Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 16
Potential Role and Expression Level of Urinary CXCL8 in Different Stages of Incipient Diabetic Nephropathy with Undiminished Creatinine Clearance: A Pilot Study
Authors He Y, Li H, Wang R, Ma N, Liu L, Shi R, Zhang B, Lin N, Tian Y
Received 5 March 2023
Accepted for publication 3 June 2023
Published 17 June 2023 Volume 2023:16 Pages 1783—1790
DOI https://doi.org/10.2147/DMSO.S410638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yang He,1 Huili Li,2 Rui Wang,3 Ning Ma,3 Lianyuan Liu,4 Rui Shi,5 Bohua Zhang,3 Ningning Lin,6 Yiming Tian3
1Hemodialysis Room, The First Hospital of Qinhuangdao, Qinhuangdao, 066000, People’s Republic of China; 2Department of Endocrinology, The First Hospital of Qinhuangdao Affiliated to Hebei Medical University, Qinhuangdao, 066000, People’s Republic of China; 3Department of Endocrinology, The First Hospital of Qinhuangdao, Qinhuangdao, 066000, People’s Republic of China; 4Functional Examination Department, The First Hospital of Qinhuangdao, Qinhuangdao, 066000, People’s Republic of China; 5Rheumatology and Immunology Department, The First Hospital of Qinhuangdao, Qinhuangdao, 066000, People’s Republic of China; 6Department of Dermatology, The First Hospital of Qinhuangdao, Qinhuangdao, 066000, People’s Republic of China
Correspondence: Yiming Tian, Department of Endocrinology, The First Hospital of Qinhuangdao, No. 258, Wenhua Road, Qinhuangdao, Hebei Province, 066000, People’s Republic of China, Email [email protected]
Background: Diabetic nephropathy (DN) is one of the most devastating microvascular complications of diabetes, with a high prevalence and poor prognosis. Early intervention is crucial to improve the outcomes of DN. CXCL8 is related to podocyte damage in incipient DN; however, the role and expression level of CXCL8 have never been elucidated, especially in those with undiminished creatinine clearance.
Methods: Consecutive inpatients with type 2 diabetes were included in this study. Patients were assigned into four groups based on the Mogensen stage, reflecting pathological features through clinical manifestations: non-DN group, hyperfiltration group, microalbuminuria group and overt DN group. Clinical and laboratory data were retrospectively collected and analyzed. Urinary CXCL8 (uCXCL8) was measured using an enzyme-linked immunosorbent assay (ELISA) method and adjusted for urinary creatinine (Cr) from the same urine sample.
Results: In total, 88 eligible consecutive inpatients with type 2 diabetes were included in this study. uCXCL8 was differentially expressed in different stages of incipient DN; it decreased in the hyperfiltration phase of incipient DN (1.40± 1.01 pg/μmol Cr) and was highly expressed in patients in the microalbuminuria stage (5.01± 4.01 pg/μmol Cr). uCXCL8 was positively correlated with age, diabetes course, cystatin C and urinary albuminuria-to-creatinine ratio, but negatively correlated with estimated glomerular filtration rate (P< 0.05). uCXCL8 was a risk factor for classic DN after adjusting for age, diabetes course and cystatin C (OR= 1.17, 95% CI 0.98– 1.4, P=0.045).
Conclusion: CXCL8 played an important role in the progression of incipient DN. The unique expression profile of uCXCL8 may provide a reference for understanding the prognosis and mechanisms of incipient DN progression. uCXCL8 was an independent risk factor for the development of classic DN.
Keywords: type 2 diabetes, diabetic nephropathies, interleukin-8, inflammation
Introduction
Diabetic nephropathy (DN) is one of the most devastating microvascular complications of diabetes. It occurs in approximately 20–40% of patients with diabetes, and has become one of the major causes of end-stage renal failure,1 leading to poor quality of life and increased mortality. The direct costs of treatment for DN are, on average, three times higher than those of patients with uncomplicated diabetes.2 As a significant global challenge, DN has a significant social and economic burden.3 A progressive decline in estimated glomerular filtration rate (eGFR) usually indicates the irreversible progression of DN, and early intervention is crucial to improve the outcomes of DN.4
Inflammation plays an important role in the progression of DN,5 especially in the early stages. CXCL8 is a member of the CXC chemokine family that plays a role in the regulation of the acute inflammatory response. It is secreted by a variety of cell types and induces chemotaxis of neutrophils and other inflammatory cells.6 CXCL8 plays an important role in DN progression by inducing podocyte damage,7 and our previous study demonstrated that an elevated neutrophil-to-lymphocyte ratio is associated with the development of incipient DN.8 However, the pattern of expression of CXCL8 in incipient DN is still controversial. Urinary CXCL8 (uCXCL8) has been shown to be elevated,7,9 or not,10 along with DN progression, and similar conflicts have also been revealed regarding serum CXCL8 expression in DN patients.9,11 On the other hand, patients in previously published studies usually presented with elevated serum creatinine or a decreased eGFR, which could not be taken as an early period of DN.9,10 Thus, the role and expression level of CXCL8 in patients with incipient DN have never been elucidated.
The Mogensen stages of diabetic renal disease emphasize the stages of incipient DN,12 and include a total of five stages corresponding to different pathological states. Stage 1 is characterized by aggravated increased urinary albumin excretion during physical exercise. Stage 2 is characterized by increased eGFR, also known as a hyperfiltration state, which predisposes to irreversible damage to the nephrons and eventually results in the development of progressive renal failure.13 Stages 3 and 4 are known as the phases of microalbuminuria and overt DN, respectively; the latter usually corresponds to a loss of high molecular weight proteins and structural disruption in the glomeruli.12 Stage 5 is end-stage renal failure with uremia due to DN. Few studies have focused on CXCL8 expression in the hyperfiltration stage, which is thought to be the very first period of classic DN.13
In this study, consecutive inpatients with type 2 diabetes were included and grouped according to Mogensen stage to investigate the role and expression level of CXCL8 in incipient DN.
Methods
Study Design and Population
This is a retrospective cross-sectional study. Consecutive patients with type 2 diabetes, over 18 years old, admitted to the Department of Endocrinology of the First Hospital of Qinhuangdao from March 8th to June 20th 2022, were included. The exclusion criteria were: 1) prior non-diabetic chronic kidney disease; 2) acute coronary artery disease or stroke; 3) acute infectious diseases; 4) neoplasm; and 5) eGFR <60 mL/min·1.73 m2.14 All participants provided written informed consent, and this study was approved by the Ethical Committee of the First Hospital of Qinhuangdao (2019D014). We declare that this study complies with the Declaration of Helsinki.
Patients were assigned into four groups based on the level of eGFR and urinary albumin-to-creatinine ratio (UACR) according to the Mogensen stage. The non-DN group included patients with UACR <30 mg/g and 90≤ eGFR <120 mL/min·1.73 m2, the hyperfiltration group included patients with UACR <30 mg/g and eGFR ≥120 mL/min·1.73 m2, the microalbuminuria group included patients with 30≤ UACR <300 mg/g and 90≤ eGFR <120 mL/min·1.73 m2, and the overt DN group included patients with UACR ≥300 mg/g or 60≤ eGFR <90 mL/min·1.73 m2, as shown in Figure 1. Classic DN was defined as UACR ≥30 mg/g or eGFR <90 mL/min·1.73 m2 in this study.
 |
Figure 1 Flowchart of the enrollment and grouping of the patients. Abbreviations: DN, diabetic nephropathy; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio. |
Measurement of Clinical and Laboratory Data
Clinical data of patients were recorded, including age, gender, body mass index (BMI), waist circumference, smoking history, course of diabetes, hypertension and oral angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB).
Laboratory examinations were measured in the central laboratory of the First Hospital of Qinhuangdao, including glycosylated hemoglobin A1c (HbA1c), serum creatinine, cystatin C, C-reactive protein (CRP), low-density lipoprotein cholesterol (LDL-c) and UACR. UACR was calculated using the average of two morning urine samples on different days, and eGFR was calculated by the Modification of Diet in Renal Disease (MDRD) formula.15
The morning midstream spot void was collected and stored at −80°C in a refrigerator. The uCXCL8 concentration was measured using a Human IL-8 enzyme-linked immunosorbent assay (ELISA) kit (QuantiCyto®) with a sensitivity of 3.9 pg/mL, and urinary creatinine concentration was measured by the sarcosine oxidase method, on the same urine sample. uCXCL8 expression was adjusted for urinary creatinine, and expressed as pg/μmol Cr.
Statistical Analysis
R version 3.6.3 was used for the statistical analysis. Normally distributed data were described as mean ± standard deviation (SD), non-normally distributed numeric data were shown as median [interquartile range], and enumeration data were expressed as number and percentage. One-way ANOVA, Kruskal–Wallis or chi-squared analysis was applied to assess the differences among different groups according to the data type. Dunn’s test was used to compare the differences among CXCL8 groups in post-hoc analysis. Spearman’s rank correlation test was used to evaluate linear correlations between independent variables. The likelihood ratio test was performed to determine the risk factors for classic DN in multiple logistic regression analysis. For power calculation, when the type 1 (α) error was considered as 0.05, two-sided and equally, the type 2 (1−β) error was estimated as 88.59%.16 P values <0.05 were considered statistically significant. Missing data were discarded in the statistical process.
Results
Demographic, Clinical and Laboratory Characteristics of Patients
In total, 88 consecutive patients with type 2 diabetes were finally included in this study (Figure 1). There were no significant differences in age, gender, BMI, smoking history, waist circumference, family history of diabetes, history of stroke and hypertension, or oral ACEI/ARB use among the different stages of incipient DN (P>0.05) (Table 1). However, the diabetes course was the longest in the microalbuminuria group and shortest in the hyperfiltration group (P=0.025) (Table 1). Regarding the laboratory data, there were no significant differences in the concentrations of HbA1c, CRP and LDL-c among the different stages of incipient DN, while concentrations of cystatin C and serum creatinine showed similar trends of distribution among the different groups, being highest in the overt DN group and lowest in the hyperfiltration group, opposite to the trends for eGFR distribution (P<0.05, Table 1).
 |
Table 1 Demographic, Clinical and Laboratory Characteristics of Patients in Different Stages of Incipient DN |
uCXCL8 Expression Level in Different Stages of Incipient DN
uCXCL8 was differentially expressed in the different stages of incipient DN (P=0.017) (Table 1, Figure 2). It was expressed at a medium level in patients in the non-DN group (3.11±4.13 pg/μmol Cr) and at an extremely low level in patients in the hyperfiltration group (1.40±1.01 pg/μmol Cr), then it dramatically increased in those in microalbuminuria group (5.01±4.01 pg/μmol Cr) and slightly decreased in patients in the overt DN group (4.41±4.60 pg/μmol Cr), as shown in Table 1 and Figure 2. Notably, uCXCL8 was the only index that was the highest in the microalbuminuria group among all the laboratory indicators investigated in our study (P<0.05). In post-hoc analysis, the uCXCL8 level of patients in the microalbuminuria and overt DN groups was significantly higher than that in the hyperfiltration group (P<0.01), but showed no significant differences compared to that in patients in the non-DN group (Figure 2).
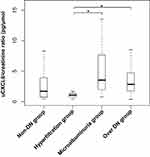 |
Figure 2 Urinary CXCL8 expression in different stages of incipient diabetic nephropathy. Abbreviations: DN, diabetic nephropathy; uCXCL8, urinary CXCL8. Note: *P<0.05 in the post-hoc analysis. |
Correlations Between uCXCL8 and Other Clinical and Laboratory Factors in Patients with Incipient DN
uCXCL8 was positively correlated with age (r=0.219, P=0.040), diabetes course (r=0.213, P=0.046), cystatin C (r=0.337, P=0.004) and UACR (r=0.233, P=0.031), and negatively correlated with eGFR (r=−0.292, P=0.006), as shown in Table 2. Other clinical and laboratory parameters showed no correlations with uCXCL8 in incipient DN. Specifically, CRP showed no linear correlation with uCXCL8 in patients with incipient DN (r=0.113, P=0.478) (Table 2).
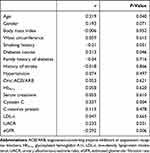 |
Table 2 Correlations Between uCXCL8 and Other Clinical and Laboratory Parameters in DN |
Associations Between uCXCL8 and Classic DN
In the crude logistic regression analysis, uCXCL8 was significantly associated with a greater risk of classic DN [OR=1.23, 95% confidence interval (CI) 1.03–1.47, P=0.023] (Table 3). After adjustment for the risks of age, diabetes course and cystatin C, uCXCL8 remained significantly associated with classic DN (OR=1.17, 95% CI 0.98–1.40, P=0.045) (Table 3).
 |
Table 3 Crude and Adjusted Analyses of Relationships Between uCXCL8 and Classic DN in Regression Analysis |
Discussion
With a rapidly increasing prevalence, diabetes and its chronic microvascular complication, nephropathy, has become a global social and economic burden.3 The inflammatory factor CXCL8 has been demonstrated to be associated with podocyte damage. CXCL8 is increased in the microalbuminuria stage of DN,7 and targeting its receptors, CXCR1 and CXCR2, can ameliorate metabolic disturbances through modulating inflammation in DN.17 However, the role and expression level of CXCL8 in incipient DN have never been elucidated, especially when based on the Mogensen stage and taking the hyperfiltration stage into account. In this study, consecutive inpatients with type 2 diabetes were included and grouped according to the Mogensen classification. As a result, uCXCL8 was demonstrated to be differentially expressed in different stages of incipient DN, significantly correlated with conventional predictors for diagnosing DN, and remained an independent risk factor for classic DN after adjusting for multiple variables.
Based on the results of this study, the uCXCL8 level was the lowest in the hyperfiltration group (Table 1, Figure 2). Since the results have been adjusted for urinary creatinine, they were not affected by individual differences in urine volume. Therefore, we can postulate that the hyperfiltration state of incipient DN may not be related to increased neutrophil chemotaxis. To our knowledge, no previous studies have explored the expression of CXCL8 in the hyperfiltration stage of incipient DN. The uCXCL8 level was highest in the microalbuminuria group and decreased slightly in the overt DN group in our study (Table 1, Figure 2), in agreement with previous clinical and animal research,7 although the CXCL8 expression was not adjusted for creatinine in the previous study. The possible cause for this may be the most intense inflammatory response occurring in the microalbuminuria stage, before the fibrosis of glomeruli. The underlying clinical implication is that we may be able to identify the pathological progression of glomeruli through the fluctuation of urinary indicators, but not through biopsy.
UACR and eGFR have become widely accepted as markers for diagnosing DN in clinical practice,4 and cystatin C is an early predictor of DN, in both serum and urine.4,18 In this study, uCXCL8 was positively correlated with UACR and negatively correlated with eGFR, in agreement with previous studies.9,11 However, whether uCXCL8 is associated with a faster decline in eGFR remains controversial.7,10 In addition, uCXCL8 was positively correlated with cystatin C in our study, and we found no relevant reports in previous studies. This makes uCXCL8 a potential early predictor of DN. Besides, there was no significant correlation between uCXCL8 and serum CRP in our study, although both of them are parameters of inflammation. This may be because CRP is a systematic inflammatory factor tested in serum and can be influenced by many other diseases and conditions, whereas CXCL8 was measured in urine samples to identify only renal problems in our study. Previous studies have not shed light on this point, to our knowledge. In summary, uCXCL8, differently from systemic inflammatory factors, was associated with conventional predictors of incipient DN; on the other hand, fluctuations in these predictors may be related to inflammatory responses.
Based on the results of our study, uCXCL8 was an independent risk factor of classic DN after adjusting for multiple variables. Although previous studies also focused on the relationship between CXCL8 and albuminuria or proteinuria,9,11 some of them did not control for the decline in eGFR,9 and some used serum but not urinary CXCL8 as a predictor.11 Therefore, our study is the first to report the independent ability of uCXCL8 to predict incipient DN with undiminished eGFR.
It is worth noting that the diabetes course was not even among the different groups in our study, and did not increase along with the progression of stages in incipient DN. Since the included patients were consecutive admissions to our hospital during a certain period, this result may reflect the epidemiological distribution of local patients to some extent, and supports the point that DN progression is not entirely dependent on the course of disease.
This study has several strengths. First, consecutive inpatients were included to minimize the selection bias of this retrospective study. Second, the Mogensen stage, with an emphasis on the stage of incipient DN, was used as the grouping standard, and the uCXCL8 level in the hyperfiltration stage, which had not been investigated in previous studies, to our knowledge, was evaluated in this study. Third, the uCXCL8 level was adjusted for urinary creatinine to avoid the influence of differential individual urine volumes, using a spot urine test, which is convenient and suitable for clinical application. Fourth, CRP was evaluated in our study as an indicator of systemic inflammation.
The study also has some limitations. First, the sample size was relatively small in this study, which may have attenuated the significance in part of the statistical analysis. Second, the observational nature of the data precluded proof of causality; they could only be considered hypothesis generating. Third, this was a cross-sectional study, and renal outcomes over time were not investigated. Prospective large-scale cohort studies are needed in the future.
Conclusion
CXCL8 plays an important role in the progression of incipient DN. The unique expression profile of uCXCL8 may provide a reference for understanding the prognosis and mechanisms of incipient DN progression. uCXCL8 correlated with conventional parameters of diagnosing DN in the early stages, and was an independent risk factor for the development of classic DN.
Data Sharing Statement
The dataset used and analyzed in current study is available from the corresponding author on reasonable request; the research proposal needs to be reviewed beforehand.
Acknowledgment
Many thanks to Dr. Tao Li, Dr. Dong Wang and Nurse Xiaoyun Wu for their assistance in specimen collection, separation and management. Thanks also to Dr. Haibo Li for sharing some brilliant R packages in the statistical analysis.
Funding
This study was nominally supported by the Medical Science Research Project of Hebei Province [grant number: 20201311], without financial support.
Disclosure
The authors declare that they have no conflict of interests.
References
1. American Diabetes Association. Standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S1–S224.
2. Gülümsek E, Keşkek Ş. Direct medical cost of nephropathy in patients with type 2 diabetes. Int Urol Nephrol. 2022;54(6):1383–1389. doi:10.1007/s11255-021-03012-4
3. Sagoo MK, Gnudi L. Diabetic nephropathy: an overview. Methods Mol Biol. 2020;2067:3–7. doi:10.1007/978-1-4939-9841-8_1
4. Thakur V, Chattopadhyay M. Early urinary markers for diabetic and other kidney diseases. Curr Drug Targets. 2018;19(7):825–831. doi:10.2174/1389450119666180319124639
5. Milas O, Gadalean F, Vlad A, et al. Pro-inflammatory cytokines are associated with podocyte damage and proximal tubular dysfunction in the early stage of diabetic kidney disease in type 2 diabetes mellitus patients. J Diabetes Complications. 2020;34(2):107479. doi:10.1016/j.jdiacomp.2019.107479
6. Pan X, Kaminga AC, Wen SW, et al. Chemokines in prediabetes and type 2 diabetes: a meta-analysis. Front Immunol. 2021;12:622438. doi:10.3389/fimmu.2021.622438
7. Loretelli C, Rocchio F, D’Addio F, et al. The IL-8-CXCR1/2 axis contributes to diabetic kidney disease. Metabolism. 2021;121:154804. doi:10.1016/j.metabol.2021.154804
8. Yiming T, Tao L, Rui W, et al. Clinical prediction models based on neutrophil to lymphocyte ratio for diabetic nephropathies in middle aged and older patients with type 2 diabetes mellitus. Int J Geriatr. 2022;43(6):714–719. doi:10.3969/j.issn.1674-7593.2022.06.016
9. Higurashi M, Ohya Y, Joh K, et al. Increased urinary levels of CXCL5, CXCL8 and CXCL9 in patients with type 2 diabetic nephropathy. J Diabetes Complications. 2009;23(3):178–184. doi:10.1016/j.jdiacomp.2007.12.001
10. Verhave JC, Bouchard J, Goupil R, et al. Clinical value of inflammatory urinary biomarkers in overt diabetic nephropathy: a prospective study. Diabetes Res Clin Pract. 2013;101(3):333–340. doi:10.1016/j.diabres.2013.07.006
11. Liu SY, Chen J, Li YF. Clinical significance of serum interleukin-8 and soluble tumor necrosis factor-like weak inducer of apoptosis levels in patients with diabetic nephropathy. J Diabetes Investig. 2018;9(5):1182–1188. doi:10.1111/jdi.12828
12. Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32(Suppl 2):64–78. doi:10.2337/diab.32.2.s64
13. Premaratne E, Verma S, Ekinci EI, et al. The impact of hyperfiltration on the diabetic kidney. Diabetes Metab. 2015;41(1):5–17. doi:10.1016/j.diabet.2014.10.003
14. Yoshida Y, Kashiwabara K, Hirakawa Y, et al. Conditions, pathogenesis, and progression of diabetic kidney disease and early decliner in Japan. BMJ Open Diabetes Res Care. 2020;8(1):e000902. doi:10.1136/bmjdrc-2019-000902
15. Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130(6):461–470. doi:10.7326/0003-4819-130-6-199903160-00002
16. Chow SC, Shao J, Wang H. Sample Size Calculations in Clinical Research.
17. Cui S, Qiao L, Yu S, et al. The antagonist of CXCR1 and CXCR2 protects db/db mice from metabolic diseases through modulating inflammation. Am J Physiol Endocrinol Metab. 2019;317(6):E1205–E1217. doi:10.1152/ajpendo.00117.2019
18. Zeng XF, Lu DX, Li JM, et al. Performance of urinary neutrophil gelatinase-associated lipocalin, clusterin, and cystatin C in predicting diabetic kidney disease and diabetic microalbuminuria: a consecutive cohort study. BMC Nephrol. 2017;18(1):233. doi:10.1186/s12882-017-0620-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
