Back to Journals » Journal of Pain Research » Volume 14
Pre-Emptive Incision-Site Infiltration with Ropivacaine Plus Dexamethasone for Postoperative Pain After Supratentorial Craniotomy: A Prospective Randomized Controlled Trial
Authors Zhao C , Wang S, Pan Y, Ji N, Luo F
Received 12 January 2021
Accepted for publication 22 March 2021
Published 19 April 2021 Volume 2021:14 Pages 1071—1082
DOI https://doi.org/10.2147/JPR.S300943
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ellen M Soffin
Chunmei Zhao,1 Shaoheng Wang,1 Yuesong Pan,2,3 Nan Ji,4 Fang Luo1
1Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 2Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China; 3China National Clinical Research Center for Neurological Diseases, Beijing, 100070, People’s Republic of China; 4Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, 100070, People’s Republic of China
Correspondence: Fang Luo
Department of Pain Management, Beijing Tiantan Hospital, Capital Medical University, No. 119 West Road, South 4th Ring Road, Fengtai District, Beijing, 100070, People’s Republic of China
Tel +86 010 59976664
Fax +86 010 67050177
Email [email protected]
Nan Ji
Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, No. 119 West Road, South 4th Ring Road, Fengtai District, Beijing, 100070, People’s Republic of China
Tel +86 010 59976516
Email [email protected]
Background: Incision-site infiltration with local anesthetics prevents pain on incision site, but pain relief is limited to the first few postoperative hours. Dexamethasone as an adjuvant to local infiltration successfully achieves better postoperative pain relief; however, this has not been studied in craniotomy patients yet.
Study Design and Methods: This is a prospective, single-center, blinded, randomized, controlled trial included patients aged between 18 and 64 years, ASA physical status of I–II, scheduled for elective supratentorial tumor craniotomy under general anesthesia. We screened patients for enrollment from April 4, 2019 through August 15, 2019. The final study visit of the last patient was conducted on February 13, 2020. We randomly assigned eligible participants (1:1) to either the dexamethasone group who received incision-site infiltration of 0.5% ropivacaine plus 0.033% dexamethasone (N=70) or the control group who received 0.5% ropivacaine alone (N=70). Primary outcome was the cumulative sufentanil consumption (μg) within 48 hours postoperatively. Primary analysis was performed based on the modified intention-to-treat (MITT) principle.
Results: Baseline characteristics were similar between the groups (p> 0.05). Sufentanil consumption during the first 48 hours postoperatively was 29.0 (10.7) μg in the dexamethasone group and 38.3 (13.7) μg in the control group (mean difference − 9.3, 95% CI − 13.4 to − 5.1; p< 0.001). There was no serious adverse effect directly associated with incision-site infiltration or local dexamethasone use.
Conclusion: The addition of dexamethasone to pre-emptive incision-site infiltration with the local anesthetic can reduce about 27% of opioids consumption and the postoperative pain scores within 72 hours after craniotomy.
Trial Registration: ClinicalTrials.Gov (NCT03618264).
Keywords: postoperative pain, craniotomy, dexamethasone, incision-site infiltration, randomized controlled trial
Introduction
After craniotomy, 55–69% of patients experience moderate to severe pain, most frequently within the first 48 postoperative hours.1,2 Inadequate analgesia may lead to postoperative complications, such as arterial hypertension, intracranial hemorrhage, prolonged hospital stay and increased mortality.3 Moreover, acute postoperative pain is associated with an increased risk of chronic pain.4,5 Therefore, optimal analgesic treatment is essential for improving the quality of pain management after craniotomy.
Systemic analgesics, such as opioids, are the mainstay of treatment for post-craniotomy pain. However, given the various side effects of systemic opioids such as postoperative nausea and vomiting (PONV), sedation, respiratory depression and interference with neurological assessments, the minimization of reliance on opioids while exploring multimodal approaches with nerve blockade or incision-site infiltration using local anesthetics and smaller doses of opioid supplements is paramount.6–9
Pain following craniotomy is thought to be primarily superficial, suggesting a more somatic than visceral origin, that arises from the pericranial muscles and soft tissues of the scalp.10 Incision-site infiltration with local anesthetics such as bupivacaine and ropivacaine prevents pain on incision site and can be a promising postoperative pain management technique for craniotomy.11–15 However, an earlier study found that, incision-site infiltration with a single anesthetic could only provide a relatively satisfactory pain relief during the first 2 postoperative hours, despite the addition of adrenaline.15 Moreover, attempts to prolong the blockade of local anesthetics by increasing dosage have provided conflicting data regarding their safety, mainly because of toxicity. The development of new drugs has also been suboptimal.
Dexamethasone is an inexpensive synthetic glucocorticoid that has a strong anti-inflammatory effect with a long half-life of 36 to 72 hours. Several randomized controlled trials evaluating the analgesic effects of adding dexamethasone to local infiltration have concluded that dexamethasone alone or as an adjuvant to local infiltration successfully achieves better pain relief after total knee arthroplasty (TKA), tonsillectomy, endodontic treatments and cesarean section.16–19 Meanwhile, no serious side effects from the local use of dexamethasone have been observed in previous studies. However, to our knowledge, no other literature exists comparing the effects of dexamethasone incorporated into local infiltration along with local anesthetics after craniotomy. Therefore, this prospective, randomized, controlled study aiming to compare postoperative pain relief during the first 48 hours following supratentorial craniotomy was conducted. We hypothesized that pre-emptive incision-site infiltration of ropivacaine plus dexamethasone could improve pain relief after craniotomy when compared with ropivacaine alone.
Materials and Methods
Ethics approval was obtained from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (KY2018-034-02). All participants were provided with a verbal explanation of the written informed consent and had signed the written informed consent to participate in the study during a preoperative visit, one day prior to the surgery. This study was registered prior to patient enrollment at ClinicalTrials.Gov website (NCT03618264, Principal investigator: Fang Luo, Date of registration: August 7, 2018). The trial was conducted in accordance with the World Medical Association’s “Declaration of Helsinki”, the principles of good clinical practice and relevant regulatory requirements. There were no substantial changes to the main study protocol after commencement of the recruitment process. The trial protocol has previously been published elsewhere.20
Study Design and Participants
This was a prospective, single-center, blinded, randomized, controlled trial, conducted at Beijing Tiantan Hospital, Capital Medical University. Patients were eligible for participation if they met the following inclusion criteria: between 18 and 64 years of age and American Society of Anesthesiologists (ASA) physical status of I-II, scheduled for elective supratentorial tumor craniotomy under general anesthesia, who were required to fix their head in a head clamp intraoperatively, had a 2 hours postoperative anticipated return of consciousness, orientation as well as cooperation, and could thereby correctly recognize and express the degree of pain. Exclusion criteria included patients with a history of craniotomy, psychiatric disorders, uncontrolled epilepsy or chronic headache, expected delay in extubation or had no plan to extubate, who were unable to use a patient-controlled analgesia (PCA) device or understand the instructions of a numeral rating scale (NRS), who had extreme body mass index (BMI) (< 15 or > 35), allergic to opioids, dexamethasone or ropivacaine, with a history of excessive alcohol or drug abuse, chronic opioid use (of more than 2 weeks), or the use of drugs with confirmed or suspected sedative or analgesic effects, who were pregnant or breastfeeding, with symptomatic cardiopulmonary, renal, or liver dysfunction or history of diabetes, a preoperative Glasgow Coma Scale (GCS) < 15, suspected intracranial hypertension, peri-incisional infection, who had received radiation therapy and chemotherapy preoperatively or with a high probability to require a postoperative radiation therapy and chemotherapy based on preoperative imaging. Participants were withdrawn from the study under the following circumstances: were not awake 2 hours after surgery, had a delayed extubation and underwent early revision within the first 48 hours, which affected the assessment of outcome measures. In addition, participants were allowed to withdraw voluntarily at any stage of the trial, for any reason.
Randomization and Masking
Eligible participants were randomly assigned (1:1) to either the dexamethasone group or the control group, via SPSS version 22.0 (International Business Machines Inc., USA). Opaque, sealed, sequentially numbered envelopes were used to ensure allocation concealment; which included the participants’ screening order outside and their assigned group inside. Before surgery, the envelope was opened by the study investigator in charge of the surgery, and the patient was assigned to undergo corresponding local infiltration. Participants were enrolled by a dedicated research nurse who was not involved in the collection of primary outcome data.
Patients, nurses, surgeons and anesthesiologists in charge of the postoperative period and pain evaluation were blinded to group assignment; except in case of serious adverse events (SAEs) potentially related to the study treatment that required unmasking. The pharmacist in charge was allowed to unmask drug allocation only in case of SAEs. The respective drugs to be used for incision-site infiltration were prepared by an independent study investigator in the two groups: 10 mg dexamethasone, 150 mg ropivacaine diluted to a total volume of 30 mL in 0.9% saline in the dexamethasone group,16,20 and 150 mg ropivacaine diluted to a total volume of 30 mL in 0.9% saline in the control group. The concentration of ropivacaine was 0.5% in both groups. Both syringes contained clear fluid, appeared identical and were labelled as “study drug”.
Procedures
On arrival in the operating room, standard monitoring of blood pressure (BP), heart rate (HR), electrocardiography, pulse oximetry (SpO2) and bispectral index (BIS) were continuously performed. Intravenous (IV) midazolam at 0.05 mg kg−1, sufentanil at 0.3–0.5 μg kg−1, propofol at 1.5–3 mg kg−1 and cisatracurium at 0.2 mg kg−1 were used for induction of anesthesia. Mechanical ventilation was adjusted at 60% oxygen and 40% air to maintain an end-tidal partial pressure of carbon dioxide at 30–40 mmHg. Anesthesia was maintained with 4 to 8 mg kg−1 hour−1 propofol and 0.1 to 0.3 µg kg−1 min−1 remifentanil intravenously. Additional doses of vasoactive drugs were administered to maintain baseline levels within the 20% of the baseline.
5 minutes before head fixation, local infiltration of the study solution was performed by the neurosurgeon in charge of the craniotomy at each pin-insertion site used for skull clamp placement. Subsequently, the same solution was infiltrated with a 22-gauge needle introduced into the skin at a 45° angle throughout the entire thickness of the scalp along the planned incision site, by the same neurosurgeon. The total volume of the study solution used for each patient was determined by the neurosurgeon based on the length of the incision and recorded by the investigator.
Patients were extubated after satisfactory hemodynamic, respiratory and neurologic evaluations were achieved. Additionally, 4 mg of ondansetron was administered to prevent PONV and patients were transferred to the post-anesthesia care unit (PACU). A PCA device (ZZB-I–150, Apon Medical Technology Co., Ltd., Jiangsu, China) containing 200 µg of sufentanil and 16 mg of ondansetron, diluted to a total volume of 100 mL in 0.9% saline. The PCA device provided a bolus of 2 μg sufentanil on demand, followed by a 10 min lockout interval; the maximum dose was limited to 8 μg per hour. Both the initial dose and background infusion of the PCA pump were set to 0. Patients pushed the PCA demand button when an NRS of 4 or more was reported and repeated until pain was relieved. Insufficient postoperative analgesia was defined as NRS score exceeding 4 after the maximum dose of sufentanil was administered with the PCA device. All aspects of the rehabilitation process were identical between the two groups.
Outcomes
Primary outcome was the cumulative sufentanil consumption (μg) via PCA pump within 48 hours postoperatively.
Secondary outcomes included the following aspects: first analgesia demand indicated by participants pressing the PCA demand button; NRS scores (0 indicating no pain, 10 indicating the most severe pain imaginable) and patient satisfaction scale (PSS) scores (0 indicating unsatisfactory, and 10 indicating very satisfactory) at 2 hours, 4 hours, 8 hours, 24 hours, 48 hours, 72 hours, 1 week, 2 weeks, 1 month, 3 months and 6 months postoperatively; PONV scores (0, absent; 1, nausea not requiring treatment; 2, nausea requiring treatment; and 3, vomiting) within 48 hours postoperatively; Wound healing score21 (total score of 3, excellent wound healing; score 4–5, good wound healing; and score 6+, suboptimal wound healing) at 3 weeks and 6 weeks postoperatively; Patient and Observer Scar Assessment Scale (POSAS) scores22 at 6 months postoperatively; the times of emergency reduction of BP within 48 hours postoperatively; postsurgical duration of hospitalization.
Safety assessments included steroid-induced complications such as wound infection, wound hematoma, impaired wound healing, chest infection or gastric ulcers during hospitalization. All the adverse events (AEs) were closely monitored and promptly treated. The research team verified suspected AEs where possible. Unrelated AEs were not recorded.
Follow-up evaluations were conducted by experienced research members who were blinded to the study.
Statistical Analysis
Based on previous studies and our clinical experience at the study center,13 we estimated that the cumulative postoperative sufentanil consumption in participants who received pre-emptive incision-site infiltration with 0.5% ropivacaine would be approximately 100±50 μg, and the postoperative pain intensity or analgesic requirements would be reduced by 30–50% with the addition of dexamethasone.16,18,19,23 Thus, we hypothesized that the sufentanil dose within 48 hours postoperatively in the dexamethasone group would be 70±50 μg. PASS V.11 software (NCSS, Kaysville, Utah, USA) was used. To detect such a reduction with 90% power at an α level of 0.05, 62 participants were required in each group. Considering a 10% withdrawal rate, the sample size was 70 in each group; the total sample size was 140 patients for this trial.
Primary analysis was performed based on the modified intention-to-treat (MITT) principle. We also prespecified a sensitivity and consistency analysis within the per-protocol (PP) population. Analyses of secondary outcomes were exploratory in nature, and therefore p values and confidence intervals were provided with no adjustments for multiple comparisons. Treatment effects were presented as risk ratios (RRs) or mean differences, with 95% confidence interval (CI). Two-tailed analyses were conducted, and a p-value of ≤ 0.05 was considered statistically significant. We further evaluated the consistency of treatment effect on primary outcome among 7 prespecified subgroups.
Safety analyses were compared in the safety data set with the incidence of AEs using Chi-square test or Fisher’s exact test, according to actual treatment received.
All statistical analyses were performed by statisticians who were masked to the entire allocation and intervention process. Data analysis was performed using the SPSS version 22.0.
Results
We screened patients for enrollment from April 4, 2019 through August 15, 2019. The final study visit of the last patient was conducted on February 13, 2020. Out of 251 patients initially assessed for eligibility, 111 (44%) patients were excluded (primarily because they did not meet one or several inclusion criteria or declined to participate); 140 (56%) patients were eligible for inclusion. We randomly assigned 70 patients to receive ropivacaine plus dexamethasone and 70 patients to receive ropivacaine alone. All participants received the allocated drug in compliance with the protocol, and were included in the mITT analysis of the primary outcome. Sixty-eight patients in the dexamethasone group and 65 patients in the control group completed the 6-month follow-up and were included in PP analysis. Figure 1 shows the trial profile.
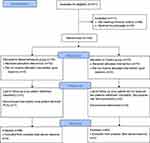 |
Figure 1 Trial profile. |
Baseline Characteristic
Demographic data and surgical variables of the two groups are shown in Table 1. Baseline characteristics were well balanced between the groups. Moreover, there were no statistically significant differences in HR (Figure 2A) and MAP (Figure 2B) intraoperatively and postoperatively between the groups (Figure 2). Eighty-three (59.3%) patients (40 patients in the dexamethasone group and 43 patients in the control group) received perioperative intravenous glucocorticoid (p=0.606).
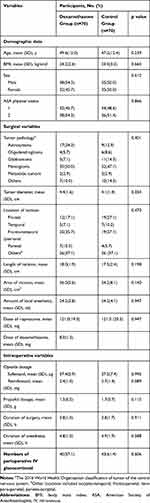 |
Table 1 Baseline Characteristics |
Primary Outcome
As shown in Table 2, dexamethasone as an adjuvant to pre-emptive incision-site infiltration significantly reduced sufentanil consumption during the first 48 hours postoperatively: 29.0 (10.7) μg in the dexamethasone group vs 38.3 (13.7) μg in the control group (p<0.001). Additionally, sensitivity analysis performed in the PP population also showed significant difference in primary outcome between the two groups (p<0.001).
 |
Table 2 Primary Outcome and Postoperative Recovery Situation |
Time of First PCA Demand
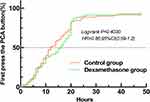
Estimated median of the first analgesia demand time indicated by participants pressing the PCA demand button was 16 hours in the dexamethasone group and 12 hours in the control group. However, analgesia demand time on the PCA device did not differ by type of intervention (hazard ratio [HR] 0.86, 95% CI 0.59 to 1.20; Log-rank p=0.40; Figure 3)
Postoperative Pain and Analgesia Satisfaction
Figure 4A and 4B show the NRS and PSS score distribution of patients after surgery. Within the first 72 postoperative hours, 69 (49.3%) patients (16 patients in the dexamethasone group and 53 patients in the control group) suffered from moderate to severe pain which was determined by NRS≥ 4 scores; the difference was statistically significant (risk ratio 0.3, 95% CI 0.2 to 0.5, p<0.001, as shown in Table 2). The pain scores (Figure 4A) decreased after 1 week postoperatively; 8 (6%) patients (4 patients in the dexamethasone group and 4 patients in the control group) were found to have chronic pain at 3 months and 6 months postoperatively. On the 2 hours, 4 hours, 8 hours, 24 hours, 48 hours and 72 hours postoperative periods, NRS scores of the control group were higher than those of the dexamethasone group [mean difference (95% CI); 0.7 (0.4 to 0.9), 0.6 (0.3 to 1.0), 1.1 (0.8 to 1.4), 1.4 (1.1 to 1.8), 1.2 (0.9 to 1.6) and 1.4 (1.0 to 1.7), respectively; p<0.001] (Figure 4A). The minimum median of PSS scores at each time point after surgery was more than 6.5 points (Figure 4B). The PSS scores were higher in the dexamethasone group than that in the control group on the 2 hours, 4 hours, 8 hours, 24 hours, 48 hours and 72 hours [mean difference (95% CI); 0.4 (0.2 to 0.7), 0.4 (0.1 to 0.8), 1.0 (0.7 to 1.3), 1.4 (1.0 to 1.7), 1.2 (0.9 to 1.5) and 0.4 (0.1 to 0.7), respectively; p < 0.05].
Wound Healing Situation
At 3 weeks postoperatively, 97 (69%) patients (53 patients in the dexamethasone and 44 patients in the control group) were reported to have very good wound healing. At 6 weeks postoperatively, the wound healing improved and a total of 92 (66%) patients achieved excellent wound healing. Three patients (4.3%) out of 70 in the control group suffered suboptimal wound healing at 6 weeks; whereas, zero patient in the dexamethasone group suffered suboptimal wound healing.
At 6 months postoperatively, a high objective evaluated outcome with a median objective general score of 2.2 was recorded. Moreover, there was a statistically significant difference between the two groups at 6 months (dexamethasone group 8.2 (0.8) vs control group 9.4 (2.8), mean difference −1.1, 95% CI, −1.9 to −0.4; p=0.003). Meanwhile, patients were also highly satisfied with their scar appearance, with a median patient general score of 2.2. However, there was no statistically significant difference between the two groups in Patient Scar Assessment Scale (PSAS) at 6 months. There was one patient with wound infection in the control group and none in the dexamethasone group.
Subgroups
The reduction of sufentanil consumption with ropivacaine plus dexamethasone incision-site infiltration was generally consistent across all prespecified subgroups, within the first 48 hours postoperatively (Figure 5). There were no significant interactions in any of the 7 predefined subgroups (P>0.10 for all comparisons).
Safety
The average volume of local anesthetic used for each patient was 24.2 mL, which was equivalent to 121 mg of ropivacaine and 8 mg of dexamethasone. The expected but unrelated AEs, such as complications of surgery, could not be attributed to study interventions. No SAEs or drug reactions were recorded during the study, which were directly associated with incision-site infiltration or local dexamethasone use.
Discussion
This is the first large-scale clinical trial examining the analgesic efficacy of dexamethasone as an adjuvant to pre-emptive incision-site infiltration in patients scheduled for elective supratentorial craniotomies. When compared to ropivacaine alone, pre-emptive incision-site infiltration with ropivacaine plus dexamethasone (average 8 mg) could reduce about 27% of sufentanil consumption via PCA within the first 48 hours after craniotomy. It could also improve the postoperative pain scores within 72 hours after craniotomies and improve patient satisfaction. However, the addition of dexamethasone to pre-emptive incision-site infiltration had no long-term effect on recovery, such as the duration of hospital stay.
The location of surgical incision (infratentorial vs supratentorial) could affect the incidence and severity of postoperative pain. Pain scores after supratentorial craniotomy are generally lower than those reported after posterior fossa procedures because of the relatively low muscle dissection.24 Subtemporal and suboccipital craniotomies yield a higher incidence of postoperative pain. Therefore, in order to minimize the influence of confounding factors that may result in high heterogeneity of the characteristics of postoperative pain, this study only included supratentorial craniotomies. Previous studies have demonstrated a common mechanism of incisional pain, which involves inflammatory mediators released after tissue damage directly stimulating the peripheral nociceptors; abnormal activation transmitted by Aδ and C-fibers causes local pain after tissue injury.25,26 Glucocorticoids inhibit inflammatory responses by blocking factors such as bradykinin, prostaglandin and leukotriene, thereby reducing inflammation levels and accompanying signs and symptoms.27 Therefore, this innovative combination of local anesthetics and dexamethasone is theoretically expected to consistently reduce incisional pain in other types of surgeries.
Although local infiltration of dexamethasone has not been used for incision-site infiltration after craniotomy yet, several clinical trials have reported the short-term benefits of the addition of dexamethasone to local infiltration analgesia in endodontic practices18 and tonsillectomy in pediatric patients.28 Despite the high heterogeneity in the design and quality of these randomized trials, the analgesic efficacy of dexamethasone appears to be definite. We demonstrated statistically significant differences in analgesic consumption within the first 48 hours after surgery. In addition, the reduction in sufentanil consumption was generally consistent across all prespecified subgroups. Moreover, there was no significant difference in the number of patients receiving systemic glucocorticoids. In addition to reducing the consumption of analgesics, there was a significant difference in NRS pain scores of greater than 1 point, especially in the 8 hours, 24 hours, 48 hours and 72 hours postoperative periods, which could be clinically important.29 The duration of the analgesic effect is consistent with the physiological effects of dexamethasone, which remains for 36–72 hours in the human body. Such a duration of effect seems to be sufficient to relieve pain after craniotomy, as it has been reported by previous studies that postcraniotomy pain peaks within the first 48 h.1,2
In this study, the duration of the analgesic effects of dexamethasone was 2–3 days following craniotomy, which is consistent with previous reports.16,30 Several studies have reported that the analgesic effects of pre-emptive local infiltration of dexamethasone alone only last 16–24 hours after endodontic treatment, cesarean section, tonsillectomy and adenoidectomy.18,19,31 Certainly, incisional pain is only one type of postoperative pain.32 The short-term analgesic effect of dexamethasone alone or as an adjuvant to local infiltration is definite on incisional pain, but might be ineffective on other types of postoperative pain. However, the characteristics of postoperative pain in different types of surgeries vary greatly.33,34 These reasons might explain the inconsistency in conclusions regarding the duration of action of dexamethasone when administered locally, alone or when mixed with local anesthetics, after different types of surgeries.
Glucocorticoid treatment complications, such as peptic ulcer, increased wound infection, and impaired wound healing, are often associated with long-term systemic use.35–37 More patients in the dexamethasone group (70%) achieved excellent wound healing than those in the control group (61%). Moreover, there was a lower wound infection rate in the dexamethasone group (0%), compared to the control group (0.7%, 1 patient). Although we did not find any adverse effects of dexamethasone on wound healing and infection, the current study is not powered for the possible side effects of the treatment. Therefore, the results of local infiltration of dexamethasone on wound healing and infection should be taken with caution.
Despite our significant findings, this study has a few limitations. First, this was a single-center study, and therefore, the generalizability of our outcomes must be established with large scale, multicentric randomized controlled trials. Second, the results of this dexamethasone study do not apply to other types of steroids.39 There are significant differences in the half-life and anti-inflammatory effects of various steroids. Third, we found a discrepancy between the estimated sufentanil consumption (100 μg) and the actual sufentanil consumption (38.3 μg) in the control group within the first 48 hours after craniotomies. The main reason may be because we only selected patients with supratentorial tumors; thus, the number of patients experiencing moderate to severe pain (37%) may have been significantly lower than previous reports (55–69%).1,2 Nevertheless, we can still guarantee the power of this study to be greater than 90% for the primary outcome. However, this reduction in the addition of dexamethasone might not really be clinically relevant based on clinical experience. Fourth, the use of painkillers at home was not considered in this study, which might have affected the severity of pain during follow-up. Fifth, we did not monitor blood glucose concentration and concentrations of dexamethasone. Finally, we had selected a single dose of dexamethasone for addition. We selected the dose that was previously reported to be safe in this setting.16 Needless to say, clinical trials on dose-dependent effects of dexamethasone need to be conducted in the future, to determine the optimal dose. The addition of other long-acting adjuvants should also be explored.
Conclusions
The addition of dexamethasone to pre-emptive incision-site infiltration with local anesthetic can reduce about 27% of opioids consumption and the postoperative pain scores within 72 hours after craniotomies. Further exploration of the ideal combination of local anesthetics and glucocorticoids is expected to provide an effective strategy for the prevention of incisional pain.
Abbreviations
MITT, modified intention-to-treat; TKA, total knee arthroplasty; ASA, American Society of Anesthesiologists physical status; PCA, patient-controlled analgesia; NRS, numeral rating scale; BMI, body mass index; GCS, Glasgow Coma Scale; SAEs, serious adverse events; BP, blood pressure; HR, heart rate; SpO2, pulse oximetry; BIS, bispectral index; IV, Intravenous; PONV, postoperative nausea/vomiting; PACU, post-anesthesia care unit; PSS, patient satisfaction scale; RSS, Ramsay Sedation Scale; POSAS, Patient and Observer Scar Assessment Scale; AEs, adverse events; IQRs, interquartile ranges; RRs, risk ratios; CI, confidence interval; PP, per-protocol.
Data Sharing Statement
Raw data were generated at Beijing Tiantan Hospital, Capital Medical University. Individual participant data that underlie the results reported in this article, after de-identification (text, tables, figures and appendices) are available. Derived data supporting the findings of this study are available from the corresponding author Fang Luo on request.
Ethics Approval and Informed Consent
Ethics approval was obtained from the Ethics Committee of Beijing Tiantan Hospital, Capital Medical University (KY2018-034-02). Written informed consent has been obtained from all patients before inclusion in the study.
Acknowledgment
We thank the patients who participated in this trial. We also thank Apon Medical Technology Co., Ltd., Jiangsu, China for lending the PCA pump equipment.
Author Contributions
CMZ and SHW contributed equally to this work and should be considered co-first authors. FL, CMZ and NJ did the literature search and were responsible for the study design. Study conduction and data collection were led by SHW. Study analysis and figure generation were done by CMZ, supervised by YSP. Writing of the paper was led by FL and CMZ with assistance from NJ and YSP. FL conceived the idea for this study and secured funding. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval for the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research was supported by Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (No. XMLX201707). The funder had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had complete access to all the data in the study; all the co-authors were responsible for making the final decision to submit for publication.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Mordhorst C, Latz B, Kerz T, et al. Prospective assessment of postoperative pain after craniotomy. J Neurosurg Anesthesiol. 2010;22(3):202–206. doi:10.1097/ANA.0b013e3181df0600
2. Gottschalk A, Berkow LC, Stevens RD, et al. Prospective evaluation of pain and analgesic use following major elective intracranial surgery. J Neurosurg. 2007;106(2):210–216. doi:10.3171/jns.2007.106.2.210
3. Basali A, Mascha EJ, Kalfas I, Schubert A. Relation between perioperative hypertension and intracranial hemorrhage after craniotomy. Anesthesiology. 2000;93(1):48–54. doi:10.1097/00000542-200007000-00012
4. Flexman AM, Ng JL, Gelb AW. Acute and chronic pain following craniotomy. Curr Opin Anaesthesiol. 2010;23(5):551–557. doi:10.1097/ACO.0b013e32833e15b9
5. Edgley C, Hogg M, De Silva A, Braat S, Bucknill A, Leslie K. Severe acute pain and persistent post-surgical pain in orthopaedic trauma patients: a cohort study. Br J Anaesth. 2019;123(3):350–359. doi:10.1016/j.bja.2019.05.030
6. Morad A, Winters B, Stevens R, et al. The efficacy of intravenous patient-controlled analgesia after intracranial surgery of the posterior fossa: a prospective, randomized controlled trial. Anesth Analg. 2012;114(2):416–423. doi:10.1213/ANE.0b013e31823f0c5a
7. Verchère E, Grenier B, Mesli A, Siao D, Sesay M, Maurette P. Postoperative pain management after supratentorial craniotomy. J Neurosurg Anesthesiol. 2002;14(2):96–101. doi:10.1097/00008506-200204000-00002
8. Dunn LK, Naik BI, Nemergut EC, Durieux ME. Post-craniotomy pain management: beyond opioids. Curr Neurol Neurosci Rep. 2016;16(10):93. doi:10.1007/s11910-016-0693-y
9. Durieux ME, Himmelseher S. Pain control after craniotomy: off balance on the tightrope? J Neurosurg. 2007;106(2):207–209. doi:10.3171/jns.2007.106.2.207
10. de Gray LC, Matta BF. Acute and chronic pain following craniotomy: a review. Anaesthesia. 2005;60(7):693–704. doi:10.1111/j.1365-2044.2005.03997.x
11. Chaki T, Sugino S, Janicki PK, et al. Efficacy and safety of a lidocaine and ropivacaine mixture for scalp nerve block and local infiltration anesthesia in patients undergoing awake craniotomy. J Neurosurg Anesthesiol. 2016;28(1):1–5. doi:10.1097/ana.0000000000000149
12. Bloomfield EL, Schubert A, Secic M, Barnett G, Shutway F, Ebrahim ZY. The influence of scalp infiltration with bupivacaine on hemodynamics and postoperative pain in adult patients undergoing craniotomy. Anesth Analg. 1998;87(3):579–582. doi:10.1097/00000539-199809000-00015
13. Song J, Li L, Yu P, Gao T, Liu K. Preemptive scalp infiltration with 0.5% ropivacaine and 1% lidocaine reduces postoperative pain after craniotomy. Acta Neurochir (Wien). 2015;157(6):993–998. doi:10.1007/s00701-015-2394-8
14. Batoz H, Verdonck O, Pellerin C, Roux G, Maurette P. The analgesic properties of scalp infiltrations with ropivacaine after intracranial tumoral resection. Anesth Analg. 2009;109(1):240–244. doi:10.1213/ane.0b013e3181a4928d
15. Law-Koune JD, Szekely B, Fermanian C, Peuch C, Liu N, Fischler M. Scalp infiltration with bupivacaine plus epinephrine or plain ropivacaine reduces postoperative pain after supratentorial craniotomy. J Neurosurg Anesthesiol. 2005;17(3):139–143. doi:10.1097/01.ana.0000171730.41008.da
16. Ikeuchi M, Kamimoto Y, Izumi M, et al. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014;22(7):1638–1643. doi:10.1007/s00167-013-2367-5
17. Basuni AS, Ezz HA, Albirmawy OA. Preoperative peritonsillar infiltration of dexamethasone and levobupivacaine reduces pediatric post-tonsillectomy pain: a double-blind prospective randomized clinical trial. J Anesth. 2013;27(6):844–849. doi:10.1007/s00540-013-1638-0
18. Shantiaee Y, Mahjour F, Dianat O. Efficacy comparison of periapical infiltration injection of dexamethasone, morphine and placebo for postoperative endodontic pain. Int Dent J. 2012;62(2):74–78. doi:10.1111/j.1875-595X.2011.00092.x
19. Maged AM, Deeb WS, Elbaradie S, et al. Comparison of local and intra venous dexamethasone on post operative pain and recovery after caesarean section. A randomized controlled trial. Taiwan J Obstet Gynecol. 2018;57(3):346–350. doi:10.1016/j.tjog.2018.04.004
20. Jia Y, Zhao C, Ren H, Wang T, Luo F. Pre-emptive scalp infiltration with dexamethasone plus ropivacaine for postoperative pain after craniotomy: a protocol for a prospective, randomized controlled trial. J Pain Res. 2019;12:1709–1719. doi:10.2147/jpr.S190679
21. Langford P, Wolfe R, Danks RA. Wound healing after craniotomy: a randomized trial comparing scalp clips to artery forceps for scalp hemostasis. J Neurosurg. 2009;111(6):1175–1178. doi:10.3171/2009.5.Jns081481
22. Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43.
23. Bayram A, Doğan M, Cihan C, Karataş D, Gökahmetoğlu G, Özcan I. The efficacy of levobupivacaine hydrochloride-dexamethasone infiltration for post-tonsillectomy pain in adults. J Craniofac Surg. 2015;26(7):e651–e653. doi:10.1097/scs.0000000000001975
24. Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth. 2007;54(7):544–548. doi:10.1007/bf03022318
25. Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008;138(2):380–391. doi:10.1016/j.pain.2008.01.017
26. Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther. 2011;13(2):210. doi:10.1186/ar3305
27. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163(1):29–43. doi:10.1111/j.1476-5381.2010.01199.x
28. Kilinc L, Türk B, Türk HS, Cinar S, Turgut S, Islamoğlu S. Peritonsillar dexamethasone-bupivacaine vs. bupivacaine infiltration for post-tonsillectomy pain relief in children: a randomized, double-blind, controlled study. Eur Arch Otorhinolaryngol. 2019;276(7):2081–2089. doi:10.1007/s00405-019-05472-y
29. Myles PS, Myles DB, Galagher W, et al. Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 2017;118(3):424–429. doi:10.1093/bja/aew466
30. El-Boghdadly K, Short AJ, Gandhi R, Chan VWS. Addition of dexamethasone to local infiltration analgesia in elective total hip arthroplasty: a double-blind, randomized control trial. Reg Anesth Pain Med. 2019;44:1003–1009. doi:10.1136/rapm-2019-100873
31. Gao W, Zhang QR, Jiang L, Geng JY. Comparison of local and intravenous dexamethasone for postoperative pain and recovery after tonsillectomy. Otolaryngol Head Neck Surg. 2015;152(3):530–535. doi:10.1177/0194599814567856
32. Blichfeldt-Eckhardt MR. From acute to chronic postsurgical pain: the significance of the acute pain response. Dan Med J. 2018;65(3).
33. Duan G, Yang G, Peng J, et al. Comparison of postoperative pain between patients who underwent primary and repeated cesarean section: a prospective cohort study. BMC Anesthesiol. 2019;19(1):189. doi:10.1186/s12871-019-0865-9
34. Tınastepe N, Oral K. Neuropathic pain after dental treatment. Agri. 2013;25(1):1–6. doi:10.5505/agri.2013.55477
35. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: gastrointestinal and endocrinologic side effects. J Am Acad Dermatol. 2017;76(1):11–16. doi:10.1016/j.jaad.2016.02.1239
36. Carolina E, Kato T, Khanh VC, et al. Glucocorticoid impaired the wound healing ability of endothelial progenitor cells by reducing the expression of CXCR4 in the PGE2 pathway. Front Med (Lausanne). 2018;5:276. doi:10.3389/fmed.2018.00276
37. Patel S, Thompson D, Innocent S, Narbad V, Selway R, Barkas K. Risk factors for surgical site infections in neurosurgery. Ann R Coll Surg Engl. 2019;101(3):220–225. doi:10.1308/rcsann.2019.0001
38. Ersayli DT, Gurbet A, Bekar A, Uckunkaya N, Bilgin H. Effects of perioperatively administered bupivacaine and bupivacaine-methylprednisolone on pain after lumbar discectomy. Spine (Phila Pa 1976). 2006;31(19):2221–2226. doi:10.1097/01.brs.0000232801.19965.a0
39. Gurbet A, Bekar A, Bilgin H, Ozdemir N, Kuytu T. Preemptive wound infiltration in lumbar laminectomy for postoperative pain: comparison of bupivacaine and levobupivacaine. Turk Neurosurg. 2014;24(1):48–53. doi:10.5137/1019-5149.Jtn.8431-13.0
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.