Back to Journals » Open Access Surgery » Volume 9
Preclinical study evaluating a novel trocar site closure system
Authors Walker P, Shah S, Wilson E
Received 29 October 2015
Accepted for publication 15 December 2015
Published 26 April 2016 Volume 2016:9 Pages 29—35
DOI https://doi.org/10.2147/OAS.S99422
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Cataldo Doria
Peter A Walker,1 Shinil K Shah,1,2 Erik B Wilson1
1Department of Surgery, University of Texas Medical School at Houston, Houston, TX, USA; 2Michael E DeBakey Institute for Comparative Cardiovascular Science and Biomedical Devices, Texas A&M University, College Station, TX, USA
Introduction: Novel trocar closure devices using subperitoneal anchor placement avoid the need for a closed loop suture, and could allow faster trocar site closure with less risk derived from less instrument penetration into the abdominal cavity while decreasing fascial ischemia and tension. We completed a series of animal experiments to compare the Carter-Thomason® suture closure device and neoClose® AnchorGuide device in order to evaluate device safety, efficacy, and ease of utilization.
Methods: A porcine model was utilized where 12 mm port sites were closed with either the Carter-Thomason® device or neoClose® AnchorGuide device. Animals were sacrificed at 14 and 56 days after trocar site closure followed by macroscopic and microscopic wound examination. A second group of animals underwent video analysis to evaluate the time required for fascial closure as well as depth of needle passage into the abdomen.
Results: All animals survived to the endpoints (14 and 56 days) without mortality or trocar site hernia. A trend towards decreased closure times was identified when using the neoClose® device (t=18.5±2.3 seconds) compared to closure with the Carter-Thomason® device (t=32.0±8.9 seconds) (P=0.153). A significant decrease in the depth of needle penetration was seen with the neoClose® device (t=31.22±1.45 mm) compared to closure with the Carter-Thomason device (t=51.0±3.1 mm) (P<0.001). Microscopic analysis showed subperitoneal anchor location at 56 days with minimal fibrosis (neoClose® device) compared to nearly complete suture degradation with deep intramuscular location (Carter-Thomason device).
Conclusion: Novel trocar closure devices could offer decreased deployment times while improving closure safety. Initial animal studies have shown that the neoClose® device yields a durable fascial closure that is easy to adopt from a technical standpoint.
Keywords: trocal closure, laparoscopy, trocar hernia, closure device
Introduction
The increasing adoption of laparoscopic surgical techniques has led to improvements in clinical outcomes including faster return to function, decreased wound infections, and decreased postoperative pain.1 While a decreased rate of incisional hernia has been observed with laparoscopic approaches,2 the incidence of trocar or “port” site hernias has been shown to be 0.4% after robotic prostatectomy,3 0.74% after gastrointestinal procedures, and up to 1.47% following colorectal procedures.4 Such retrospective analyses may underestimate the actual incidence of hernia formation. A recent observational study demonstrated a 25.9% incidence of umbilical incisional site hernia following laparoscopic cholecystectomy at a mean follow-up of 46.8 months.5 Port site hernias increase patient morbidity and have been associated with increased pain as well as bowel obstruction and strangulation.
The widespread utilization of laparoscopy has led to the development of several trocar site closure devices to improve operative times and decrease the incidence of port site complications. The Carter-Thomason® (Cooper Surgical, Trumbull, CT, USA) suture passing device is broadly utilized and has been shown to decrease closure times as well as rates of wound infection and seroma when compared to open closure techniques.6 A recent comparison with a novel trocar site closure device (the WECK EFx® Endo Fascial Closure System [Teleflex, Morrisville, NC, USA]) showed 12 mm trocar site closure times of 133.61 and 98.53 seconds for the Carter-Thomason® and WECK EFx® devices, respectively.7 However, both devices require the placement of transfascial sutures that bridge the fascial defect, which may lead to increased levels of pain and tissue ischemia.
The proprietary neoClose® device developed by neoSurgical Inc. (Newton, MA, USA) offers a novel approach to trocar site fascial closure that could potentially decrease closure times while improving pain scores. The neoClose® device obtains fascial approximation via the deployment of subfascial anchors on either side of the wound through a proprietary AnchorGuide system. The anchors approximate the defect without the need for a closed loop suture closure which decreases fascial tension after closure. In addition, the streamlined anchor deployment system does not require assistance and could lead to decreased trocar site closure times.
We completed a study using a porcine model where 12 mm port sites were closed with either the Carter-Thomason® device or neoClose® AnchorGuide device. Animals were sacrificed at 14 and 56 days after trocar site closure when macroscopic observation of the fascial defects was completed as well as histomorphologic analysis of the inflammatory reaction and wound appearance. A second group of animals (n=2) underwent closure of several 12 mm trocar sites (n=10) with either the Carter-Thomason® suture passer or neoClose® device. Video analysis was completed to evaluate the time required for fascial closure as well as depth of needle passage into the abdomen. We believe that the lack of closed loop suture closure will decrease wound tension, thereby allowing the anchors to remain and incorporate within the submuscular space of the abdominal wall. Furthermore, we believe that the neoClose® device will allow faster trocar site closure with less risk derived from less instrument penetration into the abdominal cavity. Such advantages could afford a more robust closure technique and potentially decrease the incidence of wound complications.
Materials and methods
Animal welfare and accreditation
All experiments were completed at an independent third party testing facility, CBSET Inc. (CBSET). CBSET is accredited by AAALAC International and committed to complying with all published guidelines for the care and treatment of laboratory animals. All procedures and conditions for testing were in compliance with the United States Food and Drug Administration. The protocol was reviewed and approved by the Animal Ethics committee of the CBSET Inc. facility.
Trocar site closure devices
neoClose® AnchorGuide device
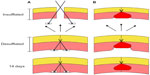
The neoClose® AnchorGuide device was utilized per the manufacturer’s protocol. Briefly, the device is used by inserting the AnchorGuide device into the wound with the guide channels perpendicular to the fascial incision (Figure 1A). The anchor driver is then inserted via the channel until penetration through the abdominal wall is visualized (Figure 1B). The suture is then released from the driver and followed by removal of the AnchorGuide. This process is repeated on the contralateral side. The sutures are then tied after desufflation with an anchor lying underneath the abdominal wall on either side. Anchor deployment allows for fascial approximation without the need for a closed loop system as seen in Figure 1C.
Carter-Thomason® device
The Carter-Thomason® suture passer with reusable suture guide was utilized as a control as per the manufacturer’s suggested protocol. Briefly, a suture was passed through one side of the fascia with a guide and pulled back through the contralateral fascia under direct visualization with a laparoscope. The sutures were tied while the abdomen was insufflated. This technique leads to fascial approximation via creation of a closed loop with suture.
Surgical procedure
Female Yucatan swine (n=4) were fasted overnight with free access to water. General anesthesia was induced via intravenous infusion of zolazepam (4–6 mg/kg, IM) followed by administration of isoflurane delivered in 100% oxygen via facemask until the animal was suitable for endotracheal intubation. Anesthesia was then maintained with continuous isoflurane in 100% oxygen. During the procedure the animal’s temperature was maintained with warming blankets and it was hydrated with crystalloid solution given through a large bore peripherally inserted intravenous catheter. Vital signs were continuously monitored with electrocardiography leads, pulse oximetry, and placement of a temperature probe and blood pressure cuff. Prior to initiation of the procedure the abdomen was prepped and draped for standard aseptic procedures.
Using a number 15 blade scalpel, an approximately 1 cm vertical incision was made at the umbilicus. The underlying fascia was grasped and divided sharply. The peritoneum was entered with blunt digital dissection. A 12 mm trocar was then inserted into the abdomen followed by insufflation to 15 mm Hg with CO2. A 10 mm/30 degree laparoscope was used for visualization during the procedure. Four additional 12 mm trocar sites were created in each animal. The trocar sites were created via incising the skin off midline with a number 15 blade scalpel followed by insertion of a 12 mm trocar under direct visualization.
After completion of fascial closure, the incisions were injected with bupivacaine followed by closure of skin with 4-0 Monocryl suture. The animals were observed for 36 hours after extubation to ensure adequate pain control. They were then transported to a long-term care facility where they remained with free access to water and nutrition until time of scheduled euthanasia.
A second set of animals (n=2) was anesthetized and prepped as previously described. Each animal underwent the creation of ten 12 mm trocar sites followed by closure with the Carter-Thomason® or first generation neoClose® device. Video of the procedure was used to document time to fascial closure (gauged by negative air leak and palpation) as well as depth of device/needle penetration. The animals used in this portion of the experiment were sacrificed upon completion of the experiment.
Necropsy
At the planned time of tissue harvest (14 and 56 days), the animals were euthanized under anesthesia with an intravenous potassium chloride solution. A limited necropsy was performed involving examination of all trocar sites with photography followed by explantation of the abdominal wall. The thickness of the abdominal wall was then measured followed by careful examination of trocar closure sites for tissue integrity and adverse events.
Histology
The explanted abdominal wall was fixed in 10% normal buffered formalin, embedded in resin (methyl methacrylate), and sectioned for light microscopy. The specimens were then stained with hematoxylin and eosin stains for analysis. Light microscopy was used for qualitative assessment of the host response including inflammatory cell infiltrates (neutrophils, eosinophils, macrophages, lymphocytes, and giant cells) and necrosis. Tissue evaluation was completed with the scoring system outlined in Table 1.
  | Table 1 Scoring system for microscopic changes observed after fascial closure |
Results
Adverse events
All four animals survived to their intended endpoint without mortality. No clinically significant adverse events were observed and no trocar site hernias were identified.
Macroscopic observations
Macroscopic observation at 14 and 56 days showed only one event after closure with the neoClose® device. The peritoneal surface of one site was associated with a focal adhesion of omental tissue. The cause of this adhesion is uncertain but may be related to a small amount of intra-operative hemorrhage/hematoma prior to the time of anchor deployment as seen in Figure 2. The adhesion was interpreted as benign with no evidence of adverse effects (eg, tissue necrosis, inflammation, torsion).
  | Figure 2 Picture of peritoneal hematoma at site of adhesion to omentum following closure of trocar site with neoClose® AnchorGuide device. |
Trocar closure times
A trend towards decreased closure times was identified when using the neoClose® device (t=18.5±2.3 seconds) compared to closure with the Carter-Thomason® device (t=32.0±8.9 seconds) (P=0.153).
Needle insertion depth
A significant decrease in the depth of needle penetration was seen with the neoClose® device (t=31.22±1.45 mm) compared to closure with the Carter-Thomason device (t=51.0±3.1 mm) (P<0.001).
Macroscopic analysis
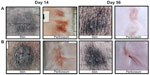
The peritoneal surface and skin were found to be intact after trocar closure with both devices at both day 14 and 56 as demonstrated in Figure 3. Measurement of abdominal wall thickness showed no difference between the two devices at both 14 (neoClose® 37.3±3.4 mm/control 36.0±2.0 mm) and 56 days (neoClose® 26.4±3.1 mm/control 25.8±3.1 mm).
Histomorphology
Review of tissue cross sections showed no difference in the local inflammatory response between the two devices at either 14 or 56 days (as measured by levels of neutrophils, eosinophils, macrophages, lymphocytes, and giant cells).
Cross sections were also completed to observe suture/anchor integration as well as local levels of fibrosis and tissue necrosis. Figure 4 reveals a subperitoneal location of the anchor with a mild surrounding fibrotic reaction at 14 days following closure with the neoClose® device (Figure 4A). Conversely, suture closure with the Carter-Thomason® (Figure 4B) showed deeper migration of the suture into the muscle layer with a more robust surrounding fibrotic reaction.
Similar observations were completed at 56 days after trocar site closure and are displayed in Figure 5. The anchors were found in subperitoneal location (Figure 5A) with the neoClose® device. The anchors appeared to be well incorporated within the abdominal wall with minimal surrounding fibrosis. Closure with the Carter-Thomason® device (5B) was associated with almost complete degradation of the suture located deep in the abdominal musculature with minimal fibrosis at 56 days.
Discussion
Our series of animal experiments has shown a potential trend towards decreased trocar closure times using the neoClose® device when compared to Carter-Thomason® control. This was also associated with a decrease in needle/device depth penetration. The expense and resources involved in conducting animate experiments limit the number of trials available, thereby increasing the difficulty of obtaining statistical significance with trocar closure times. Closure times observed in the study varied greatly from the previously published times (133.6 seconds for Carter-Thomason® closure).7 We believe that it is difficult to compare absolute times between studies secondary to the differences in operator technique; however, the comparison of groups within each study clearly shows the potential timesaving benefit that could be obtained with the development of novel trocar closure devices. Furthermore, the decreased depth of needle penetration could decrease the potential for adverse outcomes such as bowel injury.
We have also shown that anchor deployment achieved fascial closure up to 56 days after the index operation without serious adverse events. Observation revealed the anchors remain in the submuscular and subperitoneal space with adequate tissue integration. Conversely, closed loop suture closure, the Carter-Thomason®, was associated with deeper migration of suture material into the abdominal wall, as well as degradation. Furthermore, we believe that the decreased inflammatory response associated with anchor deployment seen on histopathology could lead to decreased underlying adhesion formation. Figure 6 shows a diagram outlining how subperitoneal anchor placement could potentially be associated with decreased wound tension when compared to a closed loop suture closure. This could decrease tissue ischemia, suture migration, and could lead to a decrease in the incidence of trocar site hernias. These findings indicate that fascial closure utilizing submuscular anchors offers a safe and durable alternative to classic closed loop suture closure.
Conclusion
Novel trocar closure devices could offer decreased closure times while improving closure safety. Initial animal studies have shown that the neoClose® device yields a durable fascial closure that is easy to adopt from a technical standpoint. Additional data derived from a randomized clinical trial will ensure that the observed results are translatable to the clinical setting, and validate the easy adaptability of the neoClose® AnchorGuide device.
Acknowledgment
This research was supported by neoSurgical, Limited.
Disclosure
The authors have no conflicts of interest to disclose.
References
Garbutt JM, Soper NJ, Shannon WD, Botero A, Littenberg B. Meta-analysis of randomized controlled trials comparing laparoscopic and open appendectomy. Surg Laparosc Endosc. 1999;9(1):17–26. | |
Nguyen NT, Goldman C, Rosenquist CJ, et al. Laparoscopic versus open gastric bypass: a randomized study of outcomes, quality of life, and costs. Ann Surg. 2001;234(3):279–289. | |
Kang DI, Woo SH, Lee DH, Kim IY. Incidence of port-site hernias after robot-assisted radical prostatectomy with the fascial closure of only the midline 12-mm port site. J Endourol. 2012;26(7):848–851. | |
Owens M, Barry M, Janjua AZ, Winter DC. A systematic review of laparoscopic port site hernias in gastrointestinal surgery. Surgeon. 2011;9(4):218–224. | |
Oliphant R, Drummond R, Blackhall V, et al. Re: risk factors for umbilical trocar site incisional hernia in laparoscopic cholecystectomy: a prospective 3-year follow-up study. Am J Surg. 2015;209(2):424–425. | |
Shetty A, Adiyat KT. Comparison between hand suture and Carter-Thomason needle closure of port sites in laparoscopy. Urol J. 2014;11(4):1768–1771. | |
del Junco M, Okhunov Z, Juncal S, Yoon R, Landman J. Evaluation of a novel trocar-site closure and comparison with a standard Carter-Thomason closure device. J Endourol. 2014;28(7):814–818. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.