegativity.
Logistic regression using the stepwise Wald method was used to select final variables to be included in the model. For model validation, we used the split-sample model validation method. We randomly selected 75% of nutritional factors explaining seronegativity to the different polio serotypes instead of selecting all the factors found in the initial model.
Discrimination between nutritional variables explaining polio seronegativity to the three polio serotypes was assessed using the ROC curve. We determined sensitivity, specificity, positive and negative predictive values. The performance measure of this logistic model is numerically equivalent to the area under the ROC curve.
Ethical Considerations
Ethical clearance was obtained from the ethics committee of UCLA Institutional Review Broad (IRB#18-000303) and the Kinshasa School of Public Health (approval letter No: ESP/CE/164/2021), University of Kinshasa, DRC.
Our study complies with the Declaration of Helsinki and verbal informed consent was acceptable and approved by the ethics committees.
Results
Bivariate Analysis of Seronegativity to the Three Polio Serotypes and Nutritional Status of the Children Aged 6 to 59 from Poliomyelitis Transmission High-Risk Area of the DRC
An association between underweight (p=0.003) and non-administration of vitamin A (p<0.001) was observed significantly with seronegativity to the three polio serotypes. While no significant association was found between seronegativity to the three polio serotypes with chronic malnutrition (p=0.97) and acute malnutrition (p=0.11) (Table 1).
 |
Table 1 Bivariate Analysis of Seronegativity to the Three Polio Serotypes and Nutritional Status of the Children Aged 6 to 59 from Poliomyelitis Transmission High-Risk Area of the DRC (n=968) |
Logistic Regression Model of Nutritional Variables Explaining Seronegativity to the Three Polio Serotypes
After logistic regression, three variables stand out as the predictors of seronegativity to the three polio serotypes: not receiving Vitamin A, being underweight and the age group of 12 to 59 months (Table 2). Thus, the model for predicting seronegativity to the three polio serotypes can be written as below:
 |
Table 2 Logistic Regression Model of Nutritional Variables Explaining Seronegativity to the Three Polio Serotypes |
The predictive value of seronegativity to the three polio serotypes was extracted from the matrix of the regression prediction model (Table 3). The value of the area under the ROC curve is 0.627 (Figure 1), which indicates a discrimination of its ability to predict the onset of seronegativity to the three polio serotypes.
 |
Table 3 Logistic Regression Prediction Model Confusion Matrix |
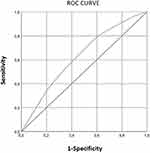 |
Figure 1 ROC curves of nutritional factors explaining seronegativity to the three polio serotypes in the initial model. |
After internal validation of this model, three variables stand out as the predictors of seronegativity to the three polio serotypes: not receiving Vitamin A, being underweight and the age group of 12 to 59 months (Table 4). Thus, the model for predicting seronegativity to the three polio serotypes can be written below:
 |
Table 4 Logistic Regression of Nutritional Variables Explaining Seronegativity to the Three Polio Serotypes by the Split Sample Validation of the Initial Sample |
The value of the area under the curve is 0.627 (Figure 2), which indicates the ability of the split model discrimination to predict the onset of seronegativity to the three polio serotypes. Comparing the ROC curves of the initial sample and the split samples, the value of the area under the two curves is 0.627 (Figure 3).
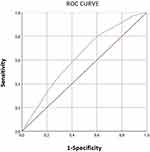 |
Figure 2 ROC curves of nutritional factors explaining seronegativity to the three polio serotypes in the split model. |
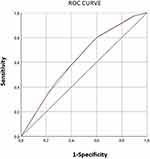 |
Figure 3 Comparison between ROC curve of the initial sample and the split sample. |
Discussion
This study suggests that being underweight, non-administration of vitamin A, and age group of 12 to 59 months are the powerful negative predictors of seroconversion to the three polio serotypes. These results are supported by Carol, Chandir and the Stoffel team in underweight children.30–32
As for the effect of Vitamin A on immunogenicity, several studies have shown that vitamin A during immunization session improves immune responses,33–38 and does not alter the immunogenicity of vaccines.39
We were not aware of any study on the model for predicting seronegativity in malnourished children at the time of writing this paper. Based on the comparison of the overlapping confidence intervals of the ROC curve, we concluded that our prediction model is valid.
The strength of our study is that, to our knowledge, it is the first study of the prediction model on seronegativity in malnourished children to the three polioserotypes. Steffel et al, in their study, on iron deficiency, predict seronegativity during administration of OPV and other antigens although their sample size was small compared to ours.30
The other advantage is that the identification of seronegativity in a poliovirus risk environment can be achieved by taking weight for age alone during preschool visits. This model will bring together health workers who deal with immunization and nutrition in health facilities and in the community, on the ease of collecting these parameters (weight and age) during a preschool visit of routine or during vaccination campaigns by health workers. Whether in a hospital setting or in the community as supported by Olivier Mukuku and his collaborators in the prediction of the risk of severe acute malnutrition.40 This model will bring on top the collaboration between the Immunization and the Nutritional programs in DRC integration policy and its replicability in other low- and middle-income countries with endemic polio virus.
Our survey is limited by its design, which reduced our ability to make causal inferences. Additionally, the laboratory method used did not allow us to make the difference between the presence of neutralizing antibodies due to vaccination or natural infection. Therefore, seroprevalence rates cannot be interpreted as only reflecting vaccination coverage in such cold chain challenging conditions.41,42 Since only 13.1% of participants had a vaccination card, did not allow us to carry out in-depth analyses. We selected children and their parents or guardians who were in the village at the moment of sample collection may have biased the study population. In addition, we had a low representation of children 35 months or older, which could have lowered our estimates of immunogenicity in the study population43. Data was not collected on the hemoglobin level or serum retinol level, birth site, or history of disease in the first months of life.
The lack of a model does not enable statistical comparisons to be made to assess the advantages or deficiencies of our model. However, compared to the standard proposed by J A Swets, with the area under the ROC curve between 0.5 and 0.7, the model could be considered not more informative.44
The Clinical implications of our findings are that a large proportion of individuals with polioseronegativity also meet diagnostic criteria for at least one malnutrition disorders.25,42 Some existing researches suggest that malnutrition is the most common reason for clinical referral.45–47 It has also been shown that having malnutrition as well as polioseronegativity may cause additional burden to the individual and lead to worse functional outcomes.48,49
Conclusion
This study of the predictive factors of seronegativity in malnourished children has made it possible to propose the first tool whose variables are easy to collect by any health worker in charge of vaccination or in charge of nutrition.
Not receiving Vitamin A, being underweight and the age group of 12 to 59 months stand out as the predictors of seronegativity to the three polio serotypes in this model.
Depending on the clinical implication, our results indicate that the assessment and diagnosis of malnutrition disorders should be a priority for clinicians caring for cases of acute flaccid paralysis, whether polio-related or not, because the diagnosis of a disorder concomitant with malnutrition can lead to a treatment plan integrating several specific intervention programs.
So, this model will bring on top, the collaboration between the Nutritional and the Immunization programs in DRC integration policy and its replicability in other low- and middle-income countries with endemic polio virus. This model lends itself to being performed in a hospital setting or in the community.
Being the first model, it may have limitations. Its improvement requires applying external validation on a large sample to generalize the practice in the same settings. We also recommend randomized control trials to verify this model and overcome the current limitations.
Acknowledgments
The authors gratefully thank the team of the Division of Viral Diseases, National Center for Immunization and Respiratory Diseases, CDC Atlanta, GA, USA: William C. Weldon, and his team who tested all samples. We also thank UCLA students, Skylar Martin, and Sydney Merritt for their thoughtful edits. We thank the DRC Ministry of Health and Provincial health authorities as well as all interviewers who participated in the collection of this data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.3
All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Bill and Melinda Gates Foundation investment OPP106684 (PI Anne W. Rimoin).
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Mbunga BK, Engebretsen IMS, Strand TA., et al. Distribution and determinants of serum zinc, copper, and selenium levels among children under five years from popokabaka, democratic republic of Congo: a cross-sectional study. Nutrients. 2022;14(3):683. doi:10.3390/nu14030683
2. Mbunga BK, Mapatano MA, Strand TA, et al. Prevalence of anemia, iron-deficiency anemia, and associated factors among children aged 1–5 years in the rural, malaria-endemic setting of popokabaka, democratic Republic of Congo: a cross-sectional study. Nutrients. 2021;13(3):1–13. doi:10.3390/nu13031010
3. Müller O, Krawinkel M. Malnutrition and health in developing countries. Cmaj. 2005;173(3):279–286. doi:10.1503/cmaj.050342
4. Faber M, Berti C, Smuts M. Prevention and control of micronutrient deficiencies in developing countries: current perspectives. Nutr Diet. 2014;6:41–57.
5. Pierar M Facteurs liés à la dénutrition chez les enfants de moins de 5 ans au Nigéria: analyse des données de l’étude National Nutrition Health Survey (NNHS) 2018. Université catholique de Louvain; 2018.
6. INS RDC. Democratic republic of the Congo: acute malnutrition situation July - December 2022 and Projection for January - June 2023; 2022. Available from: https://www.ipcinfo.org/ipc-country-analysis/details-map/en/c/1155974/.
7. Akilimali A, Banga S, Oduoye MO, et al. Malnutrition among under-five children in Democratic Republic of the Congo: a plague of the health system. Ann Med Surg. 2022;82:20–22. doi:10.1016/j.amsu.2022.104260
8. He Q, Qi X, Zhang T, Tang K. Trends in socioeconomic inequalities in malnutrition among children under 5 in the Democratic Republic of the Congo from 2001 to 2018. Nutrition. 2023;115:112182. doi:10.1016/j.nut.2023.112182
9. Rodríguez L, Cervantes E, Ortiz R. Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int J Environ Res Public Health. 2011;8(4):1174–1205. doi:10.3390/ijerph8041174
10. Opare JKL, Odoom JK, Akweongo P, Afari EA, Pappoe M. Poliovirus antibody levels and lameness among individuals in three regions of Ghana. Hum Vaccines Immunother. 2019;15(9):2050–2059. doi:10.1080/21645515.2019.1637235
11. Roberts L. Polio Outbreak Breaks the Rules. Science. 2010;330(6012):1730–1731. doi:10.1126/science.330.6012.1730
12. Bammeke P, Adamu US, Bolu O, et al. Descriptive epidemiology of poliomyelitis cases due to wild poliovirus type 1 and wild poliovirus type 3 in Nigeria, 2000–2020. Pan Afr Med J. 2023;45. doi:10.11604/pamj.supp.2023.45.2.38079
13. Alleman MM, Jorba J, Riziki Y, et al. Vaccine-derived poliovirus serotype 2 outbreaks and response in the Democratic Republic of the Congo, 2017–2021. Vaccine. 2023;41(Suppl 1):A35–A47. doi:10.1016/j.vaccine.2023.02.042
14. GPEI. Status: affected by circulating vaccine-derived poliovirus type 1 (cVDPV1) and type 2 (cVDPV2); 2022. Available from: https://polioeradication.org/where-we-work/democratic-republic-of-The-congo/.
15. Alleman MM. Vaccine-derived polioviruses outbreaks and events in 3 provinces of Democratic Republic of the Congo, 2017. Relev Epidemiol Hebd. 2018;93:117–125.
16. GPEI. DRC 2022 Status: affected by circulating vaccine-derived poliovirus type 1 (cVDPV1) and type 2 (cVDPV2) DRC. The Global Polio Eradication Initiative; 2022. Available from: https://polioeradication.org/where-we-work/democratic-republic-of-The-congo/.
17. GPEI. Global eradication of wild poliovirus type 2 declared; 2015.
18. Sutter RW, John TJ, Jain H, et al. Immunogenicity of bivalent types 1 and 3 oral poliovirus vaccine: a randomised, double-blind, controlled trial. Lancet. 2010;376(9753):1682–1688. doi:10.1016/S0140-6736(10)61230-5
19. INS RDC. Enquête par grappes à indicateurs multiples, 2017–2018, rapport de résultats de l’enquête; 2018. Available from: https://www.unicef.org/drcongo/media/3646/file/COD-MICS-Palu-2018.pdf.
20. Deshpande JM, Bahl S, Sarkar BK, et al. Assessing population immunity in a persistently high-risk area for wild poliovirus transmission in India: a serological study in Moradabad, Western Uttar Pradesh. J Infect Dis. 2014;210(suppl 1):S225–S233. doi:10.1093/infdis/jiu204
21. Congo (Democratic Republic). Ministère du plan; C. for D. C. and P. (U. S. &; M. I. D. and H. S. Enquête Démographique et de Santé, RDC 2007. Calverton, Maryland, USA: Ministère du Plan Macro Int; 2008:1–499.
22. Craig KTO, Verma H, Iliyasu Z, et al. Role of serial polio seroprevalence studies in guiding implementation of the polio eradication initiative in Kano, Nigeria: 2011–2014. J Infect Dis. 2016;213(suppl 3):S124–S130. doi:10.1093/infdis/jiv774
23. Bahl S, Estívariz CF, Sutter RW, et al. Cross-sectional serologic assessment of immunity to poliovirus infection in high-risk areas of Northern India. J Infect Dis. 2014;210(suppl_1):S243–S251. doi:10.1093/infdis/jit492
24. Gamage D, Palihawadana P, Mach O, et al. Achieving high seroprevalence against polioviruses in Sri Lanka — results from a serological survey, 2014. J Epidemiol Glob Health. 2015;5(S1):S67–S71. doi:10.1016/j.jegh.2015.06.004
25. Guindo O, Mach O, Doumbia S, et al. Assessment of poliovirus antibody seroprevalence in polio high risk areas of West Africa. Vaccine. 2018;36(8):1027–1031. doi:10.1016/j.vaccine.2018.01.022
26. Razafindratsimandresy R, Mach O, Heraud J-M, et al. Assessment of poliovirus antibody seroprevalence in high risk areas for vaccine derived poliovirus transmission in Madagascar. Heliyon. 2018;4(3):e00563. doi:10.1016/j.heliyon.2018.e00563
27. Prendergast AJ. Malnutrition and vaccination in developing countries. Philos Trans R Soc B Biol Sci. 2015;370(1671):20140141. doi:10.1098/rstb.2014.0141
28. Olofin I, Mcdonald CM, Ezzati M, Flaxman S, Black RE. Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PLoS One. 2013;8(5):e64636. doi:10.1371/journal.pone.0064636
29. Halbrook M. Population and Geospatial Risks of Vaccine-Derived Poliovirus Type-2 in theDemocratic Republic of the Congo. California, Los Angeles: UNIV; 2021.
30. Stoffel NU, Uyoga MA, Mutuku FM, et al. Iron deficiency anemia at time of vaccination predicts decreased vaccine response and iron supplementation at time of vaccination increases humoral vaccine response: a birth cohort study and a randomized trial follow-up study in Kenyan infants. Front Immunol. 2020;11. doi:10.3389/fimmu.2020.01313
31. Chandir S, Ahamed KU, Baqui AH et al. Effect of buffer on the immune response to trivalent oral poliovirus vaccine in Bangladesh: A community based randomized controlled trial. J. Infect. Dis. 2014;210(suppl_1):S390–S397. doi:10.1093/infdis/jiu378
32. Carol Susan D Risk factors associated with lack of seroprotection against type 3 poliovirus. a dissertation submitted in partial fulfillment of the declaration of the candidate; 2016.
33. Calder PC, Berger MM, Gombart AF, et al. Micronutrients to Support Vaccine Immunogenicity and Efficacy. Vaccines. 2022;10(4):1–9. doi:10.3390/vaccines10040568
34. Kaufman DR, De Calisto J, Simmons NL, et al. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol. 2011;187(4):1877–1883. doi:10.4049/jimmunol.1101248
35. Newton S, Owusu-Agyei S, Filteau S, Gyan T, Kirkwood BR. Vitamin A supplements are well tolerated with the pentavalent vaccine. Vaccine. 2008;26(51):6608–6613. doi:10.1016/j.vaccine.2008.09.037
36. Penkert RR, Rowe HM, Surman SL, et al. Influences of Vitamin A on vaccine immunogenicity and efficacy. Front Immunol. 2019;10:1–9. doi:10.3389/fimmu.2019.01576
37. Ross AC. Vitamin A Status: relationship to Immunity and the Antibody Response. Proc Soc Exp Biol Med. 1992;1(200):303–320. doi:10.3181/00379727-200-43436A
38. Strober W. Vitamin A rewrites the ABCs of oral tolerance. Mucosal Immunol. 2008;1(2):92–95. doi:10.1038/mi.2007.22
39. Deloria-Knoll M, Steinhoff M, Semba RD, Nelson K, Vlahov D, Meinert CL. Effect of zinc and vitamin A supplementation on antibody responses to a pneumococcal conjugate vaccine in HIV-positive injection drug users: a randomized trial. Vaccine. 2006;24(10):1670–1679. doi:10.1016/j.vaccine.2005.09.047
40. Mukuku O, Mutombo AM, Kamona LK, et al. Predictive Model for the Risk of Severe Acute Malnutrition in Children. J Nutri Metabol. 2019;2019:1–7. doi:10.1155/2019/4740825
41. Shemwell SA, Peratikos MB, González-Calvo L, et al. Determinants of full vaccination status in children aged 12–23 months in Gurùé and Milange districts, Mozambique: results of a population-based cross-sectional survey. Int Health. 2017;9(4):234–242. doi:10.1093/inthealth/ihx020
42. Nemat A. Seroprevalence rate of Poliovirus antibodies among the Healthy and protein energy malnutrition children. Pakistan J Med Sci. 2015;31:403–407.
43. Alfonso VH, Voorman A, Hoff NA, et al. Poliovirus immunity among adults in the Democratic Republic of the Congo: a cross-sectional serosurvey. BMC Infect Dis. 2022;22(1). doi:10.1186/s12879-021-06951-6
44. Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240(4857):1285–1293. doi:10.1126/science.3287615
45. Derseh B, Mruts K, Demie T, Gebremariam T.Co-morbidity, treatment outcomes and factors affecting the recovery rate of under -five children with severe acute malnutrition admitted in selected hospitals from Ethiopia: retrospective follow up study. Nutr. J. 2018;17:
46. Nduhukire T, Atwine D, Rachel L, Byonanebye JE, Lazzeri C. Predictors of in-hospital mortality among under-five children with severe acute malnutrition in South-Western Uganda. PLoS One. 2020;15(6):1–15. doi:10.1371/journal.pone.0234343
47. Lambebo A, Temiru D, Belachew T. Frequency of relapse for severe acute malnutrition and associated factors among under five children admitted to health facilities in Hadiya Zone South Ethiopia. PLoS One. 2021;16:1–11.
48. Ponziani FR, Coppola G, Rio P, et al. Factors influencing microbiota in modulating vaccine immune response: a long way to go. Vaccines. 2023;11(10):1609. doi:10.3390/vaccines11101609
49. Ahmed SA, Kotepui M, Masangkay FR, Milanez GD, Karanis P. Gastrointestinal parasites in Africa: a review. Adv Parasitol. 2023;119:1. doi:10.1016/bs.apar.2022.10.001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.