Back to Journals » Journal of Pain Research » Volume 11
Practical management of opioid rotation and equianalgesia
Authors Treillet E, Laurent S , Hadjiat Y
Received 5 April 2018
Accepted for publication 24 July 2018
Published 29 October 2018 Volume 2018:11 Pages 2587—2601
DOI https://doi.org/10.2147/JPR.S170269
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Erwan Treillet,1 Sophie Laurent,2 Yacine Hadjiat3
1AP-HP, Médecine de la Douleur et Médecine Palliative, Hôpital Lariboisière, Paris, France; 2Institut de Cancérologie, Institut Gustave Roussy, Villejuif, France; 3Mundipharma France, Paris, France
Purpose: To review the recent literature on opioid rotation (ie, switching from one opioid drug to another or changing an opioid’s administration route) in cancer patients experiencing severe pain and to develop a novel equianalgesia table for use in routine clinical practice.
Methods: The MEDLINE database was searched with terms “cancer pain,” “opioid rotation,” “opioid switching,” “opioid ratio,” “opioid conversion ratio,” and “opioid equianalgesia” for the major opioids (morphine, oxycodone, fentanyl, and hydromorphone) and the intravenous, subcutaneous, oral, and transdermal administration routes. Selected articles were assessed for the calculated or cited opioid dose ratio, bidirectionality, and use of the oral morphine equivalent daily dose or a direct drug-to-drug ratio.
Results: Twenty publications met our selection criteria and were analyzed in detail. We did not find any large-scale, prospective, double-blind randomized controlled trial with robust design, and most of the studies assessed relatively small numbers of patients. Bidirectionality was investigated in seven studies only.
Conclusion: The updated equianalgesic table presented here incorporates the latest data and provides information on bidirectionality. Despite the daily use of equianalgesic tables, they are not based on high-level scientific evidence. More clinical research is needed on this topic.
Keywords: opioid rotation, opioid switching, equianalgesic dose, morphine, hydromorphone, oxycodone, fentanyl
Introduction
Over the last 20 years, opioid rotation, sometimes referred to as opioid switching, has become a common practice for optimizing pain management in many fields of medicine including oncology, postsurgical care, and palliative care.1 Opioid rotation is defined as switching from one opioid drug to another or changing an opioid’s administration route. This approach may be of value if the patient starts suffering from some of the well-known adverse drug reactions associated with opioids,2 such as nausea, vomiting, constipation,3,4 acute urinary retention, myoclonus,5 respiratory depression, sedation, and cognitive impairment (including hallucinations, nightmares, agitation).6–9 These side effects can persist despite symptomatic treatment and may thus limit opioid use. Opioid rotation is also particularly useful if a patient becomes tolerant to a given opioid drug, develops hyperalgesia,7,8,10–12 or can no longer achieve sufficient pain control. Rotation may also be indicated by a change in the patient’s clinical state, such as kidney failure, liver failure, fever, and other disorders that may impair the pharmacokinetics or metabolism of opioid drugs. By way of an example, an opioid patch is contraindicated if a patient becomes feverish. Lastly, rotation may be required if oral administration is no longer feasible, such as in terminally ill patients, patients undergoing head and neck surgery, those with gastrostomies or nasogastric tubes, or those with incompatible gastrointestinal symptoms (eg, irrepressible vomiting).10,13–22
Opioid rotation is particularly relevant for patients with cancer pain: Recent advances in cancer treatment increase the survival of patients with advanced cancer.23 They require longer periods of opioid treatment and are therefore more exposed to opioid rotation.
Opioid rotation is closely linked to – but distinct from – the pharmacological concept of equianalgesia. Given that opioid drugs differ in their analgesic potency, changing from one to another will generally require re-evaluation of the dose if, for the patient’s comfort, equipotency is to be achieved. Equivalence ratios can be calculated from the difference between the two drugs’ respective potency curves.24 In routine clinical practice, opioid rotation relies on expert-validated equianalgesia tables that are available in a variety of formats: printed tables, stand-alone computer software, Internet-based converters, etc.25 However, the equivalence ratios are primarily based on data from the pharmaceutical industry or on old data that need updating or adjustment for specific disease settings (eg, for cancer pain, neurological pain). Lastly, equianalgesic ratios are not always bidirectional (a bidirectional ratio has the same value for the switch A to B as for B to A) and clinicians need to be aware that there are directional differences in opioid equivalents and that some ratios may not be “reversible” in direction.26 The mechanisms underlying this phenomenon are unclear but could be related to the generation of active metabolites.27
Although opioid rotation has been recommended by international experts8,10,13,15,19,20,24,28–35 and endorsed by a Cochrane Collaboration review published in 2010,28 these recommendations have also been criticized by pain specialists, pointing that some conversion tables do not accurately reflect the dose ratios for which evidence is available.8,24,25,29–31 Importantly, there are few publications on well-designed, randomized controlled trials (RCTs) in “ideal” study populations (ie, large number of patients, clinically stable, with stable pain).36–43 In a 2011 review of conversion ratios, Mercadante and Caraceni44 found 31 prospective papers on this topic, but the majority of these studies had methodological flaws and were not designed to explore or demonstrate equianalgesic dose data. In fact, the primary criterion for evaluation in most of opioid studies is safety or efficacy rather than equianalgesia. Given this context, we decided to review the literature on opioid rotation with a view to provide practical suggestions based on the published evidence.
Methods
The MEDLINE (via PubMed) database was searched up until April 30, 2018.
Given the lack of MESH terms for opioid rotation, opioid switching, or the opioid ratio, we used the search terms “cancer pain,” “opioid rotation,” “opioid switching,” “opioid ratio,” “opioid conversion ratio,” and “opioid equianalgesia.” The search was limited to studies of human subjects: published systematic reviews of RCTs, RCTs, other clinical trials, meta-analyses, and case reports. There were no restrictions on year, language, minimum number of study participants, or length of follow-up.
We selected articles for further analysis if at least one of the keywords was mentioned in the title or abstract. We also searched the reference lists of articles identified using this search strategy and selected those judged to be relevant. Given that our ultimate objective is to make an inventory of ratios, which could serve as a basis for devising proposals for routine clinical practice, we focused on the major opioids (morphine [M], oxycodone [OX], fentanyl [F], and hydromorphone [HM]) and the most common administration routes (oral [po], intravenous [iv], subcutaneous [sc], intramuscular [im], transdermal [td], and suppository [su]). Studies were eligible for inclusion if they involved adult patients with chronic cancer pain and contained clear data on opioid conversion ratios and equianalgesia. Evaluation of articles was performed by two pain specialists (ET and YH). Both reviewers agreed on inclusion of the specific articles. In case of disagreement, a third reviewer (SL) decided regarding the inclusion.
After reading the full text of each selected article, we noted i) the calculated opioid ratio (when this was a trial end point) or the cited ratio (when used in the study design), ii) whether dose dependency (ie, whether the ratio differed according to the dose of the first opioid) or directionality (ie, whether the ratio differed according to the direction of the switch) had been investigated, and iii) whether the ratios were based on the oral morphine equivalent daily dose (MEDD) or on direct (drug-to-drug) ratios.
Results
Twenty publications met our selection criteria (Figure 1) and were analyzed in detail. Our search did not identify any meta-analyses or large RCTs. The main reasons for excluding studies were methodological limitations (ie, studies not designed to explore or demonstrate equianalgesic dose data), mixed or unclear pain etiology (cancer and noncancer pain, ie, nociceptive, neuropathic, and postsurgical pain), and rotational studies to or from molecules not included in our scope (ie, methadone, oxymorphone, tapentadol). Most of the selected studies had been published within the past 20 years.
  | Figure 1 Flow diagram for the selection of studies. |
Morphine
In most patients requiring an opioid for moderate to severe pain, morphine is both efficacious and acceptable and is the drug of choice according to the World Health Organization guidelines45 and the opioid against which all others are measured. The effective analgesic dose of morphine varies considerably partly because of individual variations in systemic bioavailability. In addition to its pharmacologically active parent compound, morphine is glucuronidated to two metabolites with potentially important differences in efficacy: morphine-6-glucuronide, with potent analgesic activity, and morphine-3-glucuronide, which lacks analgesic activity.
Six randomized controlled, double-blind, crossover studies, three uncontrolled prospective, and five retrospective studies reported data on conversion ratios between morphine (po, sc, su, iv) and other opioids targeted in our search (Table 1).
Babul et al compared the efficacy and safety of a controlled-release suppository of morphine (Msu) and controlled-release morphine tablets (Mpo) in a randomized double-blind crossover study, in 27 patients with cancer pain.46 There were no significant differences between treatments in overall scores for pain intensity, visual analog scale, ordinal pain intensity, sedation, and mean daily rescue analgesic use. The author concluded that Msu provides pain control comparable with that provided by Mpo when given every 12 hours at a 1:1 dose ratio.
Bruera et al compared the clinical efficacy and safety of Msu every 12 hours and subcutaneous morphine (Msc) every 4 hours for 4 days each, in a randomized double-blind crossover study using a 2.5:1 analgesic equivalence ratio.47 Twenty-three patients with cancer pain completed the study, and the results showed that the mean calculated ratio of rectal to sc morphine dose was 2.4:1 (mean daily morphine dose 326+69 mg [range, 60–1,200] for Msu and 138±28 mg [range, 24–480] for Msc).
Kalso and Vainio switched 20 patients with uncontrolled cancer pain who were taking “weak” opioids (codeine, dextropropoxyphene, etc) to intravenous morphine or oxycodone in a randomized, double-blinded, crossover design.48 Patients first titrated themselves pain-free using IV patient-controlled analgesia for 48 hours. After 48 hours, they were switched to the oral form of either morphine or oxycodone. Following the switch in route, patients were able to adjust their oral doses to improve pain control during 48 hours. This phase was followed by the crossover phase to the alternative opioid. The median calculated oral:IV potency ratios (giving comparable analgesia) were 0.31 for morphine and 0.70 for oxycodone.
Five studies compared morphine administered orally,49–51 subcutaneously,36 or intravenously52 vs subcutaneous (Fsc) or transdermal fentanyl (Ftd). Two crossover studies compared Mpo with oxycodone per os (OXpo)38,53 and one compared Msc with OXsc.32 Three studies assessed the analgesic equivalence ratio between morphine (po or sc) and hydromorphone (po, sc, or iv).34,54,55
These studies are detailed below, and the results are summarized in Table 1 and Figure 2.
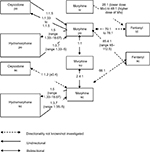  | Figure 2 Conversion ratios of morphine to and from other opioids. Abbreviations: iv, intravenous; po, oral; sc, subcutaneous; su, suppository; td, transdermal. |
Fentanyl
Fentanyl is a phenylpiperidine opioid agonist. Its low molecular weight is low and high lipid solubility provides excellent bioavailability (90%) via the transdermal or transmucosal routes, although the oral bioavailability is low (<30%). Fentanyl is predominantly converted by CYP3A4-mediated N-dealkylation to norfentanyl, a nontoxic and inactive metabolite. The mean elimination half-life is approximately 17 hours. According to the summary of product characteristics (fentanyl 50 μg/mL solution for injection), a dose of 100 μg (2 mL) per day transdermal has an analgesic action that is comparable with 10 mg oral morphine.
One randomized controlled, double-blind, crossover study, three prospective studies, and three retrospective studies reported data on conversion ratios between fentanyl (td, sc, iv) and other opioids targeted in our search (Table 2). These studies demonstrate considerable variability in conversion ratios, both within and across studies, and underscore the need for caution in applying ratios during opioid rotation from and to fentanyl.
Two published studies on the dose conversion ratio between the transdermal fentanyl patch (Ftd) and morphine preparations have been identified. Kato et al published in 2004 the results of their retrospective observational study of 144 patients with chronic pain of cancer origin and with difficulty tolerating oral morphine, undergoing rotation to fentanyl patch.49 The authors calculated the conversion ratio from the regression equation obtained from the daily oral morphine dose prior to rotation and daily fentanyl patch delivered dose in subjects undergoing opioid rotation from oral morphine to fentanyl patch (R2=0.68), reporting a daily oral morphine dose/daily fentanyl patch delivered dose ratio of 78:1.
Donner et al performed a multicenter study with 98 patients (38 patients were treated according to protocol and their data were analyzed) with controlled cancer pain with the aim of studying the most convenient conversion ratio when switching from morphine to transdermal fentanyl.51 Initial doses were calculated with the Mpo:Ftd ratio of 100:1. The regression analysis comparing fentanyl dosages at the end of the study (day 15) with the morphine dosages at day 1 demonstrated a mean Mpo:Ftd ratio of 70:1 (R2=0.82). This result implicates that a calculation table with a ratio 100:1 is by 30% subequianalgesic but may be appropriate with allowance of a safety margin.
Watanabe et al reviewed the charts of 22 patients with cancer-related pain who were switched from a variety of opioids to subcutaneous fentanyl by continuous infusion.50 In 13 patients who were switched from morphine or hydromorphone and reached dose stability (defined as no dose changes and no more than two rescue doses per day for 48 hours), the median relative potency Mpo:Fsc ratio (n=4 patients who stabilized) was 85.4:1 (range 65:1–112.5:1). The authors noted a wide range of dose ratios and suggested that a more cautious morphine to fentanyl potency ratio of 100:1 may be advisable and acknowledged that the small size of the study was a major limitation.
In a prospective study of 23 hospice cancer patients (published in 1999), Hunt et al compared subcutaneous morphine to subcutaneous fentanyl in a trial designed to evaluate pain control and side effects.36 In this study, they used and then confirmed a conversion ratio of 10 mg Msc:150 µg Fsc. This equates to an Msc:Fsc conversion ratio of 66:1. Although this was a crossover double-blind trial, bidirectionality was not investigated. Furthermore, the crossover was performed early (on day 3), which complicated the interpretation of adverse events, and the small number of subjects in this report limits the conclusions that can be made.
Kawano et al examined dose conversion ratios in patients undergoing opioid rotation from morphine injection (continuous IV administration [Miv]) to transdermal fentanyl patches in a prospective observational study of 45 patients with chronic pain of cancer origin.52 The results supported the feasibility of rotation from continuous endovenous morphine to fentanyl patches, with a conversion ratio of 50:1, but also showed that fentanyl doses decreased with the equivalent target morphine dose, ratio ranging from 28:1 (lower dose Miv) to 48:1 (higher dose Miv). These results suggest that in opioid rotation from low dose, 50:1 is not enough for the fentanyl patch. Similarly, Matsumura et al reported retrospective data from 122 patients, suggesting that the typical conversion OXpo:Ftd ratio was 95:1 but was significantly reduced in patients taking a daily oral morphine equivalent dose of <45 mg/d and in patients with poor pain control to 52:1 and 64:1, respectively (study using MEDD for conversion, not selected in our analysis).56
In 2016, Reddy et al reported a ratio of 1:100 for Fsc mg/d to MEDD (n=47 cancer patients)57 and 100:1 MEDD to Fsc mg/d (n=129) in two retrospective studies.58 As these ratios had been calculated using the MEDD conversion method, we did not retain these results for our analysis.
Two prospective studies evaluated the conversion from IV to transdermal fentanyl. Results demonstrated that this conversion can be accomplished safely and effectively using a 1:1 (Fiv:Ftd) conversion ratio. Two different conversion strategies are proposed. Kornick et al used a 12-hour conversion method in 15 patients with cancer pain: the fentanyl infusion dose was decreased in half 6 hours after patch application, then completely stopped after 12 hours.59 Samala et al proposed a continuous 6-hour overlap method as a safe and effective alternative strategy, which may be simpler and may eliminate Fiv dose adjustments during conversion to Ftd.60
The above findings are summarized in Table 2 and Figure 3.
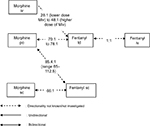  | Figure 3 Conversion ratios of fentanyl to and from other opioids. Abbreviations: iv, intravenous; po, oral; sc, subcutaneous; td, transdermal. |
Oxycodone
Oxycodone is a synthetic derivative of thebaine and is structurally related to codeine. Oxycodone is well absorbed when orally administered, and compared with morphine, it has a higher oral bioavailability (up to 87%). The central opioid effects of oxycodone are governed primarily by the parent drug, with a negligible contribution from its circulating oxidative and reductive metabolites. The relative oral bioavailability of controlled-release to immediate-release oral dosage forms is 100%.61 According to the summary of product characteristics (oxycodone prolonged release tablets), patients receiving oral morphine before oxycodone therapy should have their daily dose based on the following ratio: 10 mg of oral oxycodone is equivalent to 20 mg of oral morphine.
We identified five double-blind crossover studies and one prospective observational study with relevant data regarding equianalgesic conversion ratios to and from oxycodone in cancer pain (Table 3).
Two double-blind, randomized, crossover studies have attempted to measure the relative potency between oral oxycodone and oral morphine, using an initial 1:1.5 ratio. Heiskanen and Kalso confirmed that the total opioid consumption ratio of oxycodone to morphine was 1:1.5 when oxycodone was administered first but found that the appropriate ratio was 1.33:1 when oxycodone was administered after morphine.53 One year later, Bruera et al tested and confirmed a 1:1.5 conversion ratio between controlled-release oxycodone and controlled-release morphine but did not find oxycodone-morphine’s directionality established by Heiskanen and Kalso.38
Beaver et al published in 1978 the results of a double-blind crossover comparison of oral with intramuscular oxycodone.62 In total, the data showed that oral oxycodone is half as potent as intramuscular oxycodone.
Our selection also included Kalso and Vainio study48 (presented above, in the “Morphine” section), and two other trials32,37 comparing oxycodone and hydromorphone, which are discussed below.
The above findings are summarized in Table 3 and Figure 4.
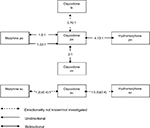  | Figure 4 Conversion ratios of oxycodone to and from other opioids. Abbreviations: iv, intravenous; im, intramuscular; po, oral; sc, subcutaneous; td, transdermal. |
Hydromorphone
Hydromorphone is a hydrogenated ketone analogue of morphine. As with morphine, there is great interindividual variation in oral bioavailability (10%–65%). Some metabolites may have greater analgesic activity than hydromorphone itself but are unlikely to contribute to the pharmacological activity of hydromorphone.63 According to the summary of product characteristics (hydromorphone hydrochloride 1.3 mg and 2.6 mg capsules), 1.3 mg of hydromorphone has an efficacy approximately equivalent to 10 mg of morphine given orally.
We identified one double-blind crossover study, three prospective studies, and three retrospective studies with relevant data regarding equianalgesic conversion ratios to and from hydromorphone (po, sc) in cancer pain (Table 4). In a study by Wallace et al, patients with chronic cancer pain were enrolled in an open-label conversion trial, from various opioids to HMpo, using an oral M:HM ratio of 5:1 for a MEDD conversion.40 Of the 127 patients who received HMpo, 85 (67%) completed the study. The majority of patients who achieved stabilization did so without titration or with just one or two titration steps. The conclusion was that the 5:1 ratio was safe and effective when converting from morphine to hydromorphone. Wallace et al did not evaluate converting patients back from hydromorphone to oral morphine.
Two retrospective studies (published in 1996 by Bruera et al and in 1997 by Lawlor et al) investigated the ratios for HMpo, Mpo, and Msc and their directionality.27,34 Both studies found that the po ratio was not bidirectional. According to Bruera et al, the ratio for Mpo:HMpo was 5.33:1 and the ratio for HMpo:Mpo was 1:3.57. The results of Lawlor et al were similar: Expressing all ratios as M:HM, the median dose ratios (lower-upper quartiles) for sc and po rotations were 4.92 (4.1–5.9) vs 5.76 (4.9–5.8) for M to HM (P=0.28, NS) and 4.0 (3.1–4.8) vs 3.45 (2.8–4.2) for HM to M (P=0.4, NS), respectively. These data suggest that HM is five times more potent than M when given second (M to HM) but is only 3.7 times more potent when given first (HM to M). The authors therefore recommended a ratio of 5 for M:HM in rotating from M to HM and ratio of 3.7 for M:HM when rotating from HM to M in patients exposed to chronic intake of these opioids. This directional difference in potency between M and HM is accepted by expert groups and may apply to both oral and parenteral dosing and may be independent of prior opioid exposure.8,24,35,64,65
Hagen and Babul compared the clinical efficacy and safety of an OXpo with that of HMpo in patients with stable cancer pain in a double-blind crossover study (31 patients completed the study).37 Based on their results, the calculated ratio for HMpo:OXpo is 1:4.13 and is bidirectional.
Gagnon et al prospectively collected the data of cancer patients who were rotated from another strong opioid to OXsc to establish conversion ratios.32 For the 11 patients who were switched from HMsc to OXsc, the authors reported an equianalgesic ratio of 0.5 (±0.4):1.
Inoue et al investigated the efficacy and safety of switching from oral morphine to hydromorphone immediate-release tablets at the HM to M conversion ratio of 1:5 or 1:8 in cancer patients with adequate pain control with oral morphine (60 or 90 mg/d).54 This multicenter, active-controlled, randomized, double-blind, parallel-group, comparative study enrolled 30 adults patients in conversion ratio 1:5 group and 40 patients in conversion ratio 1:8 group. The results showed no statistical difference in pain control between the conversion ratio groups (P=0.1298), and no intergroup difference was observed in the incidence of adverse events or serious adverse events.
The authors concluded that a conversion ratio between 1:5 and 1:8 is considered clinically appropriate for a switch from oral morphine to oral hydromorphone for pain control in cancer patients.
Reddy et al retrospectively reviewed the charts of consecutive inpatient palliative care consultations (2010–2014), in order to determine the conversion ratio from intravenous hydromorphone to oral hydromorphone and other oral opioids.55 From that cohort, they reviewed patients who were subsequently converted to oral hydromorphone (extended or immediate release or both) or to oral formulations of morphine and oxycodone and successfully discharged home without readmission within 1 week for uncontrolled pain. Among 394 patients on HMiv, 147 underwent conversion to HMpo and 247 underwent rotation to Mpo (163) or OXpo (84). Conversion ratio was defined as 24-hour oral opioid dose before discharge/net IV hydromorphone dose for each patient.
They found that the median (interquartile range) conversion ratio from HMiv to HMpo was 2.5 (2.14–2.75) with correlation of 0.95 (P<0.0001). They also found that a dose-dependency as the conversion ratio from HMiv to HMpo was significantly lower in patients receiving ≤30 mg of hydromorphone/d (2.07 vs 2.5).
When patients were rotated to other opioids, the observed equianalgesic ratio from HMiv to OXpo was 1:8.06 and the ratio from HMiv to Mpo was 1:11. The ratios were significantly lower for doses of HMiv ≥30 mg/d. This finding may suggest possible hyperalgesia with high doses of hydromorphone, wherein a lower conversion ratio to other opioids would be required.
The above findings are summarized in Table 4 and Figure 5.
  | Figure 5 Conversion ratios of hydromorphone to and from other opioids. Abbreviations: po, oral; sc, subcutaneous. |
Other opioids
The equivalence ratios for methadone will not be discussed here, as they have already been extensively studied. It has been found that the ratio varies markedly as a function of the initial opioid dose (expressed as the oral MEDD) and the reason for rotation (side effects, uncontrolled pain, tolerance, cost, etc).21,31,66 Other drugs (such as buprenorphine, hydrocodone, tapentadol, meperidine, and step 2 analgesics of the World Health Organization analgesic ladder) are not reviewed here because they are not part of the usual armamentarium or are too recent.
The use of short-acting transmucosal fentanyl warrants comment. In France, the indication for this formulation is restricted to breakthrough cancer pain.67 In fact, the dose required to achieve complete analgesia is obtained by titration, which has to be performed iteratively and is therefore not taken into account in the estimation of the equivalent dose. This methodology is subject to debate and illustrates the difficulty of performing well-designed, scientifically robust studies in the field of pain.
Discussion
Obstacles to clinical and pharmacological research on pain and analgesics
Although many clinical trials have been published, clinicians should be aware that research in this field faces a large number of obstacles. We have classified these limitations into three groups: limitations related to pharmacological studies of equivalence ratios, those related to the design of clinical trials with rotation, and those related to the characteristics of the patients and their pain.
Limitations related to the assessment of pharmacological ratios
- The most reliable method for ratio calculation is based on the evaluation of pain after a single injection of an opioid.68 In general, this ratio is calculated in a postsurgical setting, where patients are usually opioid-naïve. However, this situation does not match everyday practice in cancer pain management, where the patients are rarely opioid-naïve and long-term treatment is necessary.24,36,69
- Some researchers consider that the ratio for a given opioid may change over time (ie, during long-term treatment).70 This constitutes a further argument against the “single injection” method of ratio calculation.
- The equianalgesic ratio for hydromorphone is unidirectional.34 It is well-known that the ratio for the switch from hydromorphone to morphine is lower than for the switch in the opposition direction. However, directionality has rarely been investigated for other opioids.
- In general, the equianalgesic ratios proposed in the literature are median values that do not always confer reliable equivalence; for example, the ratio for switching from methadone to oral morphine depends on the initial opioid dose. In such a case, using a median ratio would be a mistake. The dose-dependency may apply to transdermal fentanyl and perhaps other opioids, as this has been recently reported for intravenous hydromorphone at doses ≥30 mg/d.55 This must be tested.
- The published equianalgesic ratios are often derived from pharmaceutical industry data and have not been confirmed by clinical trials or studies of routine clinical practice.
- MEDD is often used as the common denominator for opioid rotation, rather than direct drug-to-drug ratios, but this method may be less safe in patients receiving high doses of opioids. For example, patients switching from high-dose oxycodone to transdermal fentanyl will need two approximations (to MEDD and from MEDD) to calculate the final dose.
Limitations related to trial design
- We did not find any large-scale, prospective, double-blind RCT with robust design. Most of the trials in this field studied small numbers of patients and/or were retrospective.
- A crossover design has occasionally been used. Unfortunately, the crossover was usually performed early (eg, on day 3),36 which complicates the interpretation of side effects.
- The presence or absence of concomitant nonopioid analgesic use is not always mentioned in study publications, even though an impact on pain control is highly probable.
- The assessment of a small number of patients may lead to extremely wide dosage ranges (eg, a mean [range] level of 96 mg [4.5–660] for oxycodone).32 This may constitute a source of bias when calculating an equianalgesic ratio.
Limitations related to the characteristics of the patients and their pain
- Very few studies have examined primary care for cancer patients, despite the fact that primary care physicians also prescribe opioids.71,72
- Many variability factors complicate the interpretation of equianalgesic ratios, and not all of these factors can be accounted for in a pain analysis. Gender, ethnic origin,73 clinical history, changes over time in pain,74 psychological factors,75,76 opioid-naïve status, inter- and intrasubject variability in pharmacodynamics and pharmacokinetics,21,22 drug–drug interactions,8,24,29 and genetic factors21 all influence pain perception.77
- The pain profile may depend on the disease, so appropriate postsurgical pain relief may not be relevant for cancer pain.
- Increased pain levels due to disease progression also complicate the calculation of equianalgesic ratios because the latter requires stable pain. The few studies that attempted to tackle this limitation have seen their patient population collapse.51
- A number of factors seem to be significantly associated with interindividual variability in the conversion ratios of fentanyl in opioid switching: For instance, higher modified Glasgow Prognostic Score, breast cancer, total protein, alanine aminotransferase, advanced age, and male sex have been identified as significant predictors of a need for higher dose transdermal fentanyl.78,79
Expert guidelines on opioid rotation
In view of the abovementioned limitations, the pain specialist may have trouble deciding on the best choice for clinical practice. Hence, a number of general rules and recommendations have been suggested by expert panels, with a view to improve overall safety and effectiveness. However, these recommendations are not supported by high levels of scientific evidence. Equianalgesia tables are nevertheless useful in routine practice and physicians must continue to use them, while bearing in mind the inherent limitations of these tables.10,24,29,80 Fine and Portenoy8 proposed guidelines for safe, two-step rotations with the available ratios:
- Step 1: A 25% to 50% dose decrease in the new opioid (relative to the dose suggested by the equianalgesic table), except when the latter is methadone (with a decrease of 75%–90%), transdermal fentanyl (no decrease), or short-acting transmucosal fentanyl (for which titration is essential).
- Step 2: Apply an additional increase or decrease of 15%–30%, depending on the clinical context (hyperalgesia, sedation, age, etc).
In 2012, Webster and Fine81 suggested a potentially safer method of opioid rotation that obviates the need to use a conversion table: The dose of the original opioid is slowly decreased (by about 10%–25% per week), while the new opioid dose is slowly titrated beginning at a dose that would normally be used in an opioid-naïve patient or at the lowest available dose for the formulation. Webster and Fine recommend providing sufficient immediate-release opioid throughout the rotation to prevent withdrawal and/or increased pain if the dosing changes prove insufficient. In most instances, the complete switch can occur within 3–4 weeks.
A practical tool for applying clinically observed ratios
In order to take the abovementioned limitations into account, we propose the use of a table that takes into consideration the direction of the switching (ie, from the initial opioid to the target opioid) and calculating the direct dose equivalence without having to link to the MEDD (Table 5). The table is based on the clinical studies reviewed here. The principle is the same as a “distance chart”. The reader should note the absence of published data for many pairs of opioids.
Conclusion
Although most experts recommend the use of equianalgesia tables (due to their ease of use, clear clinical value, and good long-term safety record), they also acknowledge that currently available tools are not ideal. Hence, we consider that this aspect of pain medicine warrants a solid scientific basis. Although the table presented in this article is far from perfect, it does attempt to incorporate scientific uncertainties and data ranges so that the practitioner is aware of the limitations of this approach and can decide on possible alternatives (based on the latest data). The table also provides information on directionality (when available), which has rarely been investigated and may not apply to all opioids. Completion and optimization of the table will be a difficult but worthwhile task.
Acknowledgments
The authors acknowledge the assistance of Pascal Fangio (Poissy Hospital, Poissy, France) and Anna Patrikidou (Institut Gustave Roussy, Villejuif, France) in the development of the present review’s search strategy. The authors thank Philippe Mayran of PM Sante (Garches, France) for providing medical writing support, which was funded by Mundipharma, Paris, France.
Disclosure
Yacine Hadjiat is an employee of Mundipharma and reports no other conflicts of interest in this work. Erwan Treillet and Sophie Laurent report no conflicts of interest in this work.
References
Mercadante S, Bruera E. Opioid switching in cancer pain: From the beginning to nowadays. Crit Rev Oncol Hematol. 2016;99:241–248. | ||
Lawlor PG, Bruera E. Side-effects of opioids in chronic pain treatment. Curr Opin Anaesthesiol. 1998;11(5):539–545. | ||
Walters JB, Montagnini M. Current concepts in the management of opioid-induced constipation. J Opioid Manag. 2010;6(6):435–444. | ||
Reimer K, Hopp M, Zenz M, et al. Meeting the challenges of opioid-induced constipation in chronic pain management - a novel approach. Pharmacology. 2009;83(1):10–17. | ||
Mccann S, Yaksh TL, von Gunten CF. Correlation between myoclonus and the 3-glucuronide metabolites in patients treated with morphine or hydromorphone: A pilot study. J Opioid Manag. 2010;6(2):87–94. | ||
de Schepper HU, Cremonini F, Park MI, Camilleri M. Opioids and the gut: pharmacology and current clinical experience. Neurogastroenterol Motil. 2004;16(4):383–394. | ||
Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain. 2002;18(Supplement):S3–S13. | ||
Fine PG, Portenoy RK. Establishing “Best Practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38(3):418–425. | ||
Kurita GP, Lundorff L, Pimenta CA, Sjøgren P. The cognitive effects of opioids in cancer: a systematic review. Support Care Cancer. 2009;17(1):11–21. | ||
Mercadante S, Bruera E. Opioid switching: A systematic and critical review. Cancer Treat Rev. 2006;32(4):304–315. | ||
Silverman SM. Opioid induced hyperalgesia: clinical implications for the pain practitioner. Pain Physician. 2009;12(3):679–684. | ||
Mitra S. Opioid-induced hyperalgesia: pathophysiology and clinical implications. J Opioid Manag. 2008;4(3):123–130. | ||
Müller-Busch HC, Lindena G, Tietze K, Woskanjan S. Opioid switch in palliative care, opioid choice by clinical need and opioid availability. Eur J Pain. 2005;9(5):571–579. | ||
de Leon-Casasola OA. Current developments in opioid therapy for management of cancer pain. Clin J Pain. 2008;24(Supplement 10):S3–S7. | ||
Mercadante S. Opioid rotation for cancer pain: rationale and clinical aspects. Cancer. 1999;86(9):1856–1866. | ||
Cherny NJ, Chang V, Frager G, et al. Opioid pharmacotherapy in the management of cancer pain: a survey of strategies used by pain physicians for the selection of analgesic drugs and routes of administration. Cancer. 1995;76(7):1283–1293. | ||
Levy MH. Pharmacologic treatment of cancer pain. N Engl J Med Overseas Ed. 1996;335(15):1124–1132. | ||
Maddocks I, Somogyi A, Abbott F, Hayball P, Parker D. Attenuation of morphine-induced delirium in palliative care by substitution with infusion of oxycodone. J Pain Symptom Manage. 1996;12(3):182–189. | ||
Mercadante S, Ferrera P, Villari P, Casuccio A, Intravaia G, Mangione S. Frequency, indications, outcomes, and predictive factors of opioid switching in an acute palliative care unit. J Pain Symptom Manage. 2009;37(4):632–641. | ||
Ripamonti C, Dickerson ED. Strategies for the treatment of cancer pain in the new millennium. Drugs. 2001;61(7):955–977. | ||
Smith HS. Variations in opioid responsiveness. Pain Physician. 2008;11(2):237–248. | ||
Slatkin NE. Opioid switching and rotation in primary care: implementation and clinical utility. Curr Med Res Opin. 2009;25(9):2133–2150. | ||
Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. | ||
Knotkova H, Fine PG, Portenoy RK. Opioid rotation: the science and the limitations of the equianalgesic dose table. J Pain Symptom Manage. 2009;38(3):426–439. | ||
Berdine HJ, Nesbit SA. Equianalgesic dosing of opioids. J Pain Palliat Care Pharmacother. 2006;20(4):79–84. | ||
Davis MP. Cancer pain. In: Olver IN, editor. The MASCC Textbook of Cancer Supportive Care and Survivorship. Boston, MA: Springer US; 2011:11–22. | ||
Lawlor P, Turner K, Hanson J, Bruera E. Dose ratio between morphine and hydromorphone in patients with cancer pain: a retrospective study. Pain. 1997;72(1–2):79–85. | ||
Quigley C. Opioid switching to improve pain relief and drug tolerability. Cochrane Database Syst Rev. 2004;3:CD004847. | ||
Anderson R, Saiers JH, Abram S, Schlicht C. Accuracy in equianalgesic dosing. conversion dilemmas. J Pain Symptom Manage. 2001;21(5):397–406. | ||
Patanwala AE, Duby J, Waters D, Erstad BL. Opioid conversions in acute care. Ann Pharmacother. 2007;41(2):255–267. | ||
Vissers KCP, Besse K, Hans G, Devulder J, Morlion B. Opioid rotation in the management of chronic pain: where is the evidence? Pain Practice. 2010;10(2):85–93. | ||
Gagnon B, Bielech M, Watanabe S, Walker P, Hanson J, Bruera E. The use of intermittent subcutaneous injections of oxycodone for opioid rotation in patients with cancer pain. Support Care Cancer. 1999;7(4):265–270. | ||
Narabayashi M, Saijo Y, Takenoshita S, et al. Opioid rotation from oral morphine to oral oxycodone in cancer patients with intolerable adverse effects: an open-label trial. Jpn J Clin Oncol. 2008;38(4):296–304. | ||
Bruera E, Pereira J, Watanabe S, Belzile M, Kuehn N, Hanson J. Opioid rotation in patients with cancer pain. A retrospective comparison of dose ratios between methadone, hydromorphone, and morphine. Cancer. 1996;78(4):852–857. | ||
de Stoutz ND, Bruera E, Suarez-Almazor M. Opioid rotation for toxicity reduction in terminal cancer patients. J Pain Symptom Manage. 1995;10(5):378–384. | ||
Hunt R, Fazekas B, Thorne D, Brooksbank M. A comparison of subcutaneous morphine and fentanyl in hospice cancer patients. J Pain Symptom Manage. 1999;18(2):111–119. | ||
Hagen NA, Babul N. Comparative clinical efficacy and safety of a novel controlled-release oxycodone formulation and controlled-release hydromorphone in the treatment of cancer pain. Cancer. 1997;79(7):1428–1437. | ||
Bruera E, Belzile M, Pituskin E, et al. Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol. 1998;16(10):3222–3229. | ||
Wirz S, Wartenberg HC, Elsen C, Wittmann M, Diederichs M, Nadstawek J. Managing cancer pain and symptoms of outpatients by rotation to sustained-release hydromorphone: a prospective clinical trial. Clin J Pain. 2006;22(9):770–775. | ||
Wallace M, Rauck RL, Moulin D, Thipphawong J, Khanna S, Tudor IC. Conversion from standard opioid therapy to once-daily oral extended-release hydromorphone in patients with chronic cancer pain. J Int Med Res. 2008;36(2):343–352. | ||
Ahmedzai S, Brooks D. Transdermal fentanyl versus sustained-release oral morphine in cancer pain: preference, efficacy, and quality of life. The TTS-Fentanyl Comparative Trial Group. J Pain Symptom Manage. 1997;13(5):254–261. | ||
Morita T, Takigawa C, Onishi H, et al. Opioid rotation from morphine to fentanyl in delirious cancer patients: an open-label trial. J Pain Symptom Manage. 2005;30(1):96–103. | ||
Clemens KE, Klaschik E. Clinical experience with transdermal and orally administered opioids in palliative care patients-a retrospective study. Jpn J Clin Oncol. 2007;37(4):302–309. | ||
Mercadante S, Caraceni A. Conversion ratios for opioid switching in the treatment of cancer pain: a systematic review. Palliat Med. 2011;25(5):504–515. | ||
World Health Organization. Cancer Pain Relief: With a Guide to Opioid Availability. 2nd ed. Geneva, Switzerland: World Health Organization; 2009. | ||
Babul N, Provencher L, Laberge F, Harsanyi Z, Moulin D. Comparative efficacy and safety of controlled-release morphine suppositories and tablets in cancer pain. J Clin Pharmacol. 1998;38(1):74–81. | ||
Bruera E, Fainsinger R, Spachynski K, Babul N, Harsanyi Z, Darke AC. Clinical efficacy and safety of a novel controlled-release morphine suppository and subcutaneous morphine in cancer pain: a randomized evaluation. J Clin Oncol. 1995;13(6):1520–1527. | ||
Kalso E, Vainio A. Morphine and oxycodone hydrochloride in the management of cancer pain. Clin Pharmacol Ther. 1990;47(5):639–646. | ||
Kato K, Mizaki T, Yamazaki S, et al. A study of transdermal fentanyl in cancer pain at Aichi-Cancer Center. Yakugaku Zasshi. 2004;124(5):287–291. | ||
Watanabe S, Pereira J, Hanson J, Bruera E. Fentanyl by continuous subcutaneous infusion for the management of cancer pain: a retrospective study. J Pain Symptom Manage. 1998;16(5):323–326. | ||
Donner B, Zenz M, Tryba M, Strumpf M. Direct conversion from oral morphine to transdermal fentanyl: a multicenter study in patients with cancer pain. Pain. 1996;64(3):527–534. | ||
Kawano C, Hirayama T, Kuroyama M. Dose conversion in opioid rotation from continuous intravenous infusion of morphine hydrochloride injection to fentanyl patch in the management of cancer pain. Yakugaku Zasshi. 2011;131(3):463–467. | ||
Heiskanen T, Kalso E. Controlled-release oxycodone and morphine in cancer related pain. Pain. 1997;73(1):37–45. | ||
Inoue S, Saito Y, Tsuneto S, Aruga E, Ogata T, Uemori M. A double-blind, randomized comparative study to investigate the morphine to hydromorphone conversion ratio in Japanese cancer patients. Jpn J Clin Oncol. 2018;48(5):442–449. | ||
Reddy A, Vidal M, Stephen S, et al. The conversion ratio from intravenous hydromorphone to oral opioids in cancer patients. J Pain Symptom Manage. 2017;54(3):280–288. | ||
Matsumura C, Yamada M, Fujihara S, Chisaki Y, Takahashi K, Yano Y. Indication of adequate transdermal fentanyl dose in opioid switching from oral oxycodone in patients with cancer. Am J Hosp Palliat Med. 2016;33(2):109–114. | ||
Reddy A, Yennurajalingam S, Reddy S, et al. The opioid rotation ratio from transdermal fentanyl to “Strong” opioids in patients with cancer pain. J Pain Symptom Manage. 2016;51(6):1040–1045. | ||
Reddy A, Tayjasanant S, Haider A, et al. The opioid rotation ratio of strong opioids to transdermal fentanyl in cancer patients. Cancer. 2016;122(1):149–156. | ||
Kornick CA, Santiago-Palma J, Khojainova N, Primavera LH, Payne R, Manfredi PL. A safe and effective method for converting cancer patients from intravenous to transdermal fentanyl. Cancer. 2001;92(12):3056–3061. | ||
Samala RV, Bloise R, Davis MP. Efficacy and safety of a six-hour continuous overlap method for converting intravenous to transdermal fentanyl in cancer pain. J Pain Symptom Manage. 2014;48(1):132–136. | ||
Coluzzi F, Mattia C. Oxycodone. Pharmacological profile and clinical data in chronic pain management. Minerva Anestesiol. 2005;71(7-8):451–460. | ||
Beaver WT, Wallenstein SL, Rogers A, Houde RW. Analgesic studies of codeine and oxycodone in patients with cancer. I. Comparisons of oral with intramuscular codeine and of oral with intramuscular oxycodone. J Pharmacol Exp Ther. 1978;207(1):92–100. | ||
Sarhill N, Walsh D, Nelson KA. Hydromorphone: pharmacology and clinical applications in cancer patients. Support Care Cancer. 2001;9(2):84–96. | ||
Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. | ||
Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. | ||
Weschules DJ, Bain KT, Richeimer S. Actual and potential drug interactions associated with methadone. Pain Medicine. 2008;9(3):315–344. | ||
Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage. 2000;20(2):87–92. | ||
Eddy NB, Lee LE, Jr. The analgesic equivalence to morphine and relative side action liability of oxymorphone (14-hydroxydihydro morphinone). J Pharmacol Exp Ther. 1959;125(2):116–121. | ||
Woodhouse A, Hobbes AFT, Mather LE, Gibson M. A comparison of morphine, pethidine and fentanyl in the postsurgical patient-controlled analgesia environment. Pain. 1996;64(1):115–121. | ||
Dunbar PJ, Chapman RC, Buckley PF, Gavrin JR. Clinical analgesic equivalence for morphine and hydromorphone with prolonged PCA. Pain. 1996;68(2):265–270. | ||
Reid MC, Henderson CR, Papaleontiou M, et al. Characteristics of older adults receiving opioids in primary care: treatment duration and outcomes. Pain Medicine. 2010;11(7):1063–1071. | ||
Buckley DI, Calvert JF, Lapidus JA, Morris CD. Chronic opioid therapy and preventive services in rural primary care: an Oregon rural practice-based research network study. Ann Fam Med. 2010;8(3):237–244. | ||
Vallerand AH, Hasenau S, Templin T, Collins-Bohler D. Disparities between black and white patients with cancer pain: the effect of perception of control over pain. Pain Medicine. 2005;6(3):242–250. | ||
Wool MS, Mor V. A multidimensional model for understanding cancer pain. Cancer Invest. 2005;23(8):727–734. | ||
Syrjala KL, Chapko ME. Evidence for a biopsychosocial model of cancer treatment-related pain. Pain. 1995;61(1):69–79. | ||
Keefe FJ, Abernethy AP, C Campbell L. Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol. 2005;56(1):601–630. | ||
Hanks GW, Reid C. Contribution to variability in response to opioids. Support Care Cancer. 2005;13(3):145–152. | ||
Jia SS, Shang L, Me L, Zhao DM, Wh X, Wang YQ. Modified Glasgow prognostic score predicting high conversion ratio in opioid switching from oral oxycodone to transdermal fentanyl in patients with cancer pain. Int J Clin Exp Med. 2015;8(5):7606–7612. | ||
Kanbayashi Y, Hosokawa T, Okamoto K, et al. Factors predicting requirement of high-dose transdermal fentanyl in opioid switching from oral morphine or oxycodone in patients with cancer pain. Clin J Pain. 2011;27(8):664–667. | ||
Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. a critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22(2):672–687. | ||
Webster LR, Fine PG. Overdose deaths demand a new paradigm for opioid rotation. Pain Med. 2012;13(4):571–574. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





