Back to Journals » Infection and Drug Resistance » Volume 16
Potentiation and Mechanism of Berberine as an Antibiotic Adjuvant Against Multidrug-Resistant Bacteria
Authors Zhou H , Wang W , Cai L , Yang T
Received 18 July 2023
Accepted for publication 27 October 2023
Published 21 November 2023 Volume 2023:16 Pages 7313—7326
DOI https://doi.org/10.2147/IDR.S431256
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Hongjuan Zhou, Wenli Wang, Long Cai, Tingting Yang
Clinical Laboratory Experiment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Tingting Yang; Long Cai, Clinical Laboratory Experiment Center, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, No. 208 East Huancheng Road, Hangzhou, 310003, People’s Republic of China, Email [email protected]; [email protected]
Abstract: The growing global apprehension towards multi-drug resistant (MDR) bacteria necessitates the development of innovative strategies to combat these infections. Berberine (BER), an isoquinoline quaternary alkaloid derived from various medicinal plants, has surfaced as a promising antibiotic adjuvant due to its ability to enhance the effectiveness of conventional antibiotics against drug-resistant bacterial strains. Here, we overview the augmenting properties and mechanisms of BER as an adjunctive antibiotic against MDR bacteria. BER has been observed to exhibit synergistic effects when co-administered with a range of antibiotics, including β-lactams, quinolones, aminoglycosides, tetracyclines, macrolides, lincosamides and fusidic acid. The adjunctive properties of BER led to an increase in antimicrobial effectiveness for these antibiotics against the corresponding bacteria, a decrease in minimal inhibitory concentrations, and even the reversal from resistance to susceptibility sometimes. The potential mechanisms responsible for these effects included the inhibition of antibiotic efflux, the disruption of biofilm formation, the modulation of host immune responses, and the restoration of gut microbiota homeostasis. In brief, BER demonstrated significant potential as an antibiotic adjuvant against MDR bacteria and is a promising candidate for combination therapy. Further research is necessary to fully elucidate its mechanism of action and address the challenges associated with its clinical application.
Keywords: berberine, antibiotic adjuvant, multidrug-resistant bacteria, efflux pump, biofilm
Introduction
Antimicrobial resistance (AMR) poses a significant threat to human, animal and environmental health, as well as the global economy and development.1 It was reported that an estimated 4.95 million deaths were associated with bacterial AMR in 2019, including 1.27 million deaths directly attributable to bacterial AMR.2 The Review on Antimicrobial Resistance, commissioned by the UK Government, has warned that AMR could result in the deaths of 10 million people annually by 2050.3 The widespread utilization, overuse and inappropriate use of antimicrobials has resulted in the proliferation of antimicrobial resistance over the past eighty years.4 The escalating global prevalence of drug-resistance renders antibiotics progressively ineffective. The absence of effective measures for the prevention and appropriate management of drug-resistant infections, coupled with insufficient accessibility to both novel and established antimicrobial agents that meet quality standards, will result in a surge in the population of individuals experiencing treatment failure or succumbing to infectious diseases.5 Consequently, there is an urgent requirement for novel antibacterial agents or a new medication regimen.
Antibiotic adjuvants present a viable and complementary strategy to the discovery of novel antibiotics and the optimization of current ones.6 These adjuvants, which may include compounds or herbal products, do not possess direct bactericidal properties but rather augment antibiotic efficacy through various mechanisms such as resistance blockade, intracellular antibiotic accumulation enhancement, complementary bactericidal pathways, signaling and regulatory pathway inhibition or boosting the host response to bacterial infection.7
Berberine (BER), an isoquinoline quaternary alkaloid, has been extracted from several medicinal plants, including Hydrastis canadensis, Berberis aristata, Coptis chinensis, Coptis rhizome, Coptis japonica, Phellodendron amurense, and Phellodendron chinense schneid.8–10 It gained more attention as a potential adjuvant of antibiotics against multi-drug resistant (MDR) bacterial antibiotics recently.11–14 BER effectively reduced the minimum inhibitory concentrations (MICs) and enhanced bactericidal activity of some antibiotics against MDR bacteria, as well as inhibiting of bacterial adhesion and intracellular invasion. This review summarizes the synergistic effects and underlying mechanisms observed when BER was used in combination with conventional therapeutic antibiotics against MDR bacteria.
The History and Medicinal Lineage of BER
BER is the primary bioactive compound in the traditional Chinese medicinal herb Huanglian. Huanglian is a widely utilized Chinese herb that has long history of medicinal use. The earliest documented mention of Huanglian dates back to the “Shen Nong Ben Cao Jing”, which was written in 200 A.D. In “Note of Elite Physicians”, Hongjing Tao was the first to document the anti-diabetic effects of Huanglian around 1500 years ago.15
Modern pharmacological research has demonstrated that BER exhibits inhibitory effects on a broad range of Gram-positive and Gram-negative bacteria, rendering it effective in treating gastrointestinal infections and bacterial dysentery. Subsequent research on the pharmacological effects and mechanisms of BER has revealed its additional activities, including anti-tumor, cardiovascular protection, anti-inflammatory and anti-Alzheimer’s disease effects.16 Consequently, it has achieved broad application in the management of gastrointestinal disorders,17,18 infectious diseases19–21 and specific tumors.22,23
The Structure and Pharmacological Attributes of BER
The chemical structure of BER consists of a fused ring system of dihydroisoquinoline and isoquinoline, exhibiting notable planar characteristics (Figure 1). The skeleton can be categorized into four rings, denoted as A, B, C and D. In the A ring, the C2 and C3 positions form a methylenedioxy group. The C ring features a quaternary ammonium structure, wherein a positively charged nitrogen atom resides in the aromatic ring. This quaternary ammonium structure is essential for the antibacterial activity of BER. The D ring is marked by the attachment of a methoxy group to both the C9 and C10 positions.24
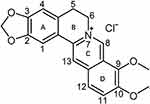 |
Figure 1 Chemical structure of berberine. Adapted from Ai X, Yu P, Peng L, et al. Berberine: a review of its pharmacokinetics properties and therapeutic potentials in diverse vascular diseases. Front Pharmacol. 2021;12:762654. Creative Commons.24 |
The main clinical application of BER is its hydrochloride salt, administered orally.16 However, pharmacokinetic studies have shown that BER has low oral bioavailability and intestinal absorption rates, measuring less than 5%.25 This may be partly attributed to the presence of the strong hydrophilic quaternary ammonium group in the structure, impeding the transmembrane transport and intestinal absorption of BER.26 Furthermore, hepatic and biliary excretion, self-aggregation and interaction with the P-glycoprotein pump may also contribute to the limited bioavailability.16 Various strategies have been developed to enhance the bioavailability of berberine, including co-administration with other substances or the use of lipid nanoparticles for BER delivery.27
The BER metabolism occurs in two stages. Phase I metabolism includes demethylation, demethylenation and reduction, leading to the formation of multiple metabolites including berberrubine (M1), thalifendine (M4), demethyleneberberine (M2), hydroxylated berberine, jatrorrhizine (M3), columbamine isomer and dhberberine. In the subsequent phase, BER is subjected to glucuronidation and sulfation, resulting in the formation of Phase II metabolites, which are subsequently excreted through bile and urine. BER metabolism can take place in the liver, intestine and gastrointestinal microbiota. However, the liver serves as the primary site of metabolism.27
Several studies have indicated that BER exhibits low toxicity in the human body. Phase I clinical trials have demonstrated the safety of consuming excessive amounts of BER. BER has minimal toxicity towards healthy cells. While BER may induce adverse reactions such as constipation or nausea, they are typically not severe. Ceasing BER use leads to the disappearance of the most common constipation symptoms.28
The Synergistic Effect of BER on Antibiotics
Combining antibiotics was a commonly employed tactic by clinicians to combat MDR bacteria or multiple infections. The potent synergy between the constituents of the combination presented an opportunity to rejuvenate existing antibiotics.29,30 The quantification of synergy could be achieved through the implementation of the straightforward checkerboard strategy, which involved the systematic dilution of concentrations of both agents to determine the optimal concentrations that result in the most effective interaction. The fraction inhibitory concentration index (FICI) was a mathematical tool utilized to determine the interaction between two compounds, A and B. The FICI was calculated using the formula FICI = MIC (A when combination with B)/MIC (A alone) + MIC (B when combination with A)/MIC (A alone). The FICI ≤0.5 indicates a synergistic effect, 0.5< FICI ≤1 indicating an additive effect, 1< FICI ≤2 indicating an indifference, and FICI >2 indicates antagonism.31,32 In cases where adjuvants do not have measurable MIC, the concentration of the adjuvant that reduces MIC of the combined antibiotic fourfold is a reliable indicator of potency. What is more significant is the degree of adjuvant concentration that reduces the MIC of antibiotics in resistant bacteria to a level equivalent to or below the breakpoint concentration.32
We used the aforementioned approaches for assessing the effectiveness of BER as an adjuvant in combination with other antibiotics against clinically prevalent MDR bacteria, encompassing gram-negative bacteria (eg, Pseudomonas aeruginosa, Acinetobacter baumannii, Salmonella and Klebsiella pneumoniae), gram-positive bacteria (eg, Methicillin-resistant Staphylococcus aureus and Clostridium difficile), and non-tuberculous mycobacteria (eg, Mycobacterium avium complex and Mycobacterium abscessus).
Gram-Negative Bacteria
The World Health Organization (WHO) released a list of bacteria that require urgent development of new antibiotics in 2017. The list specifically emphasized the danger posed by gram-negative bacteria that exhibit resistance to multiple antibiotics. Notably, all bacterial strains categorized as “Priority Critical” were carbapenem-resistant gram-negative bacteria, which encompass carbapenem-resistant A. baumannii, carbapenem-resistant P. aeruginosa and third-generation cephalosporin-resistant Enterobacteriaceae.33 Based on the recent national bacterial resistance surveillance data from China, it has been observed that approximately 70% of clinical isolates resistant to antibiotics were gram-negative bacteria.34 The treatment of infections caused by such bacteria poses a significant challenge for medical practitioners.35 There was a growing gap between the clinical need for new antibiotics and new drug discovery and development. The unique impermeable outer membrane barriers hindered the discovery of effective antibiotics against gram-negative bacteria.36 Consequently, there existed a pressing necessity for novel antibiotics and alternative approaches to combat infections of this nature. Recent research has demonstrated that BER can enhance the efficacy of conventional therapeutic antibiotics against MDR gram-negative bacteria, including P. aeruginosa, A. baumannii, Salmonella and K. pneumoniae (Tables S1–S3). These findings offered crucial insights for the management of infections caused by such bacteria.
P. aeruginosa
A variety of antibiotics, including β-lactam/β-lactamase inhibitor combinations, carbapenems, fluoroquinolones and/or aminoglycosides, have been conventionally utilized as the preferred treatment options against resistant P. aeruginosa isolates responsible for infections.37 Furthermore, the macrolide antibiotic azithromycin was frequently administered in combination with the aforementioned drugs to treat biofilm-associated cystic fibrosis infections caused by P. aeruginosa.38,39 However, the emergence of MDR and extensively drug-resistant (XDR) organisms has diminished the efficacy and reliability of these antibiotics.37
BER has been reported to exhibit synergistic effects with the carbapenem antibiotic imipenem, the macrolide antibiotic azithromycin, and several aminoglycoside antibiotics in in vitro susceptibility tests against MDR or XDR P. aeruginosa (Tables 1 and S1). The FICI of BER combining with imipenem was 0.375. The addition of 1/4 MIC BER (128μg/mL) resulted in 8-fold reduction of the MIC of P. aeruginosa to imipenem. The combination of BER and azithromycin showed an FICI of 0.13~0.5 and led to a 4~16-fold reduction in MICs to azithromycin under 128μg/mL of BER. In the infection model, there was a marked increase in mice survival and a great improvement in the inflammation of infected lungs at 0.8 mg/kg of azithromycin combined with 3.2 mg/kg of BER.40 For aminoglycoside antibiotics, such as amikacin, arbekacin, gentamicin and tobramycin, the combination of BER led to a 2~8-fold reduction in MICs when combating MDR P. aeruginosa.41 However, the efficacy of the combination of BER and tobramycin varies among different strains. Compared to the administration of tobramycin alone, the co-administration demonstrated a twofold increase in inhibitory activity and a two to four logarithmic increase in killing activity against 13 of the 28 P. aeruginosa clinical isolates tested. However, no synergistic effects were observed in the remaining strains.42,43 In conclusion, BER exhibited significant potential in reducing the resistance of imipenem, azithromycin, amikacin, arbekacin and gentamicin against MDR/XDR P. aeruginosa. Nonetheless, the extent of reduction in tobramycin resistance varies depending on the strains.
 |
Table 1 The Effect of Sub-MIC Berberine on Antibiotics Against Common Multidrug-Resistant Bacteria |
A. baumannii
Numerous strains of MDR A. baumannii have demonstrated resistance to clinically significant antibiotics, including ceftazidime/avibactam, ampicillin/sulbactam, and piperacillin/tazobactam.55,56 BER, when used alone, exhibits limited antibacterial activity against MDR A. baumannii, with a MIC range of 256 to 1024 μg/mL (Table S1).45 However, the combination of BER with other antibiotics has been shown to significantly decrease the MICs of MDR A. baumannii (Tables 1 and S1). Synergistic effects (FICI <0.5) were observed in the combinations of BER/sulbactam and BER/meropenem for the MDR strains. The addition of BER even resulted in the re-sensitization of MDR strains to various antibiotics, including ciprofloxacin (MIC reduced from 32 to 1 μg/mL), sulbactam (MIC reduced from 64 to 4 μg/mL), and meropenem (MIC reduced from 128 to 2 μg/mL). In a murine infection model, the combination therapy of 20 mg/kg BER and 400 mg/kg sulbactam exhibited superior antimicrobial efficacy against MDR strains when compared to monotherapy.45 This observation highlighted the potential of BER to reverse antibiotic resistance or augment the susceptibility of MDR A. baumannii to multiple antibiotics that have lost their effectiveness. The utilization of BER as an adjuvant presented a promising approach to reintroduce off-the-treatment-list antibiotics, such as sulbactam and ciprofloxacin, for the treatment of MDR A. baumannii infections.
Salmonella
Salmonella spp. remains a significant bacterial pathogen responsible for foodborne illnesses. The FDA has approved three antibiotics, namely ciprofloxacin, ceftriaxone and azithromycin, for the treatment of Salmonella infections in the United States.57 However, resistance to these antibiotics has become increasingly prevalent in recent years, particularly in Asia. It was noteworthy that the escalation in the rate of ciprofloxacin resistance was evident across all serotypes of Salmonella.57,58 The co-administration of BER and ciprofloxacin exhibited an additive effect against MDR Salmonella, with an FICI of 0.75 (Tables 1 and S1). The MIC to ciprofloxacin was reduced by 4-fold (from 2.56 to 0.64 μg/mL), transitioning from resistant to an intermediate level (Table S1).46 This combination therapy may reduce the dosage of ciprofloxacin required for treatment, conferring the advantage of preventing drug resistance and minimizing adverse effects. This suggests that the combination could represent a promising strategy for the management of Salmonella infection.
K. pneumoniae
A significant proportion of K. pneumoniae acquires resistance to multiple antimicrobials, in addition to inherently resisting penicillins.59 BER has been shown to enhance the susceptibility of MDR K. pneumoniae to ciprofloxacin, reducing the incidence of drug resistance (Tables 1 and S1). The presence of BER resulted in a 50~75% reduction in the concentration of ciprofloxacin compared to the use of ciprofloxacin alone. The combination of the two drugs was demonstrated synergistic (15%) and additive (80%) effects against most K. pneumoniae isolates. It significantly inhibited the growth of bacteria in the time-kill assay.47 These indicated that BER has potential in the development of antibiotic treatment regimens targeting MDR K. pneumoniae.
Gram-Positive Bacteria
Globally, treatment failure caused by gram-positive cocci infections posed a new clinical dilemma after gram-negative bacilli. WHO has recently designated gram-positive vancomycin-resistant Enterococcus (VRE), methicillin-resistant Staphylococcus aureus (MRSA), and vancomycin-resistant Staphylococcus aureus (VRSA) as high priority categories requiring new antimicrobial treatments.33 The clinical urgency for novel antimicrobial agents or effectively therapeutic strategies to address the therapeutic dilemma of gram-positive cocci were pressing. BER, when utilized as an antibiotic adjuvant, exhibits the capacity to augment the susceptibility of certain gram-positive drug-resistant bacteria to particular antibiotics, offering promising prospects for its therapeutic utilization.
MRSA
The global dissemination of MRSA has resulted in its emergence as a predominant cause of bacterial infections in healthcare and community settings. This pathogen exhibits resistance to a wide range of antibiotics, including β-lactams and cephalosporins.60 The addition of BER has been shown to restore the antimicrobial activity of various antibiotics against MRSA, including β-lactams (ampicillin and oxacillin), rifamycins (rifampicin), macrolides (azithromycin), lincosamides (clindamycin), and fusidic acid (Tables 1 and S2). The combination of BER and these antibiotics exhibited synergistic effects, resulting in a 2~16 folds reduction in the MICs of antibiotics against MRSA, except ampicillin.48–51 The effects of combination of BER and ampicillin vary among two studies. One study demonstrated an additive effect (FICI = 0.625) and led to 8-fold reduction in MICs to ampicillin,48 while the other reported indifferent effects (FICI = 1.5~2.0).49 This indicates the need for further research to elucidate the interaction between these two drugs. The combination of BER and the β-lactam antibiotic CFZ has not been found to yield significant results against MRSA.
C. difficile
Another increasingly common gram-positive MDR bacterium is C. difficile, which has been classified as an “Urgent” level to public health in 2019 by the Centers for Disease Control and Prevention.61 Clinically, C. difficile was responsible for causing approximately 10~25% of antibiotic-associated diarrhea, 50~75% of antimicrobial-associated colitis, and 90~100% of pseudomembranous colitis.62 The combination of BER and vancomycin, a peptide antibiotic, has been shown to exhibit an additive effect (FICI = 0.625~0.75) and reduced the MICs of MDR C. difficile to vancomycin by 4~8-fold (Tables 1 and S2).52
Mycobacteria
Mycobacterial-induced pulmonary diseases have been identified as a significant contributor to morbidity and mortality in humans, such as tuberculosis caused by Mycobacterium tuberculosis.63 Recent epidemiological studies revealed that the global incidence of non-tuberculous mycobacteria (NTM) infections was on the rise, posing a new critical public health concern.64 Among the NTM species, Mycobacterium avium complex and Mycobacterium abscessus have been identified as the most commonly encountered pathogens.65 The treatment of pulmonary infections caused by NTM has always been a challenge due to the intrinsic resistance of these bacteria to many commonly used antibiotics. In particular M. abscessus infections, which are resistant to most classes of antibiotics, including macrolides, aminoglycosides, rifamycins, tetracyclines and β-lactams.64
Recent studies have shown that the combination of BER with anti-NTM drugs may reduce the MICs of M. avium complex and M. abscessus to certain antibiotics, such as the frequently employed therapeutic drugs clarithromycin and linezolid. The addition of BER resulted in a reduction of the MICs of M. avium complex to clarithromycin by 2 to 8192-fold (median: 4-fold) (Tables 1 and S3). Some high-level clarithromycin-resistant strains even reverted to clarithromycin susceptibility or intermediate levels with MICs decreasing from 2048 to 0.25~16 μg/mL (128~8192-fold). Moreover, in clarithromycin-susceptible M. avium complex strains, the concomitant use of BER also demonstrated a synergistic effect, resulting in a significant reduction of clarithromycin MIC by 4~8 fold.53
In the cases of M. abscessus, BER exhibited the ability to decrease the MIC of clarithromycin and linezolid by 4-fold (from 0.5 to 0.125 μg/mL) and 8-fold (from 32 to 4 μg/mL), respectively (Tables 1 and S3).54 The combination of BER reversed linezolid resistance to susceptibility. Additionally, when combined with 1/2 MIC BER, the MICs of M. abscessus to other antibiotics could also be reduced, such as methoxybenzyl/pyrimethamine, amikacin, tigecycline, imipenem, ceftazidime and ciprofloxacin. However, no significant effect was observed in the combination with tigecycline, doxycycline, minocycline, ceftazidime, amoxicillin and moxifloxacin.54 These results indicated that BER enhances the bacteriostatic effects of certain antibiotics and could offer new therapeutic options for the treatment of NTM infections.
Mechanism of the Synergistic Effect of Berberine
Antibiotic adjuvants were classified into two distinct groups based on their intended target, namely Class I agents that act on the pathogen, and Class II agents that act on the host.32 BER functioned as a dual adjuvant and exhibited both Class I and Class II adjuvant activities. On the one hand, it reduced the development of antibiotic resistance by inhibiting bacterial efflux pumping and biofilm formation. On the other hand, it interacted with host defense mechanisms and restored the host’s gut microbiota to augment the action of antibiotics.
Action on the Pathogens as Class I Adjuvant
Inhibition of Antibiotic Efflux
Efflux pumps were transmembrane proteins that facilitate the transportation of a diverse range of toxic compounds, including antibiotics, across bacterial membranes in an energy-dependent manner.66 The majority of efflux systems were capable of transporting multiple unrelated substances, thereby potentially contributing to multidrug resistance.4 This form of resistance primarily impacted antibiotics impeding protein and DNA biosynthesis within the cell, particularly macrolides, tetracyclines and fluoroquinolones.6
BER impeded the efflux of antibiotics through direct inhibition of the expression of efflux pump genes or competition with the binding sites of efflux pump substrates. The reduction in efflux raised the concentration of antibiotics in bacteria and reduced the incidence of drug resistance. Recent studies have identified that BER primarily targets the MexXY efflux pump, including MexXY-like or MexXY-dependent efflux pumps.41–43 By inhibiting efflux systems, BER resulted in a reduction in MexXY-dependent resistance to aminoglycosides in P.aeruginosa, Acinetobacter xylosoxidans and Burkholderia cepacia (Figure 2A). This effect was also observed for other classes of antibiotics, such as cephalosporins (cefepime), macrolides (erythromycin) and lincosamides (lincomycin).41 Additionally, the inhibition of the MexXY-OprM efflux pump system by BER caused imipenem-resistant P. aeruginosa to re-sensitize to the drug. The combination of BER and imipenem significantly reduced the expression of mexX, mexY, mexZ and oprM.44
 |
Figure 2 The synergistic mechanism of berberine (BER) in inhibiting antibiotic efflux. (A) BER inhibits the expression of efflux pump genes, preventing antibiotic efflux. In Pseudomonas aeruginosa, Acinetobacter xylosoxidans and Burkholderia cepacia, BER significantly reduces the expression of the MexXY multidrug efflux system, leading to a decrease in efflux of various antibiotics, including aminoglycosides (amikacin, arbekacin, gentamicin and tobramycin), β-lactams (cefepime and imipenem), macrolides (erythromycin), and lincosamides (lincomycin). (B) BER acts as a competitor in inhibiting antibiotic efflux. In Acinetobacter baumannii, BER enhances the expression of the AdeABC efflux pump gene adeB, and in multidrug-resistant Klebsiella pneumoniae, BER upregulates the expression of the acrA, acrB, tolC and acrR genes associated with the AcrAB-TolC efflux pump. BER exhibits a greater affinity for these efflux pumps and is preferentially pumped out, thereby reducing the efflux of other corresponding antibiotics. The figure was created with BioRender.com (https://app.biorender.com). |
On the other hand, BER, as an amphoteric cation, was a preferable efflux substrate for certain MDR bacteria.67 The combination of BER could diminish the efflux of other antibiotics and maintain their concentrations in cells since BER was pumped out first (Figure 2B). In the treatment of A. baumannii, BER was more likely a pump competitor to restore antibiotic sensitivity than an inhibitor. It significantly enhanced the expression of the AdeABC efflux pump gene adeB and exhibited a greater affinity than antibiotics.45 In MDR P. aeruginosa, BER upregulated the expression of genes acrA, acrB, tolC and acrR associated with the AcrAB-TolC efflux pump, ultimately expanding the antimicrobial efficacy of ciprofloxacin against this bacterium.47
Inhibition of Biofilm Formation
Biofilms are a microbial community enmeshed in a self-generated matrix of extracellular polymeric substances (EPS) and attached to either biotic or abiotic surfaces.68 Compared to planktonic ones, bacteria within biofilms exhibit greater resistance (10~1000 times) to sanitizers and disinfectants.69 This resistance is attributed to the reduction of permeability, the decrease of target expression caused by reduced metabolic activity, and the production of large numbers of persisters.66 Inhibiting biofilm formation has been found to have a noteworthy impact on the reversal of bacterial resistance.70
BER, when used in combination with certain antibiotics, has demonstrated the ability to impede biofilm formation in corresponding bacteria. The combination of BER and fusidic acid, clindamycin and rifampicin prevented the formation of MRSA biofilm and disrupted the biofilm completion;50,51 the combination of BER with azithromycin inhibited P. aeruginosa biofilm formation;40,71 and the combination of BER with linezolid reduced the biofilm formation in M. abscessus.54
BER has the potential to inhibit biofilm formation at multiple stages, including bacterial attachment, microcolony formation, biofilm maturation and biofilm dispersal.72 It can impact the expression of the type I fimbriae gene fimA in S. typhimurium, resulting in reduced quantities of type I fimbriae and consequently decreasing bacterial activity and adhesion (Figure 3A).73 BER can reduce the formation of Salmonella biofilms by 31.20%.74 It interacts with the quorum-sensing receptors LasR and RhlR in P. aeruginosa, effectively inhibiting the formation and maturation of biofilms (Figure 3B).74 The combination of BER and azithromycin has been shown to significantly reduce the levels of QS molecules in P. aeruginosa, as well as the expression of key genes involved in biofilm establishment and structural stability, such as lasI, lasR, rhlI, rhlR, eDNA68 and the alginate-related regulatory genes algG, algD and algR.71 BER reduced the relative expression levels of biofilm-related genes (sarA, fnbA, rbf, eno, lrgA, strA, cidA and agr) in S. aureus, consequently impacting biofilm formation at stages including bacterial attachment, aggregation, structural maturation and dispersal (Figure 3C).75–77 These findings highlight the potential of BER as a promising therapeutic agent for the prevention and treatment of biofilm-associated infections.
 |
Figure 3 Berberine (BER) enhances antibiotic resistance by inhibiting the biofilm formation. (A) BER reduces the expression and quantity of type I fimbriae by affecting the expression of the fimA gene, thereby decreasing the activity and adhesion of Salmonella typhimurium. (B) BER affects the bacterial attachment, microcolony and biofilm maturation of Pseudomonas aeruginosa by reducing the levels of QS molecules and the expression of key genes involved in biofilm establishment and structural stability, such as lasI, lasR, rhlI, rhlR, eDNA and the alginate-related regulatory genes algG, algD and algR. (C) BER inhibits the attachment, microcolony formation and biofilm maturation, and promotes biofilm dispersal of Staphylococcus aureus by significantly downregulating the expression of genes associated with biofilm formation, such as srtA, agr, sarA, fnbA, rbf, lrgA, cidA and eno. These genes affect the production of polysaccharide intercellular adhesin (PIA), multiple extracellular proteases, and bacterial cell wall anchoring (CWA) proteins, etc. The figure was created with BioRender.com (https://app.biorender.com). |
Other Mechanisms of Action on Pathogens
BER has been observed to potentially exhibit synergistic antibacterial effects through additional mechanisms, such as the increase of cell membrane permeability and the disruption of the bacterial cell wall and cytoplasmic membrane. In an alkaline pH environment, BER enhanced bacterial cell membrane permeability and disrupted the proton motive force, implying a potential mechanism by which it can synergistically interact with other antibacterial compounds under milder conditions78,79 It has been demonstrated that the presence of BER facilitates the intracellular penetration of antibiotics such as clindamycin and levofloxacin in MRSA, leading to an increase in drug concentration within the bacteria and subsequent antibacterial activity. This phenomenon may be attributed to the ability of BER to compromise the structural integrity of the bacterial cell wall and membrane in MRSA,50 but further evidence is required.
Action on the Host as Class II Adjuvants
BER has the potential to augment the effectiveness of antibiotics in the host through two ways, by modulating host immunity or by restoring the host’s gut microbiota to modulate its inflammatory response to infection. For example, when utilized in combination with rifampicin and isoniazid, BER enhanced the efficacy of anti-tuberculosis treatment by modulating the host’s immune status.80 When used as an adjuvant therapy in a mouse model of pulmonary TB, BER mitigated pulmonary inflammation by selectively targeting immune cell recruitment and reducing inflammatory cytokines, while avoiding the induction of granulomatous lung pathology or caseous necrosis.80 In the P. aeruginosa infection models, the administration of 0.8 mg/kg of azithromycin in combination with 3.2 mg/kg of BER resulted in a significant increase in the mice survival, notable improvements in lung inflammation, reduced levels of IL-6 and IL-8, and increased levels of IL-10.40
Additionally, BER has the ability to reverse both the structural and quantitative changes of the gut microbiota in pathological conditions.81 It eliminates harmful bacteria in the intestines while enhancing the composition of beneficial bacteria, including Bifidobacterium adolescentis and Lactobacillus acidophilus. There were two main ways in which the gut microbiota interacts directly with BER: BER regulates the gut microbiota, and the gut microbiota transforms BER. The reported function of BER as a Class II adjuvant was mainly attributed to the former, such as restoring the imbalance in bacterial communities caused by vancomycin through the modulation of the structure and quantity of the gut microbiota, effectively preventing the recurrence of C. difficile infection.82
Conclusion
BER, as an antibiotic adjuvant, can reduce the resistance of many notoriously MDR bacteria to specific antibiotics and even reverse their resistance phenotypes. The combination with BER resulted in an enhanced antibacterial effect of many antibiotics, including β-lactams (sulbactam, meropenem, oxacillin and imipenem), quinolones (ciprofloxacin and levofloxacin), aminoglycosides (amikacin, arbekacin and gentamicin), tetracyclines (tigecycline), rifamycins (rifampicin), macrolides (clarithromycin and azithromycin), lincosamides (clindamycin), and fusidic acid. This offers novel perspectives for the treatment of prevalent MDR gram-negative bacteria (P. aeruginosa, A. baumannii, Salmonella and K. pneumoniae), gram-positive bacteria (MRSA and C. difficile), and mycobacteria (M. abscessus and M. avium). The known mechanisms by which BER augments the bactericidal efficacy of antibiotics include mitigation of antibiotic efflux, inhibition of biofilm formation, and regulation of host immunity and gut microbiota. The genes implicated in these mechanisms comprise those associated with the AdeABC efflux pump (adeB), MexXY efflux pump (mexX, mexY, mexZ and oprM), QS system (lasI, lasR, rhlI and rhlR), and biofilm components (algG, algD and algR).
There were limitations in the current studies on BER as an antibiotic adjuvant, such as the incomplete and unsystematic design of the combination drug susceptibility experiments, and the lack of mechanistic studies. The limited sample sizes and exclusive focus on a single antibiotic or antibiotic class hinder the comprehensive assessment of the collective synergistic effects of BER with commonly used therapeutic antibiotics on the targeted multidrug-resistant bacteria. The precise mechanisms underlying the adjuvant function of BER have yet to be fully elucidated through mechanistic studies. For example, it remains unclear whether the disparity in the synergistic effect of BER between the rrs A2059C mutant strain of M. avium and the wild-type strain.53 Moreover, the utilization of BER in conjunction with antibiotics for the treatment of MDR bacterial infections poses several obstacles, such as the feasibility of achieving a synergistic effect in vivo. The solution to this predicament entails consideration of intricate pharmacology, pharmacokinetics, and in vivo drug metabolism, among other pivotal determinants. This suggests that prior to clinical trials, it is imperative to conduct a thorough toxicological evaluation. Despite the challenges and complexities, these attempts are worthwhile owing to BER’s offering a new strategy in addressing MDR bacterial infections amidst limited antibiotic development and the continued rise of drug resistance.
Acknowledgments
This study was supported by Project of Hangzhou Municipal Health Commission (A20210201).
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organization. Global antimicrobial resistance and use surveillance system (GLASS) report: 2022; 2022. Available from: https://www.who.int/publications-detail-redirect/9789240062702.
2. Murray CJL, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0
3. O’Neill J Tackling drug-resistant infections globally: final report and recommendations. (Review on Antimicrobial Resistance, London). 2016. Available from: https://wellcomecollection.org/works/thvwsuba.
4. Christaki E, Marcou M, Tofarides A. Antimicrobial resistance in bacteria: mechanisms, evolution, and persistence. J Mol Evol. 2020;88(1):26–40. doi:10.1007/s00239-019-09914-3
5. World Health Organization. Antimicrobial resistance; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
6. Douafer H, Andrieu V, Phanstiel O, Brunel JM. Antibiotic adjuvants: make antibiotics great again! J Med Chem. 2019;62(19):8665–8681. doi:10.1021/acs.jmedchem.8b01781
7. Liu Y, Li R, Xiao X, Wang Z. Antibiotic adjuvants: an alternative approach to overcome multi-drug resistant Gram-negative bacteria. Crit Rev Microbiol. 2019;45(3):301–314. doi:10.1080/1040841X.2019.1599813
8. Song D, Hao J, Fan D. Biological properties and clinical applications of berberine. Front Med. 2020;14(5):564–582. doi:10.1007/s11684-019-0724-6
9. Kong Y, Li L, Zhao LG, Yu P, Li DD. A patent review of berberine and its derivatives with various pharmacological activities (2016–2020). Expert Opin Ther Pat. 2022;32(2):211–223. doi:10.1080/13543776.2021.1974001
10. Li Z, Wang Y, Xu Q, et al. Berberine and health outcomes: an umbrella review. Phytother Res. 2023;37(5):2051–2066. doi:10.1002/ptr.7806
11. Hobson C, Chan AN, Wright GD. The antibiotic resistome: a guide for the discovery of natural products as antimicrobial agents. Chem Rev. 2021;121(6):3464–3494. doi:10.1021/acs.chemrev.0c01214
12. Millar BC, Rao JR, Moore JE. Fighting antimicrobial resistance (AMR): Chinese herbal medicine as a source of novel antimicrobials – an update. Lett Appl Microbiol. 2021;73(4):400–407. doi:10.1111/lam.13534
13. Jadimurthy R, Mayegowda SB, Nayak SC, Mohan CD, Rangappa KS. Escaping mechanisms of ESKAPE pathogens from antibiotics and their targeting by natural compounds. Biotechnol Report. 2022;34:e00728. doi:10.1016/j.btre.2022.e00728
14. Herman A, Herman AP. Herbal products and their active constituents used alone and in combination with antibiotics against multidrug-resistant bacteria. Planta Med. 2023;89(02):168–182. doi:10.1055/a-1890-5559
15. Feng X, Sureda A, Jafari S, et al. Berberine in cardiovascular and metabolic diseases: from mechanisms to therapeutics. Theranostics. 2019;9(7):1923–1951. doi:10.7150/thno.30787
16. Patel P. A bird’s eye view on a therapeutically ‘wonder molecule’: berberine. Phytomed Plus. 2021;1(3):100070. doi:10.1016/j.phyplu.2021.100070
17. Chen C, Yu Z, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract — a review of actions and therapeutic implications. Am J Chin Med. 2014;42(05):1053–1070. doi:10.1142/S0192415X14500669
18. Zhang L, Wu X, Yang R, et al. Effects of berberine on the gastrointestinal microbiota. Front Cell Infect Microbiol. 2020;10:588517. doi:10.3389/fcimb.2020.588517
19. Chu M, Zhang MB, Liu YC, et al. Role of berberine in the treatment of methicillin-resistant staphylococcus aureus infections. Sci Rep. 2016;6(1):24748. doi:10.1038/srep24748
20. Jamshaid F, Dai J, Yang LX. New development of novel berberine derivatives against bacteria. MRMC. 2020;20(8):716–724. doi:10.2174/1389557520666200103115124
21. Ghareeb DA, Saleh SR, Seadawy MG, et al. Nanoparticles of ZnO/Berberine complex contract COVID-19 and respiratory co-bacterial infection in addition to elimination of hydroxychloroquine toxicity. J Pharm Investig. 2021;51(6):735–757. doi:10.1007/s40005-021-00544-w
22. Zhang C, Sheng J, Li G, et al. Effects of berberine and its derivatives on cancer: a systems pharmacology review. Front Pharmacol. 2019;10:1461. doi:10.3389/fphar.2019.01461
23. Devarajan N, Jayaraman S, Mahendra J, et al. Berberine—A potent chemosensitizer and chemoprotector to conventional cancer therapies. Phytother Res. 2021;35(6):3059–3077. doi:10.1002/ptr.7032
24. Ai X, Yu P, Peng L, et al. Berberine: a review of its pharmacokinetics properties and therapeutic potentials in diverse vascular diseases. Front Pharmacol. 2021;12:762654. doi:10.3389/fphar.2021.762654
25. Singh S, Pathak N, Fatima E, Negi AS. Plant isoquinoline alkaloids: advances in the chemistry and biology of berberine. Eur J Med Chem. 2021;226:113839. doi:10.1016/j.ejmech.2021.113839
26. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–523. doi:10.1002/ptr.6252
27. Khoshandam A, Imenshahidi M, Hosseinzadeh H, et al. Pharmacokinetic of berberine, the main constituent of Berberis vulgaris L: a comprehensive review. Drug Deliv and Transl Res. 2022;36(11):4063–4079. doi:10.1002/ptr.7589
28. Xiong RG, Huang SY, Wu SX, et al. Anticancer effects and mechanisms of berberine from medicinal herbs: an update review. Molecules. 2022;27(14):4523. doi:10.3390/molecules27144523
29. Coates ARM, Hu Y, Holt J, Yeh P. Antibiotic combination therapy against resistant bacterial infections: synergy, rejuvenation and resistance reduction. Expert Rev Anti Infect Ther. 2020;18(1):5–15. doi:10.1080/14787210.2020.1705155
30. Liu Y, Tong Z, Shi J, Li R, Upton M, Wang Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics. 2021;11(10):4910–4928. doi:10.7150/thno.56205
31. Norden CW, Wentzel H, Keleti E. Comparison of techniques for measurement of in vitro antibiotic synergism. J Infect Dis. 1979;140(4):629–633. doi:10.1093/infdis/140.4.629
32. Wright GD. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol. 2016;24(11):862–871. doi:10.1016/j.tim.2016.06.009
33. World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed; 2019. Available from: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed.
34. Hu F, Guo Y, Yang Y, et al. Resistance reported from China antimicrobial surveillance network (CHINET) in 2018. Eur J Clin Microbiol Infect Dis. 2019;38(12):2275–2281. doi:10.1007/s10096-019-03673-1
35. Koulenti D, Song A, Ellingboe A, et al. Infections by multidrug-resistant gram-negative bacteria: what’s new in our arsenal and what’s in the pipeline? Int J Antimicrob Agents. 2019;53(3):211–224. doi:10.1016/j.ijantimicag.2018.10.011
36. Song M, Liu Y, Huang X, et al. A broad-spectrum antibiotic adjuvant reverses multidrug-resistant Gram-negative pathogens. Nat Microbiol. 2020;5(8):1040–1050. doi:10.1038/s41564-020-0723-z
37. Holger D, Kebriaei R, Morrisette T, Lev K, Alexander J, Rybak M. Clinical Pharmacology of Bacteriophage Therapy: a Focus on Multidrug-Resistant Pseudomonas aeruginosa Infections. Antibiotics. 2021;10(5):556. doi:10.3390/antibiotics10050556
38. Sörensen M, Khakimov B, Nurjadi D, et al. Comparative evaluation of the effect of different growth media on in vitro sensitivity to azithromycin in multi-drug resistant Pseudomonas aeruginosa isolated from cystic fibrosis patients. Antimicrob Resist Infect Control. 2020;9(1):197. doi:10.1186/s13756-020-00859-7
39. Leroy AG, Caillon J, Caroff N, et al. Could azithromycin be part of Pseudomonas aeruginosa acute pneumonia treatment? Front Microbiol. 2021;12:642541. doi:10.3389/fmicb.2021.642541
40. Li Y, Huang J, Li L, Liu L. Synergistic activity of berberine with azithromycin against Pseudomonas Aeruginosa isolated from patients with cystic fibrosis of lung in vitro and in vivo. Cell Physiol Biochem. 2017;42(4):1657–1669. doi:10.1159/000479411
41. Morita Y, Nakashima KI, Nishino K, et al. Berberine is a novel type efflux inhibitor which attenuates the MexXY-mediated aminoglycoside resistance in pseudomonas aeruginosa. Front Microbiol. 2016;7:1223. doi:10.3389/fmicb.2016.01223
42. Laudadio E, Cedraro N, Mangiaterra G, et al. Natural alkaloid berberine activity against Pseudomonas aeruginosa MexXY-mediated aminoglycoside resistance: in silico and in vitro studies. J Nat Prod. 2019;82(7):1935–1944. doi:10.1021/acs.jnatprod.9b00317
43. Mangiaterra G, Cedraro N, Laudadio E, et al. The natural alkaloid berberine can reduce the number of Pseudomonas aeruginosa Tolerant cells. J Nat Prod. 2021;84(4):993–1001. doi:10.1021/acs.jnatprod.0c01151
44. Su F, Wang J. Berberine inhibits the MexXY‑OprM efflux pump to reverse imipenem resistance in a clinical carbapenem‑resistant Pseudomonas aeruginosa isolate in a planktonic state. Exp Ther Med. 2018;15(1):467–472. doi:10.3892/etm.2017.5431
45. Li X, Song Y, Wang L, et al. A potential combination therapy of berberine hydrochloride with antibiotics against multidrug-resistant Acinetobacter baumannii. Front Cell Infect Microbiol. 2021;11:660431. doi:10.3389/fcimb.2021.660431
46. Shi C, Li M, Muhammad I, et al. Combination of berberine and ciprofloxacin reduces multi-resistant Salmonella strain biofilm formation by depressing mRNA expressions of luxS, rpoE, and ompR. J Vet Sci. 2018;19(6):808. doi:10.4142/jvs.2018.19.6.808
47. Zhou XY, Ye XG, He LT, et al. In vitro characterization and inhibition of the interaction between ciprofloxacin and berberine against multidrug-resistant Klebsiella pneumonia e. J Antibiot. 2016;69(10):741–746. doi:10.1038/ja.2016.15
48. Yu HH, Kim KJ, Cha JD, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant Staphylococcus aureus. J Med Food. 2005;8(4):454–461. doi:10.1089/jmf.2005.8.454
49. Zuo GY, Li Y, Han J, Wang GC, Zhang YL, Bian ZQ. Antibacterial and synergy of berberines with antibacterial agents against clinical multi-drug resistant isolates of Methicillin-Resistant Staphylococcus aureus (MRSA). Molecules. 2012;17(9):10322–10330. doi:10.3390/molecules170910322
50. Xia S, Ma L, Wang G, et al. In vitro antimicrobial activity and the mechanism of berberine against methicillin-resistant Staphylococcus aureus isolated from bloodstream infection patients. IDR. 2002;15:1933–1944. doi:10.2147/IDR.S357077
51. Liang RM, Yong XL, Duan YQ, et al. Potent in vitro synergism of fusidic acid (FA) and berberine chloride (BBR) against clinical isolates of methicillin-resistant Staphylococcus aureus (MRSA). World J Microbiol Biotechnol. 2014;30(11):2861–2869. doi:10.1007/s11274-014-1712-2
52. Wultańska D, Piotrowski M, Pituch H. The effect of berberine chloride and/or its combination with vancomycin on the growth, biofilm formation, and motility of Clostridioides difficile. Eur J Clin Microbiol Infect Dis. 2020;39(7):1391–1399. doi:10.1007/s10096-020-03857-0
53. Menichini M, Lari N, Rindi L. Effect of efflux pump inhibitors on the susceptibility of Mycobacterium avium complex to clarithromycin. J Antibiot. 2020;73(2):128–132. doi:10.1038/s41429-019-0245-1
54. Tseng CY, Sun MF, Li TC, Lin CT. Effect of coptis chinensis on biofilm formation and antibiotic susceptibility in Mycobacterium abscessus. Evid Based Compl Alter Med. 2020;2020:1–9. doi:10.1155/2020/9754357
55. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18(7):392–407. doi:10.1038/s41579-020-0331-1
56. Assefa M, Amare A, Ibrahim S, Al-Saryi N, Al-Kadmy IMS, Aziz SN. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol Biol Rep. 2021;48(10):6987–6998. doi:10.1007/s11033-021-06690-6
57. Rai S, Kumar A, Darby EM, et al. Bacteriophage therapeutics to confront multidrug‐resistant Acinetobacter baumannii ‐ a global health menace. Environ Microbiol Rep. 2022;14(3):347–364. doi:10.1111/1758-2229.12988
58. Chen K, Wai Chi Chan E, Chen S. Evolution and transmission of a conjugative plasmid encoding both ciprofloxacin and ceftriaxone resistance in Salmonella. Emerg Micro Infect. 2019;8(1):396–403. doi:10.1080/22221751.2019.1585965
59. Chen K, Yang C, Dong N, et al. Evolution of ciprofloxacin resistance-encoding genetic elements in Salmonella. mSystems. 2020;5(6):e01234–20. doi:10.1128/mSystems.01234-20
60. Wyres KL, Lam MMC, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol. 2020;18(6):344–359. doi:10.1038/s41579-019-0315-1
61. Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4(1):18033. doi:10.1038/nrdp.2018.33
62. Centers for Disease Control and Prevention (U.S.). Antibiotic resistance threats in the United States, 2019. Centers for Disease Control and Prevention (U.S.); 2019. Available from: https://stacks.cdc.gov/view/cdc/82532.
63. Guery B, Galperine T, Barbut F. Clostridioides difficile: diagnosis and treatments. BMJ. 2019;366:l4609. doi:10.1136/bmj.l4609
64. Gopalaswamy R, Shanmugam S, Mondal R, Subbian S. Of tuberculosis and non-tuberculous mycobacterial infections – a comparative analysis of epidemiology, diagnosis and treatment. J Biomed Sci. 2020;27(1):74. doi:10.1186/s12929-020-00667-6
65. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
66. Darby EM, Trampari E, Siasat P, et al. Molecular mechanisms of antibiotic resistance revisited. Nat Rev Microbiol. 2023;21(5):280–295. doi:10.1038/s41579-022-00820-y
67. Lamut A, Peterlin Mašič L, Kikelj D, Tomašič T. Efflux pump inhibitors of clinically relevant multidrug resistant bacteria. Med Res Rev. 2019;39(6):2460–2504. doi:10.1002/med.21591
68. Grande R, Puca V, Muraro R. Antibiotic resistance and bacterial biofilm. Expert Opin Ther Pat. 2020;30(12):897–900. doi:10.1080/13543776.2020.1830060
69. Liu D, Huang Q, Gu W, Zeng XA. A review of bacterial biofilm control by physical strategies. Crit Rev Food Sci Nutr. 2022;62(13):3453–3470. doi:10.1080/10408398.2020.1865872
70. Dutt Y, Dhiman R, Singh T, et al. The association between biofilm formation and antimicrobial resistance with possible ingenious bio-remedial approaches. Antibiotics. 2022;11(7):930. doi:10.3390/antibiotics11070930325
71. Zhao Z, Guo M, Xu X, et al. In vitro synergistic inhibitory activity of natural alkaloid berberine combined with azithromycin against alginate production by Pseudomonas aeruginosa PAO1. Oxid Med Cell Longev. 2022;2022:1–10. doi:10.1155/2022/3858500
72. Mohammed YHE, Manukumar HM, Rakesh KP, Karthik CS, Mallu P, Qin HL. Vision for medicine: staphylococcus aureus biofilm war and unlocking key’s for anti-biofilm drug development. Microb Pathog. 2018;123:339–347. doi:10.1016/j.micpath.2018.07.002
73. Xu C, Wang F, Huang F, Yang M, He D, Deng L. Targeting effect of berberine on type I fimbriae of Salmonella Typhimurium and its effective inhibition of biofilm. Appl Microbiol Biotechnol. 2021;105(4):1563–1573. doi:10.1007/s00253-021-11116-1
74. Aswathanarayan JB, Vittal RR. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Adv. 2018;8(63):36133–36141. doi:10.1039/C8RA06413J
75. Zhang C, Li Z, Pan Q, et al. Berberine at sub-inhibitory concentration inhibits biofilm dispersal in Staphylococcus aureus. Microbiology. 2022;168(9). doi:10.1099/mic.0.001243
76. Ning Y, Wang X, Chen P, et al. Targeted inhibition of methicillin-resistant Staphylococcus aureus biofilm formation by a graphene oxide-loaded aptamer/berberine bifunctional complex. Drug Deliv. 2022;29(1):1675–1683. doi:10.1080/10717544.2022.2079768
77. Zhao N, Isguven S, Evans R, Schaer TP, Hickok NJ. Berberine disrupts staphylococcal proton motive force to cause potent anti-staphylococcal effects in vitro. Biofilm. 2023;5:100117. doi:10.1016/j.bioflm.2023.100117
78. Zhang X, Sun X, Wu J, et al. Berberine damages the cell surface of methicillin-resistant Staphylococcus aureus. Front Microbiol. 2008;11:621. doi:10.3389/fmicb.2020.00621
79. Wang JJ, Wang J, Li Y, et al. A systematic Cochrane Review of antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis. Zhongguo Zhong Yao Za Zhi. 2021;46(1):33–40. doi:10.19540/j.cnki.cjcmm.20201002.601
80. Ozturk M, Chia JE, Hazra R, et al. Evaluation of berberine as an adjunct to TB treatment. Front Immunol. 2021;12:656419. doi:10.3389/fimmu.2021.656419
81. Habtemariam S. Berberine pharmacology and the gut microbiota: a hidden therapeutic link. Pharmacol Res. 2020;155:104722. doi:10.1016/j.phrs.2020.104722
82. Lv Z, Peng G, Liu W, Xu H, Su J. Berberine blocks the relapse of Clostridium difficile Infection in C57BL/6 mice after standard vancomycin treatment. Antimicrob Agents Chemother. 2015;59(7):3726–3735. doi:10.1128/AAC.04794-14
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
