Back to Journals » Journal of Inflammation Research » Volume 16
Potential Role and Prognostic Value of Interleukin-15 for Mortality Among Elderly Patients with Sepsis
Authors Zhao J, Zhang Y, Wang J, Wei B , Liu Y
Received 5 July 2023
Accepted for publication 3 October 2023
Published 11 October 2023 Volume 2023:16 Pages 4481—4488
DOI https://doi.org/10.2147/JIR.S429080
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Jingjing Zhao,1 Ye Zhang,2 Junyu Wang,2 Bing Wei,2 Yugeng Liu1,2
1Department of Infectious Disease and Clinical Microbiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, 100043, People’s Republic of China; 2Emergency Medicine Clinical Research Center, Beijing Chaoyang Hospital & Beijing Key Laboratory of Cardiopulmonary Cerebral Resuscitation, Clinical Center for Medicine in Acute Infection, Capital Medical University, Beijing, 100043, People’s Republic of China
Correspondence: Bing Wei; Yugeng Liu, Emergency Medicine Clinical Research Center, Beijing Chaoyang Hospital, Capital Medical University, 5 Jing-Yuan Road, Beijing, 100043, People’s Republic of China, Email [email protected]; [email protected]
Background: To investigate the potential role and prognostic value of interleukin-15 (IL-15) in predicting 28-day mortality in elderly patients with sepsis.
Methods: According to the Sepsis-3.0 diagnostic criteria for sepsis, elderly patients with sepsis who were admitted to the emergency department of the Shi jingshan branch of Beijing Chaoyang Hospital between October 2021 and June 2022 were enrolled in this retrospective cohort study. After observation for 28 days, patients were divided into a survival group and a nonsurvival group. Samples for laboratory tests, baseline characteristic data, and SOFA and Acute Physiology and Chronic Health Evaluation (APACHE II) scores were collected or recorded within 24 h after admission to the emergency department. Quantitative detection of IL-15 was performed with a Luminex assay. Logistic regression analysis and receiver operating characteristic curve (ROC) analysis were conducted for comparison.
Results: In total, 220 elderly patients with sepsis were enrolled, 69 of whom were in the survival group and 151 of whom were in the nonsurvival group at the 28-day interval. Systolic pressure, high-density lipoprotein (HDL), platelets (PLT) and albumin (ALB) were significantly higher in the survival group (P< 0.05), while IL-15, SOFA, and APACHE II were significantly higher in the nonsurvival group (P< 0.05). IL-15 was an independent risk factor associated with 28-day mortality (OR=1.842, 95% CI [1.323, 2.565]). The area under the receiver operating characteristic curve (AUROC) of IL-15 alone was 0.691 (95% CI [0.618, 0.764]), with a sensitivity of 46.67% and a specificity of 85.81%. The AUROC of the combined IL-15 and SOFA reached 0.880 (95% CI [0.672, 0.812]), for which the sensitivity and specificity were 80.95% and 85.08%, respectively.
Conclusion: IL-15 possesses the prognostic value for predicting 28-day mortality in elderly patients with sepsis.
Keywords: elderly patients, sepsis, interleukin-15, platelet, albumin, SOFA, APACHE II
Introduction
According to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3.0), sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 As the most common cause of death in patients with infections, sepsis remains a major public health problem worldwide, with a mean mortality rate of 33.2%.1,2 Increased age has been shown to be an independent predictor of death among sepsis patients, especially those aged over 65 years.3 As the global aging population continues to increase, the incidence of sepsis in elderly patients is rising. Elderly patients possess low immunity,4 reduced organ reserve function, and few specific symptoms during sepsis,5 making it difficult to diagnose. Early diagnosis and treatment of sepsis can improve patient mortality.1 Although SOFA and APACHE II scores can be used to predict the outcome of patients with sepsis,6,7 little evidence on their prognostic values for elderly patients with sepsis has been shown.
As a group of endogenous inflammatory proteins, cytokines act as modulators of the inflammatory response and mediate the pathophysiology of the systemic inflammatory response syndrome (SIRS) associated with sepsis. Cytokines, such as tumor necrosis factor alpha (TNF-α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12P70, IL-13, IL-17, IL-18, IL-22, interferon gamma (IFN-γ), procalcitonin (PCT) and C-reactive protein (CRP), have been studied in SIRS and septic patients.8,9 IL-15 has been shown to play vital roles in the development and homeostasis of naive CD8+ T cells, memory T cells, and natural killer (NK) cells, which exert functions in pathogen elimination,10–12 making it an attractive therapeutic target in sepsis. However, little is known about the role and prognostic value of IL-15, especially in elderly patients with sepsis. This study aimed to investigate the potential role and prognostic value of IL-15 in predicting 28-day mortality in elderly patients with sepsis.
Patients and Methods
Ethics
The protocol of this study was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (approval number: 2021-ke-636). This study was in compliance with the Declaration of Helsinki and informed consent was obtained from all patients or their families before this study.
Patients and Inclusion Criteria
This single-center retrospective study was performed at the Shi jingshan branch of Beijing Chaoyang Hospital Affiliated to Capital Medical University, with a total of 220 septic patients (132 men and 88 women) enrolled.
Patients who were identified with suspected infection and with an acute change in total SOFA score ≥2 points were enrolled according to the Sepsis-3 criteria.1 Those who were receiving immunosuppressive therapy or with malignant tumors, connective tissue diseases, hematological diseases were excluded.
The baseline characteristics of the patients (age, sex) were collected and documented at the time of admission to the emergency department of Shi jingshan branch of Beijing Chaoyang Hospital between October 2021 and June 2022. Vital signs, such as temperature, mean arterial pressure (MAP), respiratory rate and heart rate, were also collected. SOFA and APACHE II scores were calculated on admission for all enrolled patients. According to the clinical outcomes at 28 days after admission, the patients were divided into a nonsurvival group and a survival group.
Sample Collection and Laboratory Tests
Blood samples were collected within 24 h of admission to the emergency department and used to measure the following laboratory results: PCT, CRP, white blood cell (WBC), PLT, lactate (LAC), blood urea nitrogen (BUN), creatinine (CR), total bilirubin (TRIL), aspartate transaminase (AST), alanine transaminase (ALT), albumin (ALB), high density lipoprotein (HDL), low density lipoprotein (LDL), myoglobin, lymphocytes, monocytes, and neutrophils. Circulating levels of IL-15 in plasma samples were quantified by using the Human XL Cytokine Luminex Performance Assay 46-plex Fixed Panel (LKTM014B, R&D) according to the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed by SPSS software, version 19.0 (SPSS Inc., Chicago, IL, USA). The results are presented as the prevalence rates, means ± SD, or medians and ranges in cases of a skewed distribution. The Kolmogorov‒Smirnov goodness of fit test was performed to check the parametric distribution of the data. The unpaired Student’s t-test or Mann‒Whitney U-test was used accordingly for the data analysis. Categorical data were analyzed by the χ2 test. P<0.05 was considered significant. Pearson correlation coefficient analysis was performed for the correlation analysis.
Logistic regression analysis was used to screen the risk factors for 28-day mortality in septic patients. ROC curve analysis was used to compare the prognostic value of IL-15 alone or together with SOFA or APACHE II for elderly patients with sepsis, and AUROC was used to evaluate their predictive values. Comparisons of the ROC curves were analyzed by the Z test, which was carried out by MedCalc software. P<0.05 was considered statistically significant.
Results
Patient Characteristics
In total, 220 patients (132 male, 88 female) were enrolled in this study, of whom 69 survived and 151 died within a 28-day period (Table 1). There were no significant differences in sex, age, temperature, diastolic pressure, mean arterial pressure, or heart rate between the survival group and nonsurvival group. The mean age of the survival group was 70 (60,83), and the mean age of the nonsurvival group was 75 (65,83). There was a significant difference in systolic pressure between the survival group and the nonsurvival group (P<0.05).
 |
Table 1 Comparison of Demographic Data Between the Survival Group and Non-Survival Group |
Results of Laboratory Tests and IL-15 Analysis
No significant differences were found in PCT, CRP, Lac, BUN, CR, GLU, TRIL, AST, ALT, LDL, myoglobin, WBCs, lymphocytes, monocytes, or neutrophils between the survival group and nonsurvival group. The survival group possessed significantly higher PLT, ALB, and HDL levels than the nonsurvival group (all P<0.05). The SOFA and APACHE II scores of the nonsurvival group were significantly higher than those of the survival group (all P<0.001). IL-15 was significantly higher in the nonsurvival group (mean value=1.80 pg/mL) than in the survival group (mean value=1.03 pg/mL) (P<0.001).
Results from Logistic Regression and ROC Curve Analysis
As demonstrated in Table 2, the logistic regression analysis found that IL-15 was an independent risk factor associated with 28-day mortality (OR=1.842, 95% CI [1.323, 2.565]). The 28-day mortality of elderly patients with sepsis was also independently associated with protective factors, such as PLT (OR=0.996, 95% CI [0.992, 1.000]) and ALB (OR=0.949, 95% CI [0.913, 0.986]). No significant differences were demonstrated between IL-15 and other factors by Pearson correlation analysis, as shown in Table 3.
 |
Table 2 Multivariate Logistic Regression Analysis of Risk Factors for 28-Day Mortality in Elderly Patients with Sepsis |
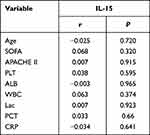 |
Table 3 Pearson Correlation Analysis of IL-15 and Other Biomarkers |
ROC curve analysis was performed to evaluate the prognostic values of IL-15 alone and together with SOFA or APACHE II scores (Figure 1). As shown in Table 4, the cutoff values of IL-15 and SOFA were 1.99 pg/mL and 5.5, respectively. The AUROC of IL-15 alone was 0.691 (95% CI [0.618, 0.764]), of which the sensitivity and specificity were 46.67% and 85.81%, respectively. The AUROC of the combination of IL-15 and APACHE II reached 0.828 (95% CI [0.771, 0.885]), for which the sensitivity and specificity were 77.70% and 80.59%, respectively. The ROC curve of IL-15 and APACHE II could be significantly distinguished from that of IL-15 (Z=3.606, P=0.0003) or APACHE II (Z=3.187, P=0.0014). The ROC curves of IL-5 and SOFA were significantly different from those of IL-15 (Z=4.620, P<0.0001), SOFA (Z=2.639, P=0.0083) and APACHE II (Z=3.172, P=0.0015). The AUROC calculated by combining IL-15 and SOFA reached 0.880 (95% CI [0.829, 0.932]), for which the sensitivity and specificity reached 80.95% and 85.08%, respectively.
 |
Table 4 Receiver Operating Characteristic Curve (ROC) Analysis of the Prognostic Value of IL-15 Together with Other Factors for 28-Day Mortality of Elderly Patients with Sepsis |
 |
Figure 1 Analysis of the prognostic value of IL-15 together with other factors for 28-day mortality of elderly patients with sepsis by using ROC curve. |
Discussion
Sepsis is a life-threatening health crisis, and the number of worldwide incident cases has increased to an estimated 50 million, with approximately 5 million deaths each year.13–16 With the advent of the aging era, more elderly patients will be confronted with this crisis.17 It is estimated that elderly patients account for 58~65% of all patients with sepsis.18 Increased age has been shown to be an independent predictor of death among patients with sepsis, especially for those over 65 years.19 The mortality rate for patients aged 60–64 years is 26% and is 38% for patients aged over 85 years.16,20 In this study, the mean ages of the survival group and nonsurvival group reached 70 and 75, respectively. Most elderly patients with sepsis are prone to respiratory failure, acute renal failure, and multiple organ dysfunction syndrome (MODS) as a result of the deterioration in their physical function and poor organ compensatory function.3 Thus, it is vital to perform early diagnosis as well as predict the clinical severity and outcomes in an accurate manner for patients with sepsis, especially for elderly individuals. However, it is difficult to diagnose elderly patients with sepsis due to their relatively few specific symptoms and signs. The roles of more potential biological markers and their prognostic values in predicting mortality for elderly patients with sepsis need to be investigated.
Recent studies have reported that one of the main reasons for high mortality in elderly septic patients is persistent T cell exhaustion and a decline in the immune response against new pathogens, resulting in secondary infections.21,22 This is characterized by reduced immunocompetent T cells, persistent lymphopenia, increased circulating regulatory T cells (Tregs) and expression of programmed death-1 (PD-1).23 Notably, T cell exhaustion in sepsis can be improved by IL-15, which plays a vital role in the development and homeostasis of naive CD8+ T cells, memory T cells, and NK cells and enhances innate and adaptive immunity.11,12 This characteristic makes IL-15 an attractive therapeutic target in sepsis.
IL-15 is a pleiotropic cytokine belonging to the four a-helix bundle family of cytokines, together with IL-2, IL-4, IL-7, IL-9, and IL-21. In contrast to IL-2, which is generated by activated T cells,24,25 IL-15 is secreted primarily by dendritic cells, monocytes, and epithelial cells during infection. IL-15 can interact with the IL-15R complex through a mechanism known as trans-presentation and induce the activation of diverse signaling pathways dependent on the cell type.26 IL-15 can act on both the innate and adaptive immune systems. For the innate immune system, IL-15 is responsible for the development and homeostatic maintenance of NK cells, maturation of macrophages and DCs in response to microbial infection, induction and enhancement of neutrophils, and support of mast cells for proliferation. For the adaptive immune system, the roles of IL-15 include the promotion of memory CD8+ T cell proliferation, induction of Tregs, and enhanced proliferation of Th17 cells and Th1/Th17 cells. It has been found that upregulation of IL-15 plays a central role in the development of several autoimmune or chronic inflammatory diseases, such as celiac disease, inflammatory bowel disease, multiple sclerosis, systemic sclerosis, and rheumatoid arthritis. Additionally, studies on the relationship between IL-15 and infectious diseases, including infection with retroviruses, hepatitis viruses, bacteria, or parasites, have revealed an association between defects in IL-15 and infectious disorders.27
Several studies have been carried out on septic mouse models to demonstrate the role of IL-15 in sepsis. IL-15 has been shown to prevent CD8+ T cell apoptosis and improve the survival rate in mouse models of sepsis.28 Overproduction of IL-15 improves resistance to live Escherichia coli challenge in mice via inhibition of apoptosis in host cells.29 In an aged mouse model of sepsis, IL-15 reinvigorated the immune response against pathogens and improved sepsis-induced persistent T cell exhaustion by inhibiting the aging-induced increase in PD-1 on CD4+ T cells and Tregs in peripheral blood. IL-15 can increase the frequency of naive CD4+ and CD8+ T cell distribution and downregulate the expression of PD-1 on T cell and Treg populations, with increasing NK cells and macrophages and phagocytosis activity in aged septic mice.30 The above results indicated that aging was associated with increased susceptibility to infection and severity of sepsis, in which IL-15 clearly exerts a positive effect on improving the immune response and eliminating pathogens.
However, few clinical studies have investigated the expression pattern and role of IL-15 in elderly septic patients. For this purpose, our study demonstrated that the serum level of endogenous IL-15 was significantly higher (1.80 vs 1.03 pg/mL) in the nonsurvival group than in the survival group of elderly septic patients. A previous clinical study, which enrolled 92 elderly patients with sepsis, indicated that patients in the severe lymphopenia group (0.3 vs 1.1×103/µL) possessed elevated plasma IL-15 levels (12.2 vs 6.4 pg/mL) and increased mortality during severe sepsis.31 Our study showed that there were no significant differences in the numbers of lymphocytes between the survival and nonsurvival groups. The serum levels of IL-15 for both groups of elderly patients were lower than those in the above study, which may be related to our patients’ average levels of lymphocytes, and the underlying reasons need to be further investigated. Similarly, upregulation of IL-15 was associated with increased mortality in elderly septic patients in both studies. Considering the boosting effect of IL-15 on the immune system and elimination of the pathogen, we conclude that the upregulation of IL-15 in the nonsurvival group of elderly patients with sepsis reflects a more severe dysfunction of the immune cells and a worse state of infection accompanied by a worsened clinical outcome. This is consistent with previous findings about the roles of IL-15. Thus, IL-15 may potentially be used as an independent indicator of the severity or clinical outcomes for elderly patients with sepsis, which helps with early judgment as well as proper treatment for emergency physicians. Administration of exogenous IL-15 may be a possible strategy to intervene in the immune functions of elderly patients in the future.
The logistic analysis also showed that IL-15 can be an independent risk factor associated with 28-day mortality in elderly septic patients. In this study, we also examined routine indices, such as platelets and albumin, in elderly patients with sepsis. Changes in platelet function and impairment of blood rheology might play a role in the pathogenesis of MODS by reducing microvascular blood flow followed by hypoperfusion.32 Albumin plays vital regulatory roles in acid‒base physiology and fluid distribution,33 and lower plasma albumin levels were found in septic patients than in nonseptic patients.34 Our results showed a significantly lower number of PLTs as well as a lowered level of albumin in elderly patients with sepsis in the nonsurvival group compared with the survival group. Similar to IL-15, PLT and albumin were also independent risk factors for 28-day mortality in elderly patients with sepsis, which can help physicians comprehensively understand the conditions of septic patients.
Next, we examined the prognostic value of IL-15 for 28-day mortality in elderly patients with sepsis. The SOFA score has been widely used for the prediction of short-term mortality in patients with sepsis35,36 and is regarded as a better tool for predicting in-hospital mortality than quick SOFA, SIRS and APACHE II scores.37,38 SOFA and APACHE II scores were also analyzed and compared with IL-15 in terms of their prognostic value for 28-day mortality in elderly patients with sepsis. Our results demonstrated that the AUROC of IL-15 for predicting 28-day mortality in elderly patients with sepsis was 0.691, while the AUROCs of APACHE II and SOFA were 0.750 and 0.842, respectively. Consistent with previous reports, the sensitivity of APACHE II was relatively low in our study. The SOFA score possesses a better balance between sensitivity and specificity and is easier to obtain and handle than the APACHE II score. As the specificity of IL-15 (85.81%) was superior to that of SOFA (78.88%), we conducted a combined analysis of IL-15 and SOFA, and the combination resulted in an AUROC of 0.880 with increased sensitivity (80.95%) and specificity (85.08%). This combination had a comparative advantage in predicting 28-day mortality for elderly patients with sepsis considering that it would be more convenient to collect IL-15 and SOFA data than APACHE II data in the clinical setting. The cutoff values of IL-15 and SOFA in this study were 1.99 pg/mL and 5.5, respectively. Based on these data, earlier and more accurate judgment on the severity of illness can be made, and proper interventions can be conducted to reduce the mortality for elderly patients with sepsis.
This study also has limitations, such as the small sample size, lack of healthy and elderly controls and the fact that it was a single-center study. Further multicenter studies with larger sample sizes as well as determination of the baseline levels of serum IL-15 in healthy controls are still needed. Future studies should focus more on the exact roles of IL-15 in elderly patients with sepsis as well as its prognostic value for long-term mortality.
Conclusion
IL-15 possesses the prognostic value for predicting 28-day mortality in elderly patients with sepsis.
Data Sharing Statement
We declare that all the data in this article are authentic, valid, and available for use on reasonable request. Dr Jingjing Zhao can be contacted ([email protected]) regarding the availability of data and materials.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Beijing Chaoyang Hospital Affiliated to Capital Medical University (Approval number: 2021-ke-636). This study was in compliance with the Declaration of Helsinki and informed consent was obtained from all patients or their families before this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
All authors declare that they have no competing interests in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The Third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):762–774. doi:10.1001/jama.2016.0288
3. Martin-Loeches I, Guia MC, Vallecoccia MS, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: a prospective, observational, multi-center cohort study. Ann Intensive Care. 2019;9(1):26. doi:10.1186/s13613-019-0495-x
4. Müller L, Di Benedetto S, Pawelec G. The immune system and its dysregulation with aging. Subcell Biochem. 2019;91:21–43. doi:10.1007/978-981-13-3681-2_2
5. Wu CC, Lan HM, Han ST, et al. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: a systematic review and meta-analysis. Ann Intensive Care. 2017;7(1):91. doi:10.1186/s13613-017-0316-z
6. Basile-Filho A, Lago AF, Menegueti MG, et al. The use of APACHE II, SOFA, SAPS 3, C-reactive protein/albumin ratio, and lactate to predict mortality of surgical critically ill patients: a retrospective cohort study. Medicine. 2019;98(26):e16204. doi:10.1097/MD.0000000000016204
7. Liu Z, Meng Z, Li Y, et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. doi:10.1186/s13049-019-0609-3
8. Jekarl DW, Kim KS, Lee S, Kim M, Kim Y. Cytokine and molecular networks in sepsis cases: a network biology approach. Eur Cytokine Netw. 2018;29(3):103–111. doi:10.1684/ecn.2018.0414
9. Tschoeke SK, Oberholzer A, Moldawer LL. Interleukin-18: a novel prognostic cytokine in bacteria-induced sepsis. Crit Care Med. 2006;34(4):1225–1233. doi:10.1097/01.CCM.0000208356.05575
10. Berard M, Brandt K, Bulfone-Paus S, Tough DF. IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol. 2003;170(10):5018–5026. doi:10.4049/jimmunol.170.10.5018
11. Kennedy MK, Glaccum M, Brown SN, et al. Reversible defects in natural killer and memory CD8+ T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi:10.1084/jem.191.5.771
12. Boyman O, Krieg C, Homann D, Sprent J. Homeostatic maintenance of T cells and natural killer cells. Cell Mol Life Sci. 2012;69(10):1597–1608. doi:10.1007/s00018-012-0968-7
13. Sibbald WJ, Vincent JL. Round table conference on clinical trials for the treatment of sepsis. Crit Care Med. 1995;23(2):394–399. doi:10.1097/00003246-199502000-00027
14. Saeed K, Wilson DC, Bloos F, et al. The early identification of disease progression in patients with suspected infection presenting to the emergency department: a multi-centre derivation and validation study. Crit Care. 2019;23(1):40. doi:10.1186/s13054-019-2329-5
15. Kumar S, Tripathy S, Jyoti A, Singh SG. Recent advances in biosensors for diagnosis and detection of sepsis: a comprehensive review. Biosens Bioelectron. 2019;124–125:205–215. doi:10.1016/j.bios.2018.10.034
16. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the global burden of disease study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
17. Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. doi:10.1164/rccm.201504-0781OC
18. Flaatten H, de Lange DW, Artigas A, et al. The status of intensive care medicine research and a future agenda for very old patients in the ICU. Intensive Care Med. 2017;43(9):1319–1328. doi:10.1007/s00134-017-4718-z
19. Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34(1):15–21. doi:10.1097/01.ccm.0000194535.82812.ba
20. Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–1250. doi:10.1097/01.CCM.0000261890.41311.E9
21. Esper AM, Moss M, Lewis CA, Nisbet R, Mannino DM, Martin GS. The role of infection and comorbidity: factors that influence disparities in sepsis. Crit Care Med. 2006;34(10):2576–2582. doi:10.1097/01.CCM.0000239114.50519.0E
22. Dwolatzky T, Clarfield AM, Berman H. Non-specific presentations of illness. In: Jones R, Britten N, Culpepper L, et al., editors. Oxford Textbook of Primary Medical Care. Oxford: Oxford University Press; 2003:1247–1250.
23. Weng NP. Aging of the immune system: how much can the adaptive immune system adapt? Immunity. 2006;24(5):495–499. doi:10.1016/j.immuni.2006.05.001
24. Hakim FT, Gress RE. Immunosenescence: deficits in adaptive immunity in the elderly. Tissue Antigens. 2007;70(3):179–189. doi:10.1111/j.1399-0039.2007.00891.x
25. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862
26. Budagian V, Bulanova E, Paus R, et al. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17(4):259–280. doi:10.1016/j.cytogfr.2006.05.001
27. Di Sabatino A, Calarota SA, Vidali F, Macdonald TT, Corazza GR. Role of IL-15 in immune-mediated and infectious diseases. Cytokine Growth Factor Rev. 2011;22(1):19–33. doi:10.1016/j.cytogfr.2010.09.003
28. Inoue S, Unsinger J, Davis CG, et al. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol. 2010;184(3):1401–1409. doi:10.4049/jimmunol.0902307
29. Hiromatsu T, Yajima T, Matsuguchi T, et al. Overexpression of interleukin-15 protects against Escherichia coli-induced shock accompanied by inhibition of tumor necrosis factor-alpha-induced apoptosis. J Infect Dis. 2003;187(9):1442–1451. doi:10.1086/374643
30. Saito M, Inoue S, Yamashita K, Kakeji Y, Fukumoto T, Kotani J. IL-15 improves aging-induced persistent t cell exhaustion in mouse models of repeated sepsis. Shock. 2020;53(2):228–235. doi:10.1097/SHK.0000000000001352
31. Chung KP, Chang HT, Lo SC, et al. Severe lymphopenia is associated with elevated plasma interleukin-15 levels and increased mortality during severe sepsis. Shock. 2015;43(6):569–575. doi:10.1097/SHK.0000000000000347
32. Alt E, Amann-Vesti BR, Madl C, Funk G, Koppensteiner R. Platelet aggregation and blood rheology in severe sepsis/septic shock: relation to the Sepsis-related Organ Failure Assessment (SOFA) score. Clin Hemorheol Micro. 2004;30(2):107–115.
33. Vincent JL, Russell JA, Jacob M, et al. Albumin administration in the acutely ill: what is new and where next? Crit Care. 2014;18(4):231. doi:10.1186/cc13991
34. Omiya K, Sato H, Sato T, et al. Albumin and fibrinogen kinetics in sepsis: a prospective observational study. Crit Care. 2021;25(1):436. doi:10.1186/s13054-021-03860-7
35. Li Y, Yan C, Gan Z, et al. Prognostic values of SOFA score, qSOFA score, and LODS score for patients with sepsis. Ann Palliat Med. 2020;9(3):1037–1044. doi:10.21037/apm-20-984
36. Godinjak A, Iglica A, Rama A, et al. Predictive value of SAPS II and APACHE II scoring systems for patient outcome in a medical intensive care unit. Acta Med Acad. 2016;45(2):97–103. doi:10.5644/ama2006-124.165
37. Probst L, Schalk E, Liebregts T, et al. Prognostic accuracy of SOFA, qSOFA and SIRS criteria in hematological cancer patients: a retrospective multicenter study. J Intensive Care. 2019;7(1):41. doi:10.1186/s40560-019-0396-y
38. Schoe A, Bakhshi-Raiez F, de Keizer N, van Dissel JT, de Jonge E. Mortality prediction by SOFA score in ICU-patients after cardiac surgery; comparison with traditional prognostic-models. BMC Anesthesiol. 2020;20(1):65. doi:10.1186/s12871-020-00975-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
