Back to Journals » Journal of Inflammation Research » Volume 16
Potential Use of Janus Kinase Inhibitors in the Treatment of Systemic Lupus Erythematosus
Authors Huo R, Huang X, Yang Y, Lin J
Received 15 November 2022
Accepted for publication 14 March 2023
Published 6 April 2023 Volume 2023:16 Pages 1471—1478
DOI https://doi.org/10.2147/JIR.S397639
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Monika Sharma
Rongxiu Huo,* Xinxiang Huang,* Yang Yang, Jinying Lin
Department of Rheumatology and Immunology, Guangxi Academy of Medical Sciences, the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinying Lin, Department of Rheumatology and Immunology, Guangxi Academy of Medical Sciences, the People’s Hospital of Guangxi Zhuang Autonomous Region, 6 Taoyuan Road, Qingxiu District, Nanning, Guangxi Zhuang Autonomous Region, 530016, People’s Republic of China, Email [email protected]
Abstract: Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease with unclear pathogenesis. One characteristic of SLE is pro-inflammatory and anti-inflammatory cytokine imbalance. Janus kinase (JAK) is an intracellular non-receptor tyrosine kinase essential for many cytokine signaling pathways. Dysregulation of the JAK/signal transduction and transcriptional activator (STAT) pathway is an important process in SLE pathogenesis. Targeting JAK/STAT proteins can simultaneously block the functions of multiple cytokines. Current SLE treatment with non-specific corticosteroids and immunosuppressants can cause many adverse reactions. Therefore, treatments designed to control specific molecular targets for SLE are desirable. JAK inhibitors (JAKis) are a potential treatment for rheumatic diseases; however, the use of targeted signaling pathways to treat SLE remains a challenge, and its efficacy has not been determined. JAKis have shown positive results in reducing the use of glucocorticoids and/or non-specific immunosuppressants for SLE. JAKis are currently undergoing several clinical trials and expected to be the next stage in the treatment of SLE. Therefore, inhibition of the JAK/STAT pathway through JAKis may improve traditional treatment strategies for SLE.
Keywords: systemic lupus erythematosus, Janus kinase, JAK/STAT pathway, JAK inhibitors
Introduction
Systemic lupus erythematosus (SLE) is a chronic systemic autoimmune disease that affects many organs and tissues, particularly the kidneys and skin. Its pathogenesis is complex and includes the overproduction of a series of cytokines. Under these conditions, loss of self-tolerance and overproduction of autoantibodies occur.1 Many of these cytokines play biological roles through the JAK/STAT cascade signaling pathway. Activation of the JAK/STAT pathway enables B cells stimulated by autoreactive T helper cells to undergo a class shift and differentiate into autoantibody-producing cells in response to interleukin (IL)-21 and other cytokines. In addition, B cells produce various cytokines, such as IL-62,3 Analysis of JAK/STAT pathway inhibition suggested that it plays a central role in reducing inflammation in SLE.1 Current SLE treatment regimens are usually based on corticosteroids and immunosuppressants. Corticosteroids therapy is widely used to significantly improve the prognosis of SLE, with reported survival rates of 90% or higher after 5 years, 70–90% after 10 years, and 50–70% after 20 years.1 However, the possible occurrence of many adverse reactions and poor efficacy reflects high morbidity and mortality of SLE.4,5 Biomolecular targeting of pro-inflammatory cytokines has improved the treatment of other autoimmune diseases;6 therefore, the JAK/STAT pathway is an ideal target for SLE treatment.7 Many JAKis have been studied for the treatment of SLE.1 JAKis block downstream signaling of type I/II interferon (IFN). Therefore, JAKis may have a variety of effects on cytokines and cells in the pathogenesis of SLE, including innate immune cells, B cells, CD8+T cells, and CD4+T cell subsets (helper T cells (Th), including Th1 cells and pathogenic Th17 cells).8–10 In recent years, studies have analyzed whether inhibition of this intracellular signaling can play a safe and effective role in patients with SLE,6 and some positive results have been achieved. Therefore, JAKis targeting pro-inflammatory cytokines may be a promising therapeutic strategy for SLE.
JAK/STAT Pathway
Four JAKs have been identified in mammals: JAK1–3 and non-receptor tyrosine-protein kinase 2 (TYK2). JAK1–2 and TYK2 are commonly expressed, whereas JAK3 is only expressed in hematopoietic cells.1 The structure of JAK consists of seven homologous regions (JH1–JH7), forming four domains (FERM, SRC homology 2 (SH2), pseudokinase, and kinase domains). The JH1 and JH2 regions are located at the C-terminus of the enzyme-encoded kinase and pseudokinase, respectively. JH2 homologs are characterized by dual kinase activity and regulate catalytic kinase activity. Seven STAT proteins have been identified: STAT1–4, STAT5A, STAT5B, and STAT6.11 STAT proteins consist of an N-terminal domain, DNA-binding domain, curling tail domain, splice domain, SH2 domain, tyramyl phosphate tail, and trans-activation domain located at the C-terminal.12,13 Each STAT field plays a unique role. The N-terminus is a conserved domain responsible for STAT phosphorylation. The DNA-binding domain forms a complex with DNA and STAT proteins, while the SH2 domain interacts with other proteins. Finally, the C-terminal domain functions as the activation center for the entire STAT molecule.14 Protein kinases are important modulators of cell function and achieve intracellular signal transduction through the linkage between JAK, TYK2 isomers, and STAT members. JAK receives signals from various cytokine receptors that are members of the IL and IFN families.15,16 The role of JAK in signaling is focused on type I and type II cytokine receptors, and the activity of each JAK depends on its specific interaction with the cytokine receptors.3 The specific cytokine receptor/JAK kinase combination is important for the generation of targeted therapies.
Pathogenesis of the JAK/STAT Pathway in SLE
In SLE, immune imbalance may activate cytokines of the innate and adaptive immune system and increase the level of pro-inflammatory factors, such as type I IFN, IL-2, IL-4, IL-6, IL-13, IL-15, IL-17, IL-23, and IL-31.17–19 These cytokines can activate the JAK/STAT pathway in immune cells (such as dendritic cells) (Figure 1), further increasing the level of pro-inflammatory cytokines and activating T and B cells. This induces T cells to release pro-inflammatory cytokines and B cells to generate autoantibodies (Figure 2),3 indicating the importance of the JAK/STAT pathway in the pathogenesis of SLE. In recent studies, IL-4, IL-6, and IL-21 have been considered potential targets for JAK inhibition in SLE.3,20,21 Disruption of the regulation between type I IFN and B cells is one of the main features of SLE that can be targeted by JAKis.22 The pathogenesis of SLE caused by the JAK/STAT pathway is shown in Figures 1 and 2.
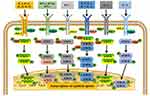 |
Figure 1 Mechanism of the JAK/STAT signaling pathway activation in immune cells in SLE. Extracellular cytokines bind to their specific receptors on immune cells (such as dendritic cells) to induce dimerization of JAKs, which are then activated and phosphorylated by tyrosine residues in the tail of their receptors to form p-JAK. Subsequently, these phosphorylation sites act as docking sites for STAT binding via the SH2 domain, resulting in tyrosine phosphorylation and STAT activation to form p-STAT. The phosphorylated STAT homo- or heterodimer is then translocated into the nucleus. There they act as transcription factors, regulating the expression of inflammatory cytokine genes. Adapted from Montero P, Milara J, Roger I, et al. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int J Mol Sci. 2021;22(12):6211. Creative Commons.23 |
Different JAKi Action Targets
JAKi is one of the newest classes of targeted synthetic disease-modifying antirheumatic drugs (TsDMARDs). JAKis are oral small-molecule drugs that can enter the cytoplasm and directly regulate intracellular signal transduction.3 JAKis suppress many pro-inflammatory cytokines by inhibiting the JAK/STAT pathway.3 In particular, TsDMARDs act by blocking ATP-binding sites in the JAK kinase-catalyzed region.24,25 This inhibition of downstream signaling pathways is related to immune regulation and can also block cytokine receptor phosphorylation and gene transcription, ultimately leading to impaired differentiation of Th1, Th2, Th17, and other cells,3,22,26 thus achieving therapeutic effects. JAKi has been extensively studied in the JAK/STAT pathway (Table 1).3 The main target of each JAKi differs; however, there are similar adverse reactions.
 |
Table 1 Overview of JAKis of Effect Targets and Adverse Effects |
The Effect of JAKi in in vitro Experiments
In vitro, T cells from patients with SLE carrying the STAT4 risk allele showed enhanced IFN-α and IL-12 phosphorylation of STAT4, leading to significant IL-12 induction of IFN-γ production in T cells. The addition of TYK2 inhibitors prevented IL-12 and IFN-α activation in T cells, and the addition of tofacitinib to block JAK2 inhibited IFN-γ-induced cell activation.21 In addition, Anti-double-stranded DNA(anti-ds-DNA) and anti-extractable nuclear antigens(ENA) specific antibody-secreting cells (ASCs) in blood samples from patients with SLE expressed plasma cell niche receptor cytokines, such as IL-6, which promote autoantibody production in a STAT3-dependent manner. These effects were suppressed after the addition of ruxolitinib.27,28 Previous in vitro studies have also shown that baricitinib mitigated B cell differentiation and restored podocyte skeletal structure damaged by inflammatory stimulation by blocking the JAK/STAT pathway.29
The Role of JAKi in in vivo Studies
Preclinical Studies
Most studies have focused on kidney and skin lesions associated with SLE, and good therapeutic results have been achieved. With respect to lupus nephritis (LN), treatment with tofacitinib (15 mg/kg/d) improved LN in Murphy Roths Large/lymphoproliferation (MRL/lpr) mice, as evidenced by decreased albuminuria and reduced renal histopathological scores, as well as improved clinical markers (reduced plasma anti-ds-DNA antibody levels).30 The mechanism of tofacitinib may be to relieve SLE by upregulating the expression of the TGFβI receptor and inhibiting the activation of CD4+T cells.30 Tofacitinib treatment also reduced glomerular, tubular, and interstitial lesions and reduced IgG and C3 renal deposition in mice. At the same time, the number of T cells and macrophages and the expression of STAT regulatory genes and several inflammatory mediators were reduced. The levels of inflammatory cytokines TNF-α, IFN-α, and IL-17 were significantly reduced.31 In another study, tofacitinib-treated MRL/lpr mice showed low levels of albuminuria, reduced frequency of severe glomerulonephritis, low glomerular scores, less pronounced interstitial nephritis, and low intensity of renal IgG and C1q deposits.32 A study using baricitinib showed the expression of structural proteins in renal podocytes was restored, and renal inflammation improved in mice while inhibiting B cell differentiation and subsequent immunoglobulin production stimulated by pro-inflammatory conditions.29 MRL/lpr mice usually develop cutaneous lupus erythematosus (CLE), and tofacitinib-treated mice had improved skin hyperplasia and rashes on the face and back, while pathology suggests a significant reduction in skin hyperplasia and inflammatory infiltration.28 In addition, TREX1−/− mice spontaneously developed CLE-like red scales and skin lesions at a certain age, and JAK1i treatment improved skin lesions and significantly reduced lupus skin activity scores.33 In addition, ruxolitinib reduced the development of lupus-associated skin lesions, inflammatory infiltration (as well as infiltrating T cells), and epidermal hyperplasia and downregulated the expression of IFN response genes.34
Clinical Research
In human SLE, tofacitinib, baricitinib, and ruxolitinib are the main drugs used for treatment and have been widely studied; therefore, these three drugs will be the focus of our discussion. For tofacitinib, 5 mg twice daily treatment in patients with SLE significantly reduced STAT phosphorylation in T cells, IFN levels in circulating immune cells, and percentage of low-density granulocytes and neutrophil extracellular trap complexes, while HDL cholesterol increased. Overall, this improved cardiac and immunological features associated with premature atherosclerosis in SLE without adverse side effects35 In patients with “refractory” CLE, the index score for the CLE disease area and severity showed significant improvement with tofacitinib treatment.36 In addition, tofacitinib improved arthritis and rashes in patients with SLE, but there was no significant improvement in serological markers, and varicella zoster was present in some patients.36
With respect to baricitinib, skin lesions improved significantly in patients with familial lupus frostbite and TREX1 mutations after treatment with 4 mg/day; however, pain associated with arthritis and skin damage was not completely alleviated, and mild respiratory infections occurred repeatedly.37 Another study showed the refractory papuloscaly rash in patients with SLE completely resolved after baricitinib treatment.37 In a recent study, baricitinib treatment with 4 mg/day was used to induce complete renal response (significant reduction in urinary protein levels) and control joint performance in patients with rhupus with type V glomerulonephritis.38
With respect to ruxolitinib, rapid clinical improvement and almost complete regression of skin lesions have been described in patients with lupus erythematosus caused by TREX1 defects associated with Aicardi–Goutieres syndrome.36 In a patient recently diagnosed with SLE, 1.5% ruxolitinib cream was used to treat a right scalp plaque. After two months, the plaque improved, and hair regrowth occurred,39 suggesting that topical ruxolitinib is an effective treatment for CLE and associated hair loss. In addition, ruxolitinib effectively blocks the production of anti-ENA and anti-dsDNA antibodies in patients with SLE.36
Clinical study results for treating SLE with tofacitinib, baricitinib, and ruxolitinib are shown in Table 2. The main studies on ongoing clinical trials of JAKi in patients with SLE are shown in Table 3.
 |
Table 2 JAKis of Clinical Trials in SLE Patients |
 |
Table 3 Ongoing Studies on the Use of JAKis in SLE Patients are Presented |
Conclusion
The JAK/STAT pathway is involved in the pathogenesis of SLE and associated with elevated levels of pro-inflammatory cytokines. JAKis play an immunomodulatory role in SLE by inhibiting various cytokine signaling pathways mediated by JAK/STAT. Thus, mechanism-based therapies targeting multiple cytokines and their signaling have brought about a paradigm shift in SLE treatment strategies. Current treatments are based on corticosteroids and immunosuppressants; however, some patients experience poor responses and require further treatments. Intensive and appropriate induction therapy is a prerequisite for achieving and maintaining SLE remission without causing organ damage. JAKis target the JAK/STAT pathway, reduce pro-inflammatory cytokine levels, inhibit inflammatory immune cells, and may ultimately lead to SLE disease remission without the use of corticosteroids. Clinical trials of JAKis in later stages of treatment have indicated that they may be effective for sustained remission, drug-free remission, or even cure in patients with SLE. Therefore, JAKis are a promising class of drugs for the treatment of SLE; however, more basic and clinical studies are needed to further confirm their efficacy and safety.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Tanaka Y. State-of-The-art treatment of systemic lupus erythematosus. Int J Rheum Dis. 2020;23(4):465–471. doi:10.1111/1756-185X.13817
2. Gupta S, Kaplan MJ. Bite of the wolf: innate immune responses propagate autoimmunity in lupus. J Clin Invest. 2021;131(3):e144918. doi:10.1172/JCI144918
3. Tanaka Y, Luo Y, O’Shea JJ, et al. Janus kinase-targeting therapies in rheumatology: a mechanisms-based approach. Nat Rev Rheumatol. 2022;18(3):133–145. doi:10.1038/s41584-021-00726-8
4. Wallace DJ, Furie RA, Tanaka Y, et al. Baricitinib for systemic lupus erythematosus: a double-blind, randomised, placebo-controlled, Phase 2 trial. Lancet. 2018;392(10143):222–231. doi:10.1016/S0140-6736(18)31363-1
5. Yurkovich M, Vostretsova K, Chen W, et al. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res. 2014;66(4):608–616. doi:10.1002/acr.22173
6. O’Shea JJ, Kontzias A, Yamaoka K, et al. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis. 2013;72 Suppl 2:ii111–5. doi:10.1136/annrheumdis-2012-202576
7. Wenzel J, van Holt N, Maier J, et al. JAK1/2 Inhibitor Ruxolitinib Controls a Case of Chilblain Lupus Erythematosus. J Invest Dermatol. 2016;136(6):1281–1283. doi:10.1016/j.jid.2016.02.015
8. Ghoreschi K, Jesson MI, Li X, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690550). J Immunol. 2011;186(7):4234–4243. doi:10.4049/jimmunol.1003668
9. Okiyama N, Furumoto Y, Villarroel VA, et al. Reversal of CD8 T-cell-mediated mucocutaneous graft-versus-host-like disease by the JAK inhibitor tofacitinib. J Invest Dermatol. 2014;134(4):992–1000. doi:10.1038/jid.2013.476
10. Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20(9):1043–1049. doi:10.1038/nm.3645
11. Chang Z, Wang Y, Zhou X, et al. STAT3 roles in viral infection: antiviral or proviral? Future Virol. 2018;13(8):557–574. doi:10.2217/fvl-2018-0033
12. Darnell JE. STATs and gene regulation. Science. 1997;277(5332):1630–1635. doi:10.1126/science.277.5332.1630
13. Kotyla PJ, Engelmann M, Giemza-Stokłosa J, et al. Thromboembolic Adverse Drug Reactions in Janus Kinase (JAK) Inhibitors: does the Inhibitor Specificity Play a Role? Int J Mol Sci. 2021;22(5):2449. doi:10.3390/ijms22052449
14. Gao Q, Liang X, Shaikh AS, et al. JAK/STAT Signal Transduction: promising Attractive Targets for Immune, Inflammatory and Hematopoietic Diseases. Curr Drug Targets. 2018;19(5):487–500. doi:10.2174/1389450117666161207163054
15. Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16(12):843–862. doi:10.1038/nrd.2017.201
16. Zarrin AA, Bao K, Lupardus P, et al. Kinase inhibition in autoimmunity and inflammation. Nat Rev Drug Discov. 2021;20(1):39–63. doi:10.1038/s41573-020-0082-8
17. Waickman AT, Park JY, Park JH. The common γ-chain cytokine receptor: tricks-and-treats for T cells. Cell Mol Life Sci. 2016;73(2):253–269. doi:10.1007/s00018-015-2062-4
18. Choy EH. Clinical significance of Janus Kinase inhibitor selectivity. Rheumatology. 2019;58(6):953–962. doi:10.1093/rheumatology/key339
19. Gadina M, Chisolm DA, Philips RL, et al. Translating JAKs to Jakinibs. J Immunol. 2020;204(8):2011–2020. doi:10.4049/jimmunol.1901477
20. Kubo S, Nakayamada S, Tanaka Y. Baricitinib for the treatment of rheumatoid arthritis and systemic lupus erythematosus: a 2019 update. Expert Rev Clin Immunol. 2019;15(7):693–700. doi:10.1080/1744666X.2019.1608821
21. Hagberg N, Joelsson M, Leonard D, et al. The STAT4 SLE risk allele rs7574865[T] is associated with increased IL-12-induced IFN-γ production in T cells from patients with SLE. Ann Rheum Dis. 2018;77(7):1070–1077. doi:10.1136/annrheumdis-2017-212794
22. You H, Xu D, Zhao J, et al. JAK Inhibitors: prospects in Connective Tissue Diseases. Clin Rev Allergy Immunol. 2020;59(3):334–351. doi:10.1007/s12016-020-08786-6
23. Montero P, Milara J, Roger I, et al. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int J Mol Sci. 2021;22(12):6211
24. Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem. 2014;57(12):5023–5038. doi:10.1021/jm401490p
25. Al Khalili A, Dutz JP. Janus Kinase Inhibition and SLE: is this a Plausible Treatment Option for SLE? Curr Treat Options Rheumatol. 2020;6:406–417. doi:10.1007/s40674-020-00155-w
26. Gadina M, Johnson C, Schwartz D, et al. Translational and clinical advances in JAK-STAT biology: the present and future of jakinibs. J Leukoc Biol. 2018;104(3):499–514. doi:10.1002/JLB.5RI0218-084R
27. de la Varga Martínez R, Rodríguez-Bayona B, Añez GA, et al. Clinical relevance of circulating anti-ENA and anti-dsDNA secreting cells from SLE patients and their dependence on STAT-3 activation. Eur J Immunol. 2017;47(7):1211–1219. doi:10.1002/eji.201646872
28. Alunno A, Padjen I, Fanouriakis A, et al. Pathogenic and Therapeutic Relevance of JAK/STAT Signaling in Systemic Lupus Erythematosus: integration of Distinct Inflammatory Pathways and the Prospect of Their Inhibition with an Oral Agent. Cells. 2019;8(8):898. doi:10.3390/cells8080898
29. Lee J, Park Y, Jang SG, et al. Baricitinib Attenuates Autoimmune Phenotype and Podocyte Injury in a Murine Model of Systemic Lupus Erythematosus. Front Immunol. 2021;12:704526. doi:10.3389/fimmu.2021.704526
30. Yan Q, Chen W, Song H, et al. Tofacitinib Ameliorates Lupus Through Suppression of T Cell Activation Mediated by TGF-Beta Type I Receptor. Front Immunol. 2021;12:675542. doi:10.3389/fimmu.2021.675542
31. È R, de Ramon L, Draibe Bordignon J, et al. JAK3-STAT pathway blocking benefits in experimental lupus nephritis. Arthritis Res Ther. 2016;18(1):134. doi:10.1186/s13075-016-1034-x
32. Ikeda K, Hayakawa K, Fujishiro M, et al. JAK inhibitor has the amelioration effect in lupus-prone mice: the involvement of IFN signature gene downregulation. BMC Immunol. 2017;18(1):41. doi:10.1186/s12865-017-0225-9
33. Fetter T, Smith P, Guel T, et al. Selective Janus Kinase 1 Inhibition Is a Promising Therapeutic Approach for Lupus Erythematosus Skin Lesions. Front Immunol. 2020;11:344. doi:10.3389/fimmu.2020.00344
34. Chan ES, Herlitz LC, Jabbari A. Ruxolitinib Attenuates Cutaneous Lupus Development in a Mouse Lupus Model. J Invest Dermatol. 2015;135(7):1912–1915. doi:10.1038/jid.2015.107
35. Hasni SA, Gupta S, Davis M, et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat Commun. 2021;12(1):3391. doi:10.1038/s41467-021-23361-z
36. Richter P, Cardoneanu A, Burlui AM, et al. Why Do We Need JAK Inhibitors in Systemic Lupus Erythematosus? Int J Mol Sci. 2022;23(19):11788. doi:10.3390/ijms231911788
37. Shi H, Gudjonsson JE, Kahlenberg JM. Treatment of cutaneous lupus erythematosus: current approaches and future strategies. Curr Opin Rheumatol. 2020;32(3):208–214. doi:10.1097/BOR.0000000000000704
38. Garufi C, Mancuso S, Spinelli FR, et al. Janus kinases inhibitors for treating patients with rhupus. Joint Bone Spine. 2020;87(6):673–674. doi:10.1016/j.jbspin.2020.05.010
39. Park JJ, Little AJ, Vesely MD. Treatment of cutaneous lupus with topical ruxolitinib cream. JAAD Case Rep. 2022;28:133–135. doi:10.1016/j.jdcr.2022.08.038
40. You H, Zhang G, Wang Q, et al. Successful treatment of arthritis and rash with tofacitinib in systemic lupus erythematosus: the experience from a single centre. Ann Rheum Dis. 2019;78(10):1441–1443. doi:10.1136/annrheumdis-2019-215455
41. Bonnardeaux E, Dutz JP. Oral tofacitinib citrate for recalcitrant cutaneous lupus. JAAD Case Rep. 2021;20:61–64. doi:10.1016/j.jdcr.2021.09.030
42. Zimmermann N, Wolf C, Schwenke R, et al. Assessment of Clinical Response to Janus Kinase Inhibition in Patients With Familial Chilblain Lupus and TREX1 Mutation. JAMA Dermatol. 2019;155(3):342–346. doi:10.1001/jamadermatol.2018.5077
43. Evaluation of Tofacitinib in Prevention of Photosensitivity in Lupus. Available from: https://clinicaltrials.gov/ct2/show/NCT05048238.
44. Use of Baricitenib to Maintain of Remission. Available from: https://clinicaltrials.gov/ct2/show/NCT05686746.
45. Study of Ruxolitinib Cream for the Treatment of Discoid Lupus Erythematosus; Available from: https://clinicaltrials.gov/ct2/show/NCT04908280.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.