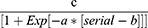Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Potential Roles of Teamwork and Unmet Needs on Surgical Learning Curves of Spinal Robotic Screw Placement
Authors Su YF , Tsai TH, Kuo KL, Wu CH, Tsai CY, Lu YM , Hwang SL, Lin PC, Lieu AS, Lin CL, Chang CH
Received 1 July 2022
Accepted for publication 26 August 2022
Published 7 September 2022 Volume 2022:15 Pages 1971—1978
DOI https://doi.org/10.2147/JMDH.S380707
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yu-Feng Su,1– 3 Tai-Hsin Tsai,2– 4 Keng-Liang Kuo,2,4 Chieh-Hsin Wu,2,4 Cheng-Yu Tsai,1,2 Yen-Mou Lu,5 Shiuh-Lin Hwang,6 Pei-Chen Lin,7 Ann-Shung Lieu,2 Chih-Lung Lin,1,2,4 Chih-Hui Chang1,2,4
1Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; 2Department of Neurosurgery, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan; 3Division of Neurosurgery, Department of Surgery, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan; 4Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan; 5Department of Orthopedics, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan; 6Department of Spinal Surgery, Chi-Hsien Spine Hospital, Kaohsiung, Taiwan; 7Department of Oral Hygiene, College of Dental Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
Correspondence: Chih-Hui Chang, Email [email protected]
Background: The aim of this study was to investigate the learning curve of robotic spine surgery quantitatively with the well-described power law of practice.
Methods: Kaohsiung Medical University Hospital set up a robotic spine surgery team by the neurosurgery department in 2013 and the orthopedic department joined the well-established team in 2014. A total of consecutive 150 cases received robotic assisted spinal surgery. The 150 cases, with 841 transpedicular screws were enrolled into 3 groups: the first 50 cases performed by neurosurgeons, the first 50 cases by orthopedic surgeons, and 50 cases by neurosurgeons after the orthopedic surgeons joined the team. The time per screw and accuracy by each group and individual surgeon were analyzed.
Results: The time per screw for each group was 9.56 ± 4.19, 7.29 ± 3.64, and 8.74 ± 5.77 minutes, respectively, with p-value 0.0017. The accuracy was 99.6% (253/254), 99.5% (361/363), and 99.1% (222/224), respectively, with p-value 0.77. Though the first group took time significantly more on per screw placement but without significance on the nonlinear parallelism F-test. Analysis of 5 surgeons and their first 10 cases of short segment surgery showed the time per screw by each surgeon was 12.28 ± 5.21, 6.38 ± 1.54, 8.68 ± 3.10, 6.33 ± 1.90, and 6.73 ± 1.81 minutes. The first surgeon who initiated the robotic spine surgery took significantly more time per screw, and the nonlinear parallelism test also revealed only the first surgeon had a steeper learning curve.
Conclusion: This is the first study to demonstrate that differences of learning curves between individual surgeons and teams. The roles of teamwork and the unmet needs due to lack of active perception are discussed.
Keywords: human computer communication, human–robot collaboration, learning curve, robotic spine surgery, teamwork
Introduction
Robotic-assisted technologies have been introduced into the practice of spinal surgery, mainly focusing on transpedicular screw placement over recent years. The accuracy of robot-assisted pedicle screw placement has been a focus of research.1 However, few studies address the learning curve of robotic spine surgery.2–11
The teamwork, including human–robot team interaction, workplace culture, and experiences of surgeons can affect the processes and outcomes of robotic endoscopic surgery.12 Interestingly, the relationship between teamwork and the learning curve has rarely been mentioned in the field of robotic spine surgery. Furthermore, active perception, one of the key components in robotics, has never been mentioned in any study or research protocol of spinal robotic surgery.
Kaohsiung Medical University Hospital (KMUH) set up the robotic spine surgery team in May 2013. Up until July 2017, the members of the team had performed 688 cases and implanted 3896 transpedicular screws. The orthopedics department joined the robotic spine surgery team in August 2014. At that time, 255 cases had been carried out and 1237 screws had been placed successfully by the well-established team, mainly cooperating with the department of neurosurgery (Figure 1). The perioperative teamwork of this team has been emphasized especially by active communication and the “triple-check process”.
This study aimed to investigate the learning curve of the robotic spine surgery via analyzing the accuracy and the surgical time of transpedicular screw placement. Case series with specific time sequences were collected for analysis to clarify the possible correlation among team, teamwork, individual surgeons, and learning curves.
Materials and Methods
Ethics Statement
This clinical study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (No: KMUHIRB-E(I)-20150167). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all participants. Prior to analysis, patient data were de-identified and anonymously analyzed. This study complied with the Declaration of Helsinki.
Patient Selection
Between May 2013 and June 2017, there were 688 consecutive patients who received thoracolumbar surgery using the Renaissance robotic system (RenaissanceTM; Mazor Robotics Ltd., Caesarea, Israel) at KMUH. A total of 3896 screws were implanted successfully. We retrospectively analyzed all the patients. Cases of spinal malignancy or spinal infection were excluded from the study. Three groups of patients were enrolled and analyzed:
(1) Neurosurgeon (NS) early 50: The first 50 cases performed by the department of neurosurgery and the robotic spine surgery team between May 2013 and July 2013.
(2) Orthopedic early 50: The first 50 cases performed by the department of orthopedics and the robotic spine surgery team between August 2014 and September 2015.
(3) NS later 50: 50 consecutive cases performed by the department of neurosurgery and the robotic spine surgery team between August 2014 and November 2014 (just after the department of orthopedics joined the team).
The characteristics of patients in the 3 groups were reviewed and analyzed (Table 1). The time per screw, assessed as the learning curve of the team, was calculated and plotted, respectively (Figure 2).
 |
Table 1 Characteristics of Patients in Three Groups |
Separately, the individual learning curves of surgeons were investigated with the short-segment surgery (2 segments, 4 pedicle screws). All the surgeons in this study are experienced spine surgeons with high surgical volume (up to 300 cases/year). The first 10 cases with a 100% accuracy for 5 individual surgeons (50 cases total, 40 screws total per surgeon) were enrolled for analysis. The time per screw was measured and plotted (Table 2 and Figure 3).
 |
Table 2 Time per Screw and Learning Curve Between 5 Doctors |
Robotic Spine Surgery Techniques
The Renaissance robot-guided system has been described in previous studies.13,14 Five main steps were carried out to place transpedicular screws with the Renaissance robot-guided system:
- Preoperative planning: spiral computed tomography (CT) scanning (1-mm intervals) of the spine was performed preoperatively to reconstruct the 3D plane of the spine and to select the optimal screw placement strategy.
- Mounting: the mounting system was attached to an appropriate bony structure of the spine to maintain stability during registration. The robotic arm of the guided system was also fixed to the bony structure to provide intraoperative guidance.
- Registration: the Renaissance robot-guided system automatically registered the intraoperative images with the preoperative CT images by comparing anteroposterior and oblique views of radiographic images.
- Drilling: the robot was attached to a mounting frame and moved to the position selected during preoperative planning. After directing the guiding tube to the pedicle, the surgeon performed the drilling. The K-wire was then placed with robotic guidance. During the process of drilling, there is no passive nor active perception process/mechanism provided by the robotic system.
- Intraoperative accuracy evaluation: after the K-wire implantation was completed, anteroposterior and lateral fluoroscopic images were obtained. The accuracy was evaluated and repositioning of the K-wire was achieved manually in cases where the accuracy was not acceptable Secondary registration, developed by the team at KMUH, with anteroposterior and oblique fluoroscopic images and the Renaissance robot-guided system, was carried out in cases after January 2015.13,14 Reposition of the K-wire was done again with the robotic system. Manual reposition was only applied when the reposition was still unacceptable.
Team Building and Teamwork
Team Building
The robotic spine surgery team at KMUH is made up of qualified surgeons (7 neurosurgeons and 3 orthopedic surgeons), coordinators, radiological technicians, specialist nurses, surgical technicians, and surgical nursing staff. Surgeons and surgical technicians are all qualified in the Renaissance robotics system training programs, including cadaver workshop. The coordinators and specialist nurses manage the preoperative preparation and perioperative care. The parameters and image quality of CT scans are examined carefully by radiological technicians and surgeons.
Human–Robot Interaction
The members meet the day prior to surgery to discuss the surgical plan. The process and possible factors influencing the placement of K-wires14 are previewed and examined. Five main steps mentioned above are carried out to place transpedicular screws with the Renaissance robot-guided system. The “triple-check process” is applied during the surgery, ie pointing, reading, and confirming all parameters of the settings and instruments with team members involved in every step. Pointing means the members should point with fingers the parameters shown on the screen of robot. Reading means the members should read together the parameters they point. Confirming means the member agree the parameters they point and read. The process and results are recorded immediately following every procedure.
Workplace Culture
The Joint Commission International’s (JCI) accreditation standards for hospitals (Joint Commission International, Oak Brook, Illinois, USA) are followed by the team perioperatively, as KMUH has partnered with the JCI. Patient-safety Reporting system has been setup, and staffs are encouraged to communicate actively and report possible adverse events with name or anonymously.
Statistical Analysis
The Chi-squared test and Wilcoxon sign rank test were applied for categorical and continuous variables. Mean values are presented as mean ± standard deviation (SD). The learning curves (team and individual) were fit and plotted on a nonlinear platform. The following equation was used:
Where a = growth rate, b = inflection point, and c = asymptote.
The parallelism of learning curves was measured and tested with the parallelism F-test. All statistical analyses were performed using the JMP 12 software (SAS Institute Inc., Buckinghamshire, UK). The level of significance was set at 0.05.
Results
The Learning Curves of the Team and Departments
Table 1 shows the characteristics of the 3 groups. There were no significant differences among the basic characteristics of patients. The orthopedic early group performed more long segment cases (≧3 levels and ≧ 6 screws). The total 150 cases and 841 screws in the 3 groups had a time per screw of 9.56 ± 4.19, 7.29 ± 3.64, and 8.74 ± 5.77 minutes for the NS early, orthopedic early, and NS later groups, respectively. The NS early group took significantly more time per screw (p = 0.0017), but this was not significant after the nonlinear parallelism test (growth rate estimate was −0.01 ± 0.01, −0.05 ± 0.17, and −0.01 ± 1.25, p = 0.85, Figure 1). The accuracy for each group was 99.6% (253/254) for NS early, 99.5% (361/363) for orthopedic early, and 99.1% (222/224) for NS later. There was no significant difference in the accuracy between the 3 groups.
The Individual Learning Curves of Surgeons
Five surgeons (4 neurosurgeons and 1 orthopedic surgeon) and their first 10 cases of short segment surgery (2 levels and 4 screws) were enrolled for the analysis of individual learning curves (Table 2). The time per screw of each surgeon was 12.28 ± 5.21, 6.38 ± 1.54, 8.68 ± 3.10, 6.33 ± 1.90, and 6.73 ± 1.81 minutes. The first surgeon who initiated the robotic spine surgery in the team took significantly more time per screw (p = 0.001), and the nonlinear parallelism test (Figure 2) also showed that only the first surgeon had a steeper learning curve (growth rate estimate was −0.13 ± 0.17, 0.08 ± 0.92, −1.41 ± 0.0, −0.57 ± 1.6, 0.75 ± 0.0, p < 0.0001).
Discussion
The Learning Curves Between Team and Individual Surgeons
This study demonstrates the learning curve of both the team as a whole as well as individual surgeons. With an established-team and standardized teamwork, the learning curve of a newly joined but qualified member of the surgical team may be parallel with the other experienced members in the team. To the best of our knowledge, this could be the first study to show the learning curves of robotic spine surgery with quantificational and nonlinear analysis.
The power law of practice is well described quantitatively for human learning curve study in psychology.15 This law states that the logarithm of the reaction time for a particular task decreases linearly with the logarithm of the number of practices. Therefore, the time per screw is viewed as an important element of the learning curve during robotic spine surgery.4,8 Our results, as the first study, demonstrated a similar power law of practice only on the learning curve of the first surgeon, who initiated the robotic spine surgery for the team. In the series by Urakov et al,8 the initial academic experiences of residents/fellows and their learning curve with robotic spine instrumentation were explored. No significance was noted regarding the speed of pedicle instrumentation under senior surgeons’ guidance. According to the theory, results and observations, a well-established team, teamwork, and supervision are important factors for the learning curve of robotic spine surgery.
However, the characteristics of the smooth power law may potentially mask more complex dynamics underpinning individual learning curves. Therefore, using a single power law to predict or analyze individual performance may obscure more complex learning dynamics.16
The Accuracy of Robotic Spine Surgery
The accuracy of spinal instrumentation is another important issue in robotic spine surgery. Previous studies have shown that the rate of successful robotic–assisted pedicle screw placement became consistent after 20 or 30 cases.6,10 In our study, the rate of accuracy did not change significantly between the 3 groups of 50 cases during 3 specific time intervals. In a meta-analysis of robotic spine surgery by Joseph et al,17 including 22 retrospective case series and prospective randomized trials, the consistency and high accuracy rates of robotic spine surgery were also recognized. Ringel et al3 also stated that accuracy did not improve through the course in their study. Obesity, osteoporosis, and congenital scoliosis have been recognized as risk factors for screw malposition and surgeons in the initial stage of using a robot are suggested to avoid performing surgery on patients with these risk factors.1
One of our previous studies developed a secondary registration protocol that increased the success rate and intraoperative accuracy by the same robotic system.13 In the studies, we published in 2016 and 201713,14 showed that the K-wire needed to be repositioned manually is 1.26% (4/317 K-wires, with secondary registration) and 0.15% (1/662 K-wires, with third registration). Factors influencing accuracy can be errors in preoperative planning, mounting, registration, drilling, or robot assembly.14 All of these factors could be eliminated or minimized by a well-established team and teamwork, according to the results of this study.
Potential Roles of Teamwork
Effective teamwork can be measured by examining the quality of output, the process and the members’ performance.18 Team dynamics are important for efficient teamwork. Team dynamics include open communication to avoid conflicts, effective coordination to avoid confusion, efficient cooperation to perform the tasks in a timely manner and produce the required results, and high levels of interdependence to maintain high levels of trust, risk-taking, and performance.19 The smooth and parallel learning curves of the team and individuals imply the potential roles of teamwork in robotic spine surgery.
Communication and workplace culture are thought to be important factors in the human–robot team interaction,12 but the evaluation and measurements are usually difficult in complex operation rooms. In the surgery with the Renaissance robot-guided system, a potential error could take place during registration due to inconsistencies between the numbers of station number set on the computer and on the mounting platform. The “triple-check process” applied in robotic spine surgery, ie pointing, reading, and confirming all parameters of settings and instruments with team members in every step, prevents our team from errors. There is little literature related with human–robot interaction in the field of robotic spine surgery. Though we did not propose new experimental study design about the human–robot interaction in this study, the high accuracy of transpedicular screw implantation in this study demonstrates not only the efficiency of surgical robot but also the efficiency of the good human–robot interaction.
KMUH has been an academic medical center in southern Taiwan since the 1970s. It has received accreditation every three to five years by the official accreditation organizations in Taiwan. Furthermore, the partnership with the international accreditation organization also encourages and regulates the team to follow rules and guidelines more strictly.
We believed this is the first article emphasizing the importance of team building, human–robot interaction and workplace culture in robotic spine surgery.
Robotic Spine Surgery – A System Lack of Active Perception and Unmet Needs
Modeling and control strategies for perception, defining active perception,20 are missing in the robotic system we used for this study. According to our previous study, skiving over a steep slope of bony surface is the main factor affecting accuracy.14 However, during the process of drilling, there is no passive nor active perception process or mechanism provided. The members of surgical team use their naked eyes and fingers to detect possible skiving of guiding tube before, during, and after the drilling procedure. Contrary to intraoperative image-guided spinal navigation, there is no guiding image or visual feedback provided by the robotic system during the drilling procedure and screw placement.21 These defects are also barriers to surgeons to use or trust this robotic system. Intelligent control strategies according to the data from detecting possible deviation of guiding tube are apparently the unmet needs for robotic spine surgery.
Conclusion
With teamwork, the learning curve of robotic spine surgery for a newly employed surgeon can be smooth and parallel with other experienced surgeons. The accuracy is also high and consistent. Communication and workplace culture are important for teamwork; meanwhile, triple-check process is advocated during robotic spine surgery. We propose that active perception is the unmet need for current robotic spine surgery.
Data Sharing Statement
The data that support the findings of this study are available from the authors, YFS, CHC, THT, and CLL, upon reasonable request.
Consent for Publication
No images, personal or clinical details of participants are presented that compromise anonymity.
Acknowledgments
Thanks to all the staff of Robotic Spine Surgery Team and Department of Neurosurgery at KMUH. This work was supported by part of grants KMUH104-4M20 and KMUH105-5T05 from Kaohsiung Medical University Hospital, and KMTTH-107-028, KMTTH-108-011, KMTTH-109-037 from Kaohsiung Municipal Ta-tung Hospital.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Zhang JN, Fan Y, Hao DJ. Risk factors for robot-assisted spinal pedicle screw malposition. Sci Rep. 2019;9(1):3025. doi:10.1038/s41598-019-40057-z
2. Devito DP, Kaplan L, Dietl R, et al. Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine. 2010;35(24):2109–2115. doi:10.1097/BRS.0b013e3181d323ab
3. Ringel F, Stuer C, Reinke A, et al. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: a prospective randomized comparison to conventional freehand screw implantation. Spine. 2012;37(8):E496–501. doi:10.1097/BRS.0b013e31824b7767
4. Onen MR, Simsek M, Naderi S. Robotic spine surgery: a preliminary report. Turk Neurosurg. 2014;24(4):512–518. doi:10.5137/1019-5149.JTN.8951-13.1
5. Hu X, Ohnmeiss DD, Lieberman IH. Robotic-assisted pedicle screw placement: lessons learned from the first 102 patients. Eur Spine J. 2013;22(3):661–666. doi:10.1007/s00586-012-2499-1
6. Hu X, Lieberman IH. What is the learning curve for robotic-assisted pedicle screw placement in spine surgery? Clin Orthop Relat Res. 2014;472(6):1839–1844. doi:10.1007/s11999-013-3291-1
7. Macke JJ, Woo R, Varich L. Accuracy of robot-assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg. 2016;10(2):145–150. doi:10.1007/s11701-016-0587-7
8. Urakov TM, Chang KH, Burks SS, Wang MY. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus. 2017;42(5):E4. doi:10.3171/2017.2.FOCUS175
9. Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally invasive robotic versus open fluoroscopic-guided spinal instrumented fusions: a randomized controlled trial. Spine. 2017;42(6):353–358. doi:10.1097/BRS.0000000000001778
10. Schatlo B, Martinez R, Alaid A, et al. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir. 2015;157(10):
11. Kim HJ, Kang KT, Park SC, et al. Biomechanical advantages of robot-assisted pedicle screw fixation in posterior lumbar interbody fusion compared with freehand technique in a prospective randomized controlled trial-perspective for patient-specific finite element analysis. Spine J. 2017;17(5):671–680. doi:10.1016/j.spinee.2016.11.010
12. Cunningham S, Chellali A, Jaffre I, Classe J, Cao CGL. Effects of experience and workplace culture in human-robot team interaction in robotic surgery: a case study. Int J Soc Robotics. 2012;5(1):75–88. doi:10.1007/s12369-012-0170-y
13. Kuo KL, Su YF, Wu CH, et al. Assessing the intraoperative accuracy of pedicle screw placement by using a bone-mounted miniature robot system through secondary registration. PLoS One. 2016;11(4):e0153235. doi:10.1371/journal.pone.0153235
14. Tsai TH, Tzou RD, Su YF, Wu CH, Tsai CY, Lin CL. Pedicle screw placement accuracy of bone-mounted miniature robot system. Medicine. 2017;96(3):e5835. doi:10.1097/MD.0000000000005835
15. Anderson JR. Cognitive Skills and Their Acquisition. L. Erlbaum Associates; 1981:xiii386.
16. Donner Y, Hardy JL. Piecewise power laws in individual learning curves. Psychon Bull Rev. 2015;22(5):1308–1319. doi:10.3758/s13423-015-0811-x
17. Joseph JR, Smith BW, Liu X, Park P. Current applications of robotics in spine surgery: a systematic review of the literature. Neurosurg Focus. 2017;42(5):E2. doi:10.3171/2017.2.FOCUS16544
18. Hackman JR. Groups That Work (and Those That Don’t): Creating Conditions for Effective Teamwork. Jossey-Bass; 1990:544.
19. Ilgen DR, Hollenbeck JR, Johnson M, Jundt D. Teams in organizations: from input-process-output models to IMOI models. Annu Rev Psychol. 2005;56:517–543. doi:10.1146/annurev.psych.56.091103.070250
20. Bajcsy R, Aloimonos Y, Tsotsos JK. Revisiting active perception. Auton Robots. 2018;42(2):177–196. doi:10.1007/s10514-017-9615-3
21. Kim HJ, Jung WI, Chang BS, Lee CK, Kang KT, Yeom JS. A prospective, randomized, controlled trial of robot-assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot. 2017;13(3):e1779. doi:10.1002/rcs.1779
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.