Back to Journals » Journal of Inflammation Research » Volume 16
Potential Role of Intrapulmonary Concomitant Lesions in Differentiating Non-Neoplastic and Neoplastic Ground Glass Nodules
Authors Fu BJ , Zhang XC, Lv FJ, Chu ZG
Received 27 August 2023
Accepted for publication 6 December 2023
Published 13 December 2023 Volume 2023:16 Pages 6155—6166
DOI https://doi.org/10.2147/JIR.S437419
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Bin-Jie Fu,1,* Xiao-Chuan Zhang,1,2,* Fa-Jin Lv,1 Zhi-Gang Chu1
1Department of Radiology, the First Affiliated Hospital of Chongqing Medical University, Chongqing, People’s Republic of China; 2Department of Radiology, Chonggang General Hospital, Chongqing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhi-Gang Chu, Department of Radiology, the First Affiliated Hospital of Chongqing Medical University, No. 1, Youyi Road, Yuanjiagang, Yuzhong District, Chongqing, 400016, People’s Republic of China, Tel +86 18723032809, Fax +86 23 68811487, Email [email protected]
Purpose: To determine the value of intrapulmonary concomitant lesions in differentiating non-neoplastic and neoplastic ground-glass nodules (GGNs).
Patients and Methods: From January 2014 to March 2022, 395 and 583 patients with confirmed non-neoplastic and neoplastic GGNs were retrospectively enrolled. Their clinical and chest CT data were evaluated. The CT features of target GGNs and intrapulmonary concomitant lesions in these two groups were analyzed and compared, and the role of intrapulmonary concomitant lesions in improving differentiation was evaluated.
Results: The intrapulmonary concomitant lesions were more common in patients with non-neoplastic GGNs than in those with neoplastic ones (87.88% vs 82.18%, P = 0.015). Specifically, patients with non-neoplastic GGNs had a higher incidence of multiple solid nodules (SNs), patchy ground-glass opacity/consolidation, and fibrosis/calcification in any lung fields (each P < 0.05). Logistic regression analysis indicated that patients < 44 years old, diameter < 7.35 mm, irregular shape, and coarse margin or ill-defined boundary for target GGN, pleural thickening, and concomitant SNs in the same lobe and fibrosis or calcification in any lung field were independent indicators for predicting non-neoplastic GGNs. The AUC of the model for predicting non-neoplastic GGNs increased from 0.894 to 0.926 (sensitivity, 83.10%; specificity, 87.10%) after including the concomitant lesions in the patients’ clinical characteristics and CT features of target GGNs (P < 0.0001).
Conclusion: Besides the patients’ clinical characteristics and CT features of target GGNs, the concomitant multiple SNs in the same lobe and fibrosis/calcification in any lung field should be considered in further differentiating non-neoplastic and neoplastic GGNs.
Keywords: ground-glass nodule, intrapulmonary concomitant lesion, differential diagnosis, tomography, X-rays
Introduction
With the increased availability of low‑dose computed tomography (LDCT) screening programs in recent years, an increasing number of pulmonary nodules have been detected.1–6 Multiple lung cancer screening programs in Western and Asian countries show that the detection rate of ground glass nodules (GGNs) accounts for 2.7% - 4.2%.7–9 Pathologically, the nature of GGNs is diverse, including neoplastic lesions and non-neoplastic lesions, such as atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma (IAC), inflammation, edema, fibrosis and hemorrhage and etc.10,11 However, the clinical treatment strategies and prognosis of the neoplastic and non-neoplastic GGNs are completely different. Therefore, the accurate diagnosis of GGNs is very important.
The differential diagnosis of the neoplastic and non-neoplastic GGN has always been a research hotspot. Previous studies on morphological features have shown that GGNs with solid components, well-defined boundary, vascular convergence sign, larger diameter, lobulation, spiculation, air cavity density, or pleural traction are highly suggestive of neoplasm, while those with irregular shape or ill-defined boundary are considered to be non-neoplastic ones.12–14 Although radiomics can also identify the neoplastic and non-neoplastic GGNs,15–17 there are still some nodules that cannot be differentiated. It is necessary to further explore other methods and indicators for improving differential diagnosis of GGNs.
GGN is one of the diverse lung lesions, and the most common nature of non-neoplastic GGNs is inflammation. Inflammatory GGN may be part of inflammation in the lungs, early manifestations of focal inflammation, or manifestations of inflammation after absorption. Therefore, there may be a certain relationship between GGN and other types of concomitant lesions in the lung. Thus, whether pulmonary concomitant lesions are present or absent and their types may be different in patients with neoplastic and non-neoplastic GGN, and these differences may provide additional information for distinguishing target GGNs. However, there are currently no reports of intrapulmonary concomitant lesions in distinguishing GGNs. Therefore, we hypothesize that the type and distribution of concomitant lung lesions may provide additional information for differentiation. The aim of this study was to determine the value of intrapulmonary concomitant lesions in differentiating non-neoplastic and neoplastic GGNs.
Materials and Methods
This study was approved by the Institutional Review Board of the First Affiliated Hospital of Chongqing Medical University, and the requirement for informed consent was waived for this retrospective study.
Patients
A retrospective data collection of patients with GGNs undergoing CT examinations in our hospital from January 2014 to March 2022 was performed. The inclusion criteria were as follows: (1) nodules were manifested as GGNs on the lung window, (2) GGNs were confirmed by pathological examination after surgical resection, or those disappeared during follow-up. Patients were excluded if CT images were of poor quality resulting in the features of nodules could not be well evaluated, or no thin-slice images with a thickness of ≤ 1 mm. A total of 1365 patients with 1388 GGNs met the criteria, of which non-neoplastic GGNs were 396, and neoplastic GGNs were 992. Because of the relatively large sample size of neoplastic GGNs, 595 GGNs in 583 patients were selected by stratified random sampling from 992 neoplastic GGNs, and no further selection was made for non-neoplastic ones. Finally, 583 patients with 595 neoplastic GGNs and 395 patients with 396 non-neoplastic GGNs were enrolled in this study.
Among these included patients, the vast majority (423 in Neoplastic GGNs, 265 in Non-neoplastic GGNs) were found by chance or through physical examination and therefore did not have obvious clinical symptoms. The remaining patients had some clinical manifestations such as cough, expectoration, fever, etc. The clinical symptoms of the enrolled patients were shown in Table 1. These 595 cases of neoplastic GGNs were pathologically confirmed as AAH (19 cases, 3.19%), AIS (171 cases, 28.74%), MIA (208 cases, 34.96%), and IAC (197 cases, 33.11%). Of the 396 non-neoplastic GGNs, 65 (16.41%) cases were disappeared during follow-up, with a follow-up interval of 181.20±170.09 (range: 19–712) days, while 331 (83.59%) cases were confirmed by postoperative pathologic examination. Among 331 pathologically confirmed non-neoplastic GGNs, 285 (86.1%) were nonspecific inflammation with pathological manifestations as fibrous tissue hyperplasia and massive acute or chronic inflammatory cells infiltration with/without granuloma, 35 (10.57%) were inflammatory pseudotumor, 8 (2.42%) were tuberculosis, 2 (0.6%) were organizing pneumonia, and one (0.3%) was cryptococcal granuloma.
 |
Table 1 Patients’ Clinical Symptoms |
CT Examinations
The chest CT scans were performed using one of the following scanners: Discovery CT 750 HD (GE Healthcare, Milwaukee, WI, USA), SOMATOM Definition Flash (Siemens Healthineers, Erlangen, Germany), and SOMATOM Perspective (Siemens Healthineers, Erlangen, Germany). All patients were placed in a supine position with raised upper limbs and performed a breath-hold exercise before image acquisition. Acquisition was obtained at the end of deep inspiration during a single breath-hold. The scan range was from the entrance of the thorax to the costophrenic angle.
The scanning parameters were set as follows: tube voltage, 100–130 kVp; tube current, 50–200 mAs (reference mAs, using automatic tube current modulation technology); scanning slice thickness and slice spacing, 5 mm; reconstruction slice thickness and interval, 0.625 (GE scanner) or 1 mm (Siemens scanners); matrix: 512 × 512; rotation time, 0.5–0.6 seconds; pitch, 0.9–1; reconstruction algorithm, standard algorithm (GE scanner) or medium-sharp algorithm (Siemens scanners).
Clinical Data and Image Analysis
The patients’ clinical data, including the patient’s pneumonitis history, gender, and age were recorded using the Electronic Medical Record System (Winning Health, China). Clinical data, including the patient’s gender and age were recorded. All clinical data were collected by the radiologists through the Electronic Medical Record System.
All image analysis were based on non-contrast scan images. Two radiologists (F.B.J. with 5 years of posttraining experience and Z.X.C. with 11 years of posttraining experience) independently evaluated the GGNs on axial images and/or multi-planar reformation using the PACS (Carestream Vue; Carestream, Rochester, NY, USA) without knowing the pathological results. All images were reviewed in the fixed lung window setting (window level, −600 HU; window width, 1500 HU) and mediastinal-window settings (level, 40 HU; width, 400 HU). In case of disagreement, a consensus was reached after a joint discussion.
Target GGN was defined as a nodule suspected by clinician or radiologist to have potential for neoplastic and requiring further follow-up or treatment. All target GGNs and intrapulmonary concomitant lesions were observed based on the most recent CT examination before surgery or disappearance during follow-up. Target GGNs were evaluated in the following aspects by the radiologists using the axial section on which the nodule showed the greatest cross-sectional area: size (the average of the nodule’s maximal diameter and the perpendicular diameter on axial images), shape (round/oval, or irregular), boundary and margin (smooth, coarse, or ill-defined), spiculation (yes/no), lobulation (yes/no), abnormal intra-nodular vessel (yes/no), pleural indentation (yes/no), pleural thickening (yes/no), vacuole (yes/no), and air bronchogram (yes/no). Spiculation was defined as the presence of strands extending from the nodule’s margin into the lung parenchyma, without contacting the pleural surface.18 Lobulation was defined as a wavy or scalloped configuration of a portion of the nodule’s surface.19 Abnormal intra-nodular vessel was considered present when intra-nodular vascular segments were dilated (ie, diameter of vascular segment greater than that of segments proximal to the vessel’s entry into the nodule, or diameter of vascular segment significantly greater than that of other vessels at the same branch level), or distorted (ie, vessel deviated from its normal route).15 Pleural indentation was defined as a linear strand radiating from the nodule, extending distal to the pleural surface.20 Vacuole was defined as small spots of round or ovoid air attenuation within the nodule.21 Air bronchogram was defined as branching or tubular air-filled bronchi within the nodule.22
The features of intrapulmonary concomitant lesions in patients with GGNs were evaluated in the following aspects: (1) the types of intrapulmonary concomitant lesions (GGN, single SN, multiple SNs, patchy ground-glass opacity (GGO)/Consolidation, and fibrosis/calcification); (2) the distribution of intrapulmonary concomitant lesions relative to target GGNs (the same lobe, ipsilateral lung, and contralateral lung).
Statistical Analysis
SPSS 21.0 (IBM, NY, USA) and MedCalc were used for statistical analyses. GraphPad Prism 5.01 was used for drawing receiver operating characteristic (ROC) curves, and nomogram model was built by rms with R4.2.3 library. Continuous data and categorical variables were expressed as mean ± standard deviation and numbers and percentages, respectively. The Student’s t-test was used to compare age and lesion size between patients with neoplastic and non-neoplastic GGNs. The Pearson chi-square test was used for comparing the frequency of patients’ clinical characteristics, CT features of target GGNs, and incidence of intrapulmonary concomitant lesions between patients with neoplastic and non-neoplastic GGNs. Logistic regression analysis was performed to construct models for predicting non-neoplastic GGNs based on parameters with statistical differences. Among them, Model A was based on CT features of target GGNs, Model B was according to CT features of intrapulmonary concomitant lesions, and Model C was based on CT features of both target GGNs and intrapulmonary concomitant lesions. The cut-off value of age and size, and prediction of the model were analyzed using the ROC curve. The AUC of different models was evaluated with the DeLong test. A P-value of <0.05 was considered statistically significant.
Results
Patients’ Clinical Characteristics and CT Features of Target GGNs
Regarding the pneumonitis history, the number of cases present in patients with neoplastic GGN and non-neoplastic GGN was 33 (5.55%) and 30 (7.58%), respectively. The patients’ clinical characteristics and CT features of target GGNs are listed in Table 2. Compared with patients with neoplastic GGNs, younger and male individuals were more common in those with non-neoplastic ones (each P < 0.0001). Regarding CT features of target GGNs, non-neoplastic GGNs had smaller size than neoplastic ones (P < 0.0001). Besides, Non-neoplastic GGNs with irregular shape, ill-defined boundary, and pleural thickening were more common than neoplastic GGNs, while the incidence of spiculation, vacuole, abnormal intra-nodular vessel, and air bronchogram were more frequent in neoplastic GGNs (each P < 0.0001).
 |
Table 2 Patients’ Clinical Characteristics and CT Features of Target GGNs |
Intrapulmonary Concomitant Lesions
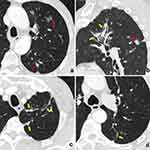
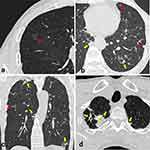
Numbers of patients with different types of intrapulmonary concomitant lesions are shown in Table 3. Compared patients with neoplastic GGNs, cases with concomitant lesions were more common in those with non-neoplastic GGNs (82.18 vs 87.88%, P = 0.015). Specifically, multiple SNs, patchy GGO/consolidation, and fibrosis/calcification were more frequently detected in patients with non-neoplastic GGNs than in those with neoplastic ones (each P < 0.05) (Figures 1 and 2). In contrast, the incidence of concomitant other GGN in neoplastic nodules was higher than that of non-neoplastic ones (44.37% vs 36.11%, P = 0.01).
 |
Table 3 Comparison of the Patients with Different Types of Pulmonary Lesions |
The distributions of different types of intrapulmonary concomitant lesions are summarized in Table 4. The concomitant multiple SNs, patchy GGO/consolidation, and fibrosis/calcification in both the same lobe and ipsilateral lung in relative to target GGN were more common in patients with non-neoplastic GGNs than in those with neoplastic ones (each P < 0.05). For the concomitant lesions in the contralateral lung, multiple SNs and fibrosis/calcification were still more frequent but GGN was less common in patients with non-neoplastic GGNs than in those with neoplastic ones (each P < 0.05). Additionally, bronchiectasis was also more common in patients with non-neoplastic GGNs than in those with neoplastic ones (each P < 0.0001).
 |
Table 4 The CT Findings of Intrapulmonary Concomitant Lesions |
Logistic Regression Model
The regression models and nomogram model were constructed according to the CT features of target GGNs and intrapulmonary concomitant lesions with statistical differences (Figures 3 and 4). Models A, B, and C for predicting non-neoplastic GGNs were performed based on CT features of target GGNs, intrapulmonary concomitant lesions, and both of them, respectively. There were significant differences in the area under curve (AUC) among model A (AUC: 0.894, 95% CI: 0.874–0.913; sensitivity: 81.3%, specificity: 86.1%; positive predictive value [PPV]: 79.50%, negative predictive value [NPV]: 87.37%), model B (AUC: 0.723, 95% CI: 0.694–0.751; sensitivity: 56.3%, specificity: 78.3%; PPV: 64.36%, NPV: 72.93%), and model C (AUC: 0.926, 95% CI: 0.908–0.941; sensitivity: 83.1%, specificity: 87.10%; PPV: 81.03%, NPV: 88.55%) (each P < 0.0001).
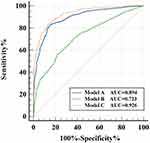 |
Figure 3 ROC curve for different regression models in predicting non-neoplastic GGNs. Abbreviations: ROC, receiver operating characteristic; GGNs, ground-glass nodules. |
Moreover, model C revealed that when patients were less than 53 years old (OR, 3.075; 95% CI, 1.779–5.314; P < 0.0001), male (OR, 2.125; 95% CI, 1.398–3.230; P < 0.0001), target GGNs with a diameter < 7.35 mm (OR, 4.470; 95% CI, 2.818–7.029; P < 0.0001), irregular shape (OR, 3.705; 95% CI, 2.011–6.828; P < 0.0001), coarse margin or ill-defined boundary (OR, 27.004; 95% CI, 16.659–43.775; P < 0.0001), pleural thickening (OR, 6.698; 95% CI, 2.768–16.209; P < 0.0001), multiple concomitant SNs in the same lobe (OR, 3.481; 95% CI, 1.142–10.607; P = 0.028), and concomitant fibrosis/calcification [the same lobe (OR, 3.691; 95% CI, 2.131–6.392); ipsilateral lung (OR, 2.370; 95% CI, 1.496–3.755); contralateral lung (OR, 2.966; 95% CI, 1.901–4.629); each P < 0.0001)] were independent indicators for predicting non-neoplastic GGNs.
Discussion
In the present study, intrapulmonary concomitant lesions were commonly detected in patients with neoplastic and non-neoplastic GGNs, while their distributions and types had some differences between them. Multiple SNs, patchy GGO/consolidation, or fibrosis/calcification in any lung fields were more commonly in patients with non-neoplastic GGNs than in patients with neoplastic ones. What’s more, the combination of intrapulmonary concomitant lesions is helpful to improve the differential diagnosis based solely on the CT features of target GGNs. To be specific, when GGNs with irregular shape, coarse margin or ill-defined boundary, pleural thickening, and intrapulmonary concomitant lesions (SNs in the same lobe and fibrosis or calcification in any lung field) occur in young patients, they are highly suggestive of non-neoplastic lesions. Therefore, the intrapulmonary concomitant lesions should also be considered when differentiating GGNs, especially for those without typical CT features.
For patients with GGNs, an accurate diagnosis could help clinicians choose reasonable treatment strategies, thereby avoiding unnecessary surgical resection of non-neoplastic ones. Previous studies have confirmed that patients’ basic clinical information and CT features of lesions could provide great value in differentiating GGNs. In the present study, old and female individuals were more common in patients with neoplastic GGNs, additionally, lesions with the larger size, well-defined boundary, abnormal intra-nodular vessels, or vacuole had a higher probability of neoplastic lesions, while those with an irregular shape, ill-defined boundary, or adjacent thickened pleura were more likely to be non-neoplastic. These findings were similar to the results revealed in previous studies.10,13–16,23 Though these clinical and CT indicators are helpful in distinguishing GGNs, some GGNs especially those lacking in CT features still cannot be well identified. Thus, further exploring other indicators for differentiating is necessary.
It was found that there are usually some different types of concomitant lesions in the lung field besides the target GGNs. What can imagine is, the target GGN may be a part of them, or they represent a background which is relevant to the presence of target GGN. Thus, the intrapulmonary concomitant lesions may provide some more information for differentiating. To our knowledge, no researchers had taken the intrapulmonary concomitant lesions into consideration in diagnosing GGNs. In this study, the intrapulmonary concomitant lesions of target GGNs were summarized, and it was found that there were some significant differences in their distribution and types between patients with non-neoplastic and neoplastic GGNs. This provides a new clue for their differential diagnosis.
With regard to intrapulmonary concomitant lesions, this study showed that the incidence of most concomitant lesions in neoplastic nodules was lower than that in non-neoplastic ones in different regions of the lung. This is mainly manifested in the higher possibility of multiple SNs, patchy GGO/consolidation, and fibrosis/calcification taking place in non-neoplastic GGNs. No matter in the same lobe or ipsilateral and contralateral lung, multiple SNs and fibrosis/calcification were more commonly detected in patients with non-neoplastic GGNs. These concomitant lesions are usually seen as incomplete absorption of chronic inflammatory or infectious diseases, thus their existence and diffuse distribution frequently indicates a potential condition of easily suffering inflammation and infection.17,24 In contrast, only patchy GGO or consolidation in the same lobe and ipsilateral lung were more commonly detected in patients with non-neoplastic GGNs. The occurrence of patchy GGO or consolidation seems more closely associated with target GGNs, probably because they are concurrent lesions, and most of non-neoplastic GGNs, patchy GGO, or consolidation were usually seen as active inflammation.25 Besides the mentioned above concomitant lesions, bronchiectasis as a structural basis of pulmonary infection was also more frequently detected in patients with non-neoplastic GGNs. Therefore, occurrence inflammatory background and lesions and anatomic basis of inflammation are indicators of benign GGNs. In contrast, GGNs in the contralateral lung were more commonly detected in patients with neoplastic GGNs. It indicates that scattered GGNs in bilateral lungs had a higher possibility of neoplasm,26,27 and at the level of genetic research, multiple GGNs are often multifocal and independent cancers.28
Regarding the efficacy of different indicators or parameters in the differential diagnosis of non-neoplastic GGNs, the AUC of model based solely on CT features of target GGNs is higher than that only based on intrapulmonary concomitant lesions, but lower than that based on the combination of CT features of target GGNs and intrapulmonary concomitant lesions. The lower AUC of intrapulmonary concomitant lesions is mainly due to absent concomitant lesions in some patients, which indicates that the CT features of concomitant lesions could only play an auxiliary role in diagnosis. In combination with concomitant lesions, the diagnostic performance of the model based on target GGNs significantly increased. The CT features of intrapulmonary concomitant lesions played an important role in differential diagnosis of GGNs, which should be evaluated with target lesions together. Specifically, the occurrence of multiple SNs in the same lobe and fibrosis or calcification were independent indicators for predicting non-neoplastic GGNs. Therefore, when evaluating the nature of GGN in clinical practice, the first consideration is to observe the CT manifestations of GGN itself, and then is to pay attention to whether there are concomitant lesions in the lungs. If there are, further evaluation of the types and distribution of accompanying lesions is needed, and a comprehensive judgment should be made based on the above steps. If the malignant signs of the target GGN are atypical and there are multiple SNs present in the same lung lobe, as well as fibrosis or calcification, then the GGN is highly suggestive of non-neoplastic. For such patients, regular follow-up or anti-inflammatory treatment can be performed and avoid aggressive surgical treatment.
The limitations of this study are as follows: Firstly, in this present study, qualitative rather than quantitative evaluation of some intrapulmonary concomitant lesions was conducted. Secondly, not all types of intrapulmonary lesions were evaluated, the rare lesions were not representative. Thirdly, for patients with multiple GGNs, the nature of the GGNs other than the target ones was unknown, so it was difficult to determine their relationships.
In conclusion, intrapulmonary concomitant lesions were commonly detected in patients with GGNs. Their types and distribution are of certain value in differentiating target GGNs and improving the diagnostic performance solely based on the morphological features of nodules. When irregular or ill-defined GGNs accompanied by multiple SNs, fibrosis or calcification in any lung lobes are present in younger or male patients, they are highly suggestive of non-neoplastic lesions. Therefore, the intrapulmonary concomitant lesions should be considered when differentiating GGNs, especially for those without typical CT features.
Abbreviations
GGNs, ground-glass nodules; SNs, solid nodules; LDCT, low‑dose computed tomography; AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; MIA, minimally invasive adenocarcinoma; IAC, invasive adenocarcinoma; GGO, ground-glass opacity; ROC, receiver operating characteristic; AUC, area under curve; PPV, positive predictive value; NPV, negative predictive value.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available because the cases are from the Picture Archiving and Communicating System of our Hospital but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study conformed to the Declaration of Helsinki on Human Research Ethics standards and was approved by the institutional review board of the First Affiliated Hospital of Chongqing Medical University (number 2019–062). The need for written, informed consent was waived by the institutional review board of the First Affiliated Hospital of Chongqing Medical University because of the retrospective design.
Funding
This work was supported by the Joint Project of Chongqing Science and Technology Commission and Chongqing Public Health Commission (2022MSXM050), and the Senior Medical Talents Program of Chongqing for Young and Middle-aged from Chongqing Health Commission (Receptor: Zhigang Chu).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Kim TJ, Goo JM, Lee KW, Park CM, Lee HJ. Clinical, pathological and thin-section CT features of persistent multiple ground-glass opacity nodules: comparison with solitary ground-glass opacity nodule. Lung Cancer. 2009;64(2):171–178. doi:10.1016/j.lungcan.2008.08.002
2. Chu ZG, Li WJ, Fu BJ, Lv FJ. CT Characteristics for Predicting Invasiveness in Pulmonary Pure Ground-Glass Nodules. AJR Am J Roentgenol. 2020;215(2):351–358. doi:10.2214/AJR.19.22381
3. Qin Y, Xu Y, Ma D, et al. Clinical characteristics of resected solitary ground-glass opacities: comparison between benign and malignant nodules. Thorac Cancer. 2020;11(10):2767–2774. doi:10.1111/1759-7714.13575
4. Ye X, Fan W, Wang Z, et al. Expert consensus on thermal ablation therapy of pulmonary subsolid nodules (2021 Edition). J Cancer Res Ther. 2021;17(5):1141–1156. doi:10.4103/jcrt.jcrt_1485_21
5. Zhang Y, Fu F, Chen H. Management of Ground-Glass Opacities in the Lung Cancer Spectrum. Ann Thorac Surg. 2020;110(6):1796–1804. doi:10.1016/j.athoracsur.2020.04.094
6. Fan L, Liu SY, Li QC, Yu H, Xiao XS. Multidetector CT features of pulmonary focal ground-glass opacity: differences between benign and malignant. Br J Radiol. 2012;85(1015):897–904. doi:10.1259/bjr/33150223
7. Kim HY, Shim YM, Lee KS, Han J, Yi CA, Kim YK. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245(1):267–275. doi:10.1148/radiol.2451061682
8. Felix L, Serra-Tosio G, Lantuejoul S, et al. CT characteristics of resolving ground-glass opacities in a lung cancer screening programme. Eur J Radiol. 2011;77(3):410–416. doi:10.1016/j.ejrad.2009.09.008
9. He XQ, Li X, Wu Y, et al. Differential Diagnosis of Nonabsorbable Inflammatory and Malignant Subsolid Nodules with a Solid Component ≤5 mm. J Inflamm Res. 2022;15:1785–1796. doi:10.2147/JIR.S355848
10. Gao F, Sun Y, Zhang G, Zheng X, Li M, Hua Y. CT characterization of different pathological types of subcentimeter pulmonary ground-glass nodular lesions. Br J Radiol. 2019;92(1094):20180204. doi:10.1259/bjr.20180204
11. Hu X, Ye W, Li Z, et al. Non-invasive evaluation for benign and malignant subcentimeter pulmonary ground-glass nodules (≤1 cm) based on CT texture analysis. Br J Radiol. 2020;93(1114):20190762. doi:10.1259/bjr.20190762
12. Mei X, Wang R, Yang W, et al. Predicting malignancy of pulmonary ground-glass nodules and their invasiveness by random forest. J Thorac Dis. 2018;10(1):458–463. doi:10.21037/jtd.2018.01.88
13. Hu H, Wang Q, Tang H, Xiong L, Lin Q. Multi-slice computed tomography characteristics of solitary pulmonary ground-glass nodules: differences between malignant and benign. Thorac Cancer. 2016;7(1):80–87. doi:10.1111/1759-7714.12280
14. Gao F, Li M, Ge X, et al. Multi-detector spiral CT study of the relationships between pulmonary ground-glass nodules and blood vessels. Eur Radiol. 2013;23(12):3271–3277. doi:10.1007/s00330-013-2954-3
15. Fu BJ, Lv FJ, Li WJ, Lin RY, Zheng YN, Chu ZG. Significance of intra-nodular vessel sign in differentiating benign and malignant pulmonary ground-glass nodules. Insights Imaging. 2021;12(1):65 doi:10.1186/s13244-021-01012-7.
16. Li F, Sone S, Abe H, Macmahon H, Doi K. Malignant versus benign nodules at CT screening for lung cancer: comparison of thin-section CT findings. Radiology. 2004;233(3):793–798. doi:10.1148/radiol.2333031018
17. de Oliveira CV, Horvat N, Testagrossa LA, Romão DDS, Rassi MB, Lee HJ. Etiological profile and main imaging findings in patients with granulomatous diseases who underwent lung biopsy. Eur J Radiol Open. 2021;8:100325. doi:10.1016/j.ejro.2021.100325
18. Fu G, Yu H, Liu J, et al. Arc concave sign on thin-section computed tomography:A novel predictor for invasive pulmonary adenocarcinoma in pure ground-glass nodules. Eur J Radiol. 2021;139:109683. doi:10.1016/j.ejrad.2021.109683
19. Wu F, Tian SP, Jin X, et al. CT and histopathologic characteristics of lung adenocarcinoma with pure ground-glass nodules 10 mm or less in diameter. Eur Radiol. 2017;27(10):4037–4043. doi:10.1007/s00330-017-4829-5
20. Hsu JS, Han IT, Tsai TH, et al. Pleural Tags on CT Scans to Predict Visceral Pleural Invasion of Non-Small Cell Lung Cancer That Does Not Abut the Pleura. Radiology. 2016;279(2):590–596. doi:10.1148/radiol.2015151120
21. Liu LH, Liu M, Wei R, et al. CT findings of persistent pure ground glass opacity: can we predict the invasiveness? Asian Pac J Cancer Prev. 2015;16(5):1925–1928. doi:10.7314/APJCP.2015.16.5.1925
22. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi:10.1148/radiol.2462070712
23. Liang ZR, Ye M, Lv FJ, et al. Differential diagnosis of benign and malignant patchy ground-glass opacity by thin-section computed tomography. BMC Cancer. 2022;22(1):1206. doi:10.1186/s12885-022-10338-4
24. Franquet T. Imaging of Community-acquired Pneumonia. J Thorac Imaging. 2018;33(5):282–294. doi:10.1097/RTI.0000000000000347
25. Li WJ, Lv FJ, Tan YW, Fu BJ, Chu ZG. Pulmonary Benign Ground-Glass Nodules: CT Features and Pathological Findings. Int J Gen Med. 2021;14:581–590. doi:10.2147/IJGM.S298517
26. Wang X, Wu M, Shen H, et al. Comparison of Clinical and Pathological Characteristics Between Extremely Multiple GGNs and Single GGNs. Front Oncol. 2021;11:725475. doi:10.3389/fonc.2021.725475
27. Xie S, Li S, Deng H, Han Y, Liu G, Liu Q. Application Value of PET/CT and MRI in the Diagnosis and Treatment of Patients With Synchronous Multiple Pulmonary Ground-Glass Nodules. Front Oncol. 2022;12:797823. doi:10.3389/fonc.2022.797823
28. Sato Y, Fujimoto D, Morimoto T, et al. Natural history and clinical characteristics of multiple pulmonary nodules with ground glass opacity. Respirology. 2017;22(8):1615–1621. doi:10.1111/resp.13089
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.