Back to Journals » Cancer Management and Research » Volume 15
Potential of Indonesian Herbal as an Anti-Cancer Therapy: A Systemic Review of in vitro Studies
Authors Permatasanti A, Hidayat W
Received 8 April 2023
Accepted for publication 8 August 2023
Published 17 August 2023 Volume 2023:15 Pages 837—850
DOI https://doi.org/10.2147/CMAR.S414457
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Ayu Permatasanti,1 Wahyu Hidayat2
1Oral Medicine Residency Program, Faculty of Dentistry, Padjadjaran University, Bandung, Indonesia; 2Department of Oral Medicine, Faculty of Dentistry, Padjadjaran University, Bandung, Indonesia
Correspondence: Wahyu Hidayat, Padjadjaran University, Jl. Sekeloa Selatan No. 1, Bandung, West Java, 40132, Indonesia, Tel +62-22-2533044, Email [email protected]
Introduction: In the world, the second most common cause of mortality is cancer, and its prevalence is increasing in both developing and developed states. Cancer therapy has severe side effects for people with cancer. The selection of natural ingredients in the form of herbal plants is expected to provide therapeutic effectiveness with low side effects. A total of 6000 plant species are utilized as herbal medicines in Indonesia by the local population for various ailments.
Objective: To describe the potential of Indonesian herbal plant products as a cancer therapy in vitro.
Methods: This systematic review is based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-SR) guidelines. The selection of articles published in the last 5 years (2018– 2023) from Pubmed, Ebsco, Garuda portal, ScienceDirect, Cochrane, and Webscience was carried out in January 2023 with the keyword “Indonesian herbal” AND/OR “Anti-cancer” AND/OR “In Vitro”. Risk of bias assessment using OHAT tools.
Results: A total of 1,816,511 articles then through inclusion, exclusion criteria screening, and the risk of bias were obtained 23 (twenty-three) low risk of bias articles. Some herbal plant products such as Soursop (Annona muricata L.), Nyamplung (Calophyllum spp.), Benalu Cengkeh/Clove Benalu (Dendrophthoe pentandra), Rumput Mutiara/Pearl Grass (Hedyotis corymbosa L.), Rasamala (Altingia excelsa), Sarang Semut/Anthill plant (Myrmecodia pendans), Basil (Ocimum sanctum Linn.), and Tepus (Zingiber griffithii) showed different potentials for activity as an in vitro anti-cancer therapy. The three Indonesian herbal plants that are most studied in vitro as anti-cancer are Soursop, Rasamala, and Benalu Cengkeh/Clove Benalu.
Conclusion: The most widely studied Indonesian herbal plant in vitro as an anti-cancer is Soursop, while the anti-cancer activity that is widely reported is by inhibiting cell proliferation through intrinsic pathways.
Keywords: Indonesian herbal, anti-cancer, in vitro
Introduction
In the world, the second most common cause of mortality is cancer, and its prevalence is increasing in both developing and developed states. One of the non-communicable diseases affecting the world’s population, including Indonesia, is cancer. In 2020, 10.3 million cancer-related deaths and 19.3 million new cases are anticipated globally.1 Basic Health Research confirms that cancer has risen to the sixth most common cause of death in Indonesia, has a prevalence of 478 cases per 100,000 individuals.2 Based on data from GLOBOCAN (Global Burden of Cancer) / IARC (International Agency of Research on Cancer) in 2020, the ten highest cancer cases in Indonesia were breast, lung, colorectal, uterine cervix, liver, nasopharynx, prostate, non-Hodgkin’s lymphoma, leukemia, and ovaries.3
Despite advancements in disease diagnosis techniques, therapy, and preventive measures, one of the leading causes of death and a severe form of metabolic syndrome is cancer. It is unclear what variables contribute to the development of cancer. A list of internal and external parameters has identified factors related to cancer. External factors including pollution, radiation, tobacco, smoking, drug effects, certain metals, and infectious agents. The World Health Organization (WHO) lists smoking, alcohol consumption (both light and hard), obesity, and physical inactivity among the primary triggers. The internal factors of cancer, which are unchangeable and endogenous in origin, are mostly associated with spontaneous mutations during deoxyribose nucleic acid (DNA), aging, hormonal changes, and inflammation.4,5
Cancer treatment consists of four major modules that integrate surgery, radiation, chemotherapy, and immunotherapy.5 Conventional strategies of cancer cell management still rely on surgery, radiotherapy, chemotherapy, and targeted therapies.6 Frequent failures in cancer treatment efforts, especially with chemotherapy, are due to the low selectivity of anti-cancer drugs and the selectivity of the cancer cells themselves to chemotherapy agents.7 Cancer conventional therapy has severe side effects for people with cancer, therefore due to side effects and expensive treatments, now research on cancer therapy is more inclined to natural sources such as herbal plants.8 This is consistent with what Mohammed et al stated, who claimed that plant-based natural products have been utilized to treat cancer and that more than 3000 plant species have been identified as having anticancer activity.4 Mohammed et al further claimed that plant-based products have provided workable replacements and adjuvants to current anti-cancer chemotherapies as a part of alternative and complementary therapy.9 Herbal plants have less side effects than conventional cancer therapies.8 A total of 6000 plant species are utilized as herbal medicines in Indonesia by the local population for various ailments.10,11
The aim of this systematic review was to describe the potential of Indonesian herbal plant products as a cancer therapy in vitro.
Method
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-SR) guidelines. The method of this systematic review used the Population, Intervention, Comparison, and Outcome (PICO) method according to the purpose of writing. The population in this systematic review is the cancer cell line; intervention with herbal plant products; comparison with control groups; the result is an Indonesian herbal plant product that has the potential to prevent the growth of cancer cells in vitro.
The search for articles to be studied in this systematic review was carried out on 6 (six) electronic databases, namely Pubmed, Ebsco, Garuda portal, Sciencedirect, Cochrane, and Webscience using the keyword “Indonesian herbal” AND/OR “Anti-cancer” AND/OR “In Vitro”.
These systematic review article search inclusion criteria are limited to articles published in the last 5 years (2018 to 2023), indexed and accredited articles, in English and Indonesian, full text and accessible, in vitro research, and human cancer cell line. The articles exclusion criteria are clinical trial research, a combination of Indonesian herbal plants, and articles that are literature review and systematic review. The next step is to evaluate each article’s risk of bias in order to measure its quality. The evaluation of research quality and bias risk according to the eligibility criteria is carried out in accordance with the Office of Health Assessment and Translation (OHAT) tool. OHAT tool requires at least 2 (two) people to assess, consisting of 6 (six) domains, and 8 (eight) questions. The response options for each question consist of “definitely low risk of bias”, “probably low risk of bias”, “probably high risk of bias”, or “definitely high risk of bias”. After answering all the questions, the value of each question is accumulated and divided by the number of questions. Then the results are divided into 3 tier consisting of low (1), moderate (2), and high risk of bias (3) results.12
Discussion
A total of 1,816,511 (one hundred and sixteen thousand five hundred and eleven) articles were obtained from the search. The next stage is applied articles from the last 5 (five) years from 2018 to 2023 so that the total articles issued are 1,225,947 (one million two hundred and twenty-five thousand nine hundred and forty-seven) articles so that the data is screened as many as 590,564 (five hundred and twenty thousand five hundred and sixty-four) articles. Then the predetermined inclusion and exclusion are applied as well as manual searches so that the final results of 23 (twenty-three) appropriate articles are obtained. Following the assessment of the risk of bias, 23 (twenty-three) articles with low risk of bias and none with high risk of bias are discovered. The process of article search and article selection can be seen in (Figure 1), while the risk of bias assessment is shown in (Table 1).
 |
Table 1 Risk of Bias Assessment Results and Quality of Articles Reviewed Using OHAT Tools |
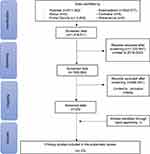 |
Figure 1 Flowchart of the Article Search Process based on the PRISMA-SR Guidelines. |
The following stage is a synopsis of each article that includes data on the author, year, journal quality, herbal plants, where they came from, model systems, different forms of cancer, test procedures, mechanisms of action, outcomes, and conclusions (Table 2).
 |
Table 2 Characteristic and Comparison Result of Researched Article |
Based on (Table 2), it is shown that the year that the most in vitro studies were carried out in cancer was in 2019. A total of 6 articles are head and neck cancer and 17 articles are not head and neck cancer. Among the cancers that are not the most head and neck cancer, namely breast cancer, as many as 6 articles. The most frequently used human cancer cell lines in the reviewed articles are SP-C1, MCF-7, and HeLa.
The most widely researched Indonesian herbal plant in vitro as an anti-cancer is Soursop (Annona muricata L.), as many as 12 (twelve) articles. The most widely taken part of Soursop is in the form of leaves as many as 10 (ten) articles, then 1 (one) article in the form of fruit, and 1 (one) article in the form of roots. Among the three forms, the fruit does not provide cytotoxic activity against cancer cells. In general, cytotoxic research uses soursop leaves more often than fruits, This is as a result of their higher phytochemical content. When compared to its presence in soursop leaves, the phytochemical content of soursop fruit, which includes alkaloids, annonaceous acetogenin compounds (AGEs), and phenols, demonstrates that each phytochemical category has a smaller variance in the fruit.17
Soursop has the potential to be anti-cancer14,15,17–19,31 because it contains various compounds such as flavonoids, AGEs, and alkaloids that have anticancer capabilities. AGEs have the ability to inhibit mitochondrial complex I which can inhibit the formation of adenosine triphosphate (ATP), resulting in a decrease in ATP in cancer cells so that it can inhibit proliferation and induce apoptosis of cancer cells.13,21,32 Preliminary research using molecular docking simulations proved that acetogenin derived from soursop has a strong bond with the B Cell Lymphoma-extra Large (BCL-xL) protein and has the potential to induce apoptosis from intrinsic pathways.32 In addition, AGEs are able to inhibit lactate dehydrogenase which is an enzyme that functions as an antioxidant so as to induce oxidative stress and trigger apoptosis and cell autophagy.21 Another active substance found in soursop leaves is quercetin. Quercetin is an anti-oxidant, however on the other hand it is able to induce apoptosis in cancer cells. The selectivity of soursop leaf extract to breast cancer cells is thought to be related to the quercetin content in soursop leaves which can trigger apoptosis in cancer cells but not in normal cells. Lamson and Brignall mentioned that some cancer cells have mechanism damage defenses against reactive oxygen species (ROS) so that these cells cannot utilize additional anti-oxidants for the repair process. This causes a buildup of free radicals in cancer cells which has an impact on the death of these cancer cells.13 In addition to AGE, flavonoids are also bioactive components that have a role in anticancer activity through apoptosis with mechanisms that are not much different from AGE. Flavonoids work as pro-oxidants in cancer cells. One of the mechanisms is to inhibit epidermal growth factor receptor/mitogen activated protein kinase (EGFR/MAPK) so that cell growth is inhibited.20
The anticancer activity of soursop not only induces apoptosis and antiproliferation, but may decrease migration, motility and invasion of cancer cells.20 Antiproliferative effects were also shown on HL-60 cells by inducing loss of cell viability, morphological changes, loss of mitochondrial potential of membranes, and capture of G0/G1 phase cells. This confirms the potential of soursop as an agent of chemotherapeutic and cytostatic activity in HL-60 cells.22 Previous research has shown that after pancreatic cancer cells FG/COLO357 are given soursop leaf extract, the motility of these cancer cells decreases. Soursop leaf extract has also shown to inhibit migration and invasion of colorectal cancer cells HT-29 and HCT-119 significantly. The cytotoxic effect of alginate nanoparticles is greater than that of soursop leaf ethanol extract against HepG2 cells. Research of Liu et al. It showed that soursop leaf ethanol extract had an Inhibitory Concentration 50 (IC50) value of about 150 μg / mL against HepG2 liver cancer cells, while in this study alginate nanoparticles of soursop leaf extract had an IC50 of 14.5 μg/mL.20 Tests using the Terminal deoxynucleotidyl transferase dUTP nick and labeling (TUNEL) method showed that soursop leaf ethanol extract is able to induce apoptosis in cancer cells.21
A total of 2 of the 12 articles with the herbal plant soursop are alginate nanoparticles of soursop leaf ethanol extract. Extract preparations are currently being developed in the form of nanoparticles. Nanoparticles have characteristics with a small size (1–300 nm). The size makes it easier for nanoparticles to extravasate through the endothelial pores of blood vessels to cancer cells, cellular internalization becomes faster due to the charge on the surface of the nanoparticles causing a stronger electrostatic bond with cancer cells thus increasing bioavailability which causes the nanoparticles to have a higher cytotoxic effect. The nanoparticle material being developed into anticancer therapy is a polymer, which can be classified into synthetic or natural polymers. One of the nanoparticle biopolymers is alginate. Alginate has good biodegradability, low toxicity and high absorption.20
Other Indonesian herbal plants in this systematic review are Calophyllum spp. (Nyamplung), Dendrophthoe pentandra (clove benalu/ benalu cengkeh), Hedyotis corymbosa L. (pearl grass/ rumput mutiara), Altingia excelsa (Rasamala), Myrmecodia pendans (anthill plant/ sarang semut), Ocimum sanctum Linn. (Basil), and Zingiber griffithii (Tepus).
Nyamplung fruit consists of seeds and shells. Polar hazardous potential substances such triterpenoids, calophyllolides, coumarins, flavonoids, saponins, and resins are known to be present in the shells and seeds of nyamplung.23 One of the anticancer substances that can be found in nature are saponin chemicals. Numerous investigations have been conducted on the cytotoxic mechanism of saponin compounds. The investigations came to the conclusion that saponins are responsible for the effects of apoptosis or non-apoptosis in cells. The intrinsic pathway of apoptosis is the apoptosis mechanism that has received the most media attention. Reduced NO generation, cytoskeleton breakdown, and autophagy-stimulated cell death are some of the non-apoptotic pathways. Triterpenoids are known to have anti-cancer activities through activating nuclear-kappa B factor and inducing apoptosis as preventive strategies.23 In (Table 2), It is discovered that whereas the ethanol extract of Nyamplung fruit shell is only moderately cytotoxic to WiDr colorectal cancer cells, the ethanol extract of Nyamplung seeds is not.
According to the polarity of the molecule to be extracted, extraction can generally be carried out using either polar solvents (water, ethanol, methanol, etc.) or non-polar solvents (petroleum ether, chloroform, etc.). This method is known as the principle of like dissolves like.17 Hexane fraction extracts contain steroid and triterpenoid compounds, which can produce almost the same cytotoxic activity as the ethyl acetate fraction. In ethanol fraction extracts, there are saponin compounds that are known to be involved in DNA replication, preventing cancer cell proliferation pathways and inhibiting cancer cell proliferation by stopping the cell cycle in the G1/S and G2/M phases.19 The most solvents from (Table 2) are ethanol as many as 14 (fourteen articles), then ethyl acetate solvents as many as 4 (four) articles, and methanol as many as 3 (three) articles.
Based on (Table 2), it was found that the most frequently used cytotoxic testing method is with 3-(4,-5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as many as 21 (twenty-one) articles, with 1 (one) article using the Cell Counting Kit-8 (CCK-8) assay method and 1 (one) article using flow cytometry analysis and quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) assay. The development of the cell cycle was determined using cytometric flow analysis after staining with propidium iodide (PI). Similar to the MTT test, various extract concentrations are applied to the cells. The fraction of cells in phases G0/G1, S, and G2/M represents the cell cycle distribution. The two stages of quantitative RT-PCR analysis are as follows: ribonucleic acid (RNA) samples are reverse transcribed into complementary deoxyribonucleic acid (cDNA), and then the qRT-PCR assay is used to analyze the results.28
Apoptosis is a highly regulated process of cell death. It takes place both during typical cell development and morphogenesis as well as as part of the cell’s response to stress and injury. Pathological cells go through necrosis, which is the death process as a result of ischemia as well as chemical, physical, and mechanical causes. A balanced process of cell division and death produces controlled cellular homeostasis and prevents aberrant proliferation. Apoptosis is preferable to necrosis because, unlike necrosis, it does not result in the surrounding cells becoming inflamed. Apoptosis, or cell death, is a closely controlled process that is initiated by either of the following two pathways: the death receptor (extrinsic) or the mitochondrial (intrinsic) pathway.28 The anti-cancer activity widely reported in this systematic review is by inhibiting cell proliferation through intrinsic pathways.
Some genes have varied patterns of expression. All biological processes, including cell death, are governed by a fundamental dogma that starts with genes. Important cell functions like apoptosis depend on the regular activation of a subset of apoptosis-related genes. BCL-2 and Bcl-2 Associated X (BAX) are two previously mentioned apoptosis-related genes. BAX is a proapoptotic effector, whereas BCL-2 is an anti-apoptosis gene (prosurvival effector). The integrity of the mitochondria’s outer membrane is regulated by genes from the BCL-2 family to govern apoptosis through the mitochondrial pathway. Complex connections that have a negative impact on the release of pro-apoptosis proteins are regulated by the interaction of the BCL-2 gene with other BCL-2 family members. Instead, BAX protein causes mitochondrial permeability by creating pores or joining with the outer mitochondrial membrane. Proteins that cause cell death are hence.28
Apoptosis also contributes to morphological changes in some images inside the cell. Chromatin condensation, nuclear fragmentation, increased cell density, and the development of cytoplasmic blebs make up the morphological picture of apoptosis. The nucleus develops an atypical shape, the nucleolar size rises, and the granules of the nucleolar enlarge and spread. DNA degradation and cell damage into fragments surrounded by a dense membrane (apoptosis body) characterize the final stage of apoptosis. Some objects of apoptosis contain fragments of the nucleus, while others contain only cytoplasm. Therefore, nuclear condensation can be used to determine the apoptosis of healthy or necrotic cells.29
IC50 or half the maximum inhibitory concentration is a measure of the effectiveness of a substance in inhibiting certain biological or biochemical functions, also defined as the concentration or amount of the drug needed to kill 50% of the cell population.15 Based on the US National Cancer Institute (NCI) crude extracts are considered to have cytotoxicity activity in vitro if they have an IC50 value of less than 20 μg/mL and less than 4 μg/mL for pure compounds.17 IC50 values of crude extracts less than 20 μg/mL have high cytotoxic activity, 21–200 μg/mL are moderate, 201–500 μg/mL are low, and more than 500 μg/mL have no cytotoxic activity.20,23
Secondary metabolite compounds in plants have many benefits for humans including as medicine. The extraction and isolation process are necessary to separate and take secondary metabolite compounds so that benefits can be obtained. The method of separation and purification of secondary metabolite compounds from plants has the purpose of looking for bioactive compounds from extracts. The goal of looking for bioactive compounds is to find the right method that can filter the source material for bioactivity such as cytotoxicity. In vitro methods are more desirable because animal experiments are expensive, require more time, and are prone to ethical controversy.33
The concentration of herbal plants is inversely proportional to the results of cell viability indicating cytotoxic cells. The average of the articles in Table 2, the higher the concentration, the lower the cell viability which indicates high cell death, indicating that it has a higher cytotoxic effect.
Limitations in this systematic review are the possibility of Indonesian herbal plants that have not been found, no information has been obtained related to the difference in the content of bioactive components from parts of Indonesian herbal plants that cause differences in the results of anti-cancer cytotoxic activity, and has not been proven the mechanism of cytotoxic activity involved from each herbal plant molecularly through in vitro examination.
Conclusion
The most widely studied Indonesian herbal plant in vitro as an anti-cancer is Annona muricata (Soursop), while the anti-cancer activity that is widely reported is by inhibiting cell proliferation through intrinsic pathways.
Acknowledgment
The authors thank Padjadjaran University for funding this review article.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Anwar R, Setiawan A, Supriatno S, Supratman U. Bioactive compounds of rasamala (altingia excelsa nornha) leaves as C-Myc proto oncogene expression suppressor of human. Dentino J Kedokt Gigi. 2018;III(2):203–210.
3. World Health Organization. Cancer in Indonesia [Internet]. The Global Cancer Observatory; 2021. Available from: https://gco.iarc.fr/today/data/factsheets/populations/360-indonesia-fact-sheets.pdf.
4. Mohammed HA, Emwas A-H, Khan RA. Salt-tolerant plants, halophytes, as renewable natural resources for cancer prevention and treatment roles of phenolics and flavonoids in immunomodulation and suppression of oxidative stress towards cancer.pdf. Int J Mol Sci. 2023;24(5171). doi:10.3390/ijms24065171
5. Chaudhary A. Natural herbs as anticancer drugs: back to the future. Chem Sci Rev Lett. 2020;9(34):481–495.
6. Anwar R, Setiawan A, Supriatno S, Supratman U. Two flavonoid compounds as antiproliferative activity against SP-C1 cancer tongue cells from the leaves of rasamala (altingia excelsa nornha). J Kim Val. 2018;4(2):75–78.
7. Anwar R, Setiawan A, Supriatno S, Supratman U. Senyawa daun rasamala (altingia excelsa nornha) sebagai penghambat proliferasi sel kanker lidah manusia in vitro [Rasamala Leaves Compound (Altingia excelsa Nornha) as an Inhibitor of Human Tongue Cancer Cell Proliferation in Vitro]. Stomatognatic - J Kedokt Gigi. 2019;16(2):42. doi:10.19184/stoma.v16i2.23090
8. Khan T, Ali M, Khan A, et al. Anticancer plants: a review of the active phytochemicals, applications in animal models, and regulatory aspects. Biomolecules. 2020;10(1):1.
9. Mohammed HA, Almahmoud SA, Arfeen M, et al. Phytochemical profiling, molecular docking, and anti-hepatocellular carcinoid bioactivity of extracts. Arab J Chem. 2022;15(7):103950. doi:10.1016/j.arabjc.2022.103950
10. Meiyanto E, Larasati YA. Correlation of serum levels of vitronectin, malondialdehyde and Hs-CRP with disease severity in coronary artery disease. Adv Pharm Bull. 2019;9(2):219–230. doi:10.15171/jcvtr.2015.24
11. Nugraha AS, Haritakun R, Lambert JM, Dillon CT, Keller PA. Alkaloids from the root of Indonesian Annona muricata L. Nat Prod Res. 2021;35(3):481–489. doi:10.1080/14786419.2019.1638380
12. Office of Health Assessment and Translation. OHAT risk of bias rating tool for human and animal studies. Natl Toxicol Progr; 2019:1–37. Available from: https://ntp.niehs.nih.gov/whatwestudy/assessments/noncancer/riskbias/index.html.
13. Fatmawati D, Suparmi S, Yusuf I. Anticancer selectivity of sirsak (Annona muricata) leaf extract on breast cancer cell lines. Bio-Site. 2018;04(2):78–83.
14. Fertilita S, Sandhika W, Suprabawati DGA. The cytotoxic activity of Annona muricata Linn Leaves Ethanolic Extract (AMEE) on T47D breast cancer cell line. Med Lab Technol J. 2020;1(1):32–39. doi:10.31964/mltj.v1i1.291
15. Suhendar U. Geographycal effect on the cytotoxic activity of annona muricata l. leaves ethanol extract against MCF-7 cancer cell. Fitofarmaka J Ilm Farm. 2018;8(2):1.
16. Ibrahim AA, Sandhika W, Budipramana VS. Uji Efektifitas Ekstrak Etanol Daun Annona Muricata Terhadap Sel Kanker Payudara Mcf-7 [The Effect of Annona Muricata’s Leaf Ethanolic Extract Against the Breast Cancer Cell Line Mcf-7]. J Manaj Kesehat Yayasan RS Dr Soetomo. 2020;6:64–72.
17. Widyanto RM, Rachmawati Putri RM, Kurniasari FN, Yunimar Y, Utomo B. Free radical scavenging and cytotoxic assay of soursop fruit juice (Annona muricata Linn.) on cervical cancer cell lines (HeLa). J Gizi Dan Diet Indones (Indonesian J Nutr Diet. 2020;7(2):51. doi:10.21927/ijnd.2019.7(2).51-57
18. Abdullah M, Desmarini D, Meilaini S, Sari P, Yunaini L, Fadilah F. The effect of ethanolic leaves extract of soursop (Annona muricata L.) on human colorectal cancer cell line: cell viability and in silico study to cyclin D1 protein. Heal Sci J Indones. 2019;10(2):96–102. doi:10.22435/hsji.v12i2.2441
19. Bhanuwati AV, Pakpahan A. Cytotoxic test of different solvents of soursop (Annona muricata) leaf extract against HSC-3 cell line. Dent J. 2022;55(3):130–136. doi:10.20473/j.djmkg.v55.i3.p130-136
20. Ramandhita AP, Hanum L. Efek Antikanker Nanopartikel Alginat Ekstrak Etanol Daun Sirsak (Annona muricata Linn) pada Kultur Sel Kanker Hepar (HepG2) [Anticancer Effects of Alginate Nanoparticles Ethanol Extract of Soursop (Annona muricata Linn) Leaves on Liver Cancer Cell Culture (HepG2)]. J Ris Kedokt. 2022;1(2):130–133.
21. Tulloh NR, Andriane Y. Sediaan Nanopartikel Alginat Ekstrak Etanol Daun Sirsak (Annona muricata Linn) Memiliki Efek Antikanker pada Kultur Sel Kanker Paru (HTB183) [Preparation of Alginate Nanoparticles Ethanol Extract of Soursop Leaves (Annona muricata Linn) Has Anticancer Effects on Lung Cancer Cell Culture (HTB183)]. J Ris Kedokt. 2022;1(2):124–129.
22. Astuti Y, Suharto A, Harimurti S, Priambodo WJ. The anti-proliferative ability of the ethanol extract of annona muricata leaves on widr cancer cells. Mutiara Med J Kedokt Dan Kesehat. 2022;22(1):38–43.
23. Fathani IJ, Miladiyah I. Cytotoxicity of ethanolic extract of fruit shells and seeds of Nyamplung (Calophyllum inophyllum L.) on WiDr colorectal cancer cells. J Kedokt Dan Kesehat Indones. 2021;12(2):166–174.
24. Mutiah R. Cytotoxic activities profile of parasite mango (Dendrophthoe pentandra) from various areas in Indonesia against T47D breast cancer cells and normal vero cell lines. J Islam Pharm. 2019;4(1):1. doi:10.18860/jip.v4i1.7726
25. Susilowati S, Anggraini TD, Kotimah N. Sitotoksisitas dan Selektivitas In Vitro Daun Benalu Cengkeh (Dendrophthoe pentandra L. Miq) terhadap Sel Kanker Serviks HeLa [In Vitro Cytotoxicity and Selectivity of Clove Benalu (Dendrophthoe pentandra L. Miq) Leaves Against Cervix Cancer Cell HeLa]. J Pharmascience. 2022;9(2):258. doi:10.20527/jps.v9i2.13514
26. Novitasari D, Handayani S, Jenie RI. Ethanolic extract of hedyotis corymbosa L. inhibits migration and MMP-9 activity on metastatic breast cancer cells. Indones J Cancer Chemoprevention. 2018;9(1):16. doi:10.14499/indonesianjcanchemoprev9iss1pp16-22
27. Supriatno S, Supratman U. Apoptosis mediated anti-proliferative activity of kaempferol and quercetine isolated from the leaves of altingia excelsa against human tongue SP-C1 cell lines. B-Dent: Jurnal Kedokteran Gigi Universitas Baiturrahmah. 2021;8(3):277–284.
28. Lestari W, Yusry WN, Iskandar SH, Ichwan SJ, Irfan NI, Suriyah WH. Gene expression of selected apoptotic markers in human oral squamous carcinoma HSC-3 cell line treated with Myrmecodia pendans plant extract. Makara J Health Res. 2019;23(2):10.
29. Wihadmadyatami H, Karnati S, Hening P, et al. Ethanolic extract Ocimum sanctum Linn. induces an apoptosis in human lung adenocarcinoma (A549) cells. Heliyon. 2019;5(11):e02772. doi:10.1016/j.heliyon.2019.e02772
30. Lutfia A, Munir E, Yurnaliza Y, Basyuni M. Chemical analysis and anticancer activity of sesterterpenoid from an endophytic fungus Hypomontagnella monticulosa Zg15SU and its host Zingiber griffithii Baker. Heliyon. 2021;7(2):e06292. doi:10.1016/j.heliyon.2021.e06292
31. Hasan AEZ, Julistiono H, Bermawie N, Riyanti EI, Arifni FR. Soursop leaves (Annona muricata L.) endophytic fungi anticancer activity against HeLa cells. Saudi J Biol Sci. 2022;29(8):103354. doi:10.1016/j.sjbs.2022.103354
32. Antony P, Vijayan R. Acetogenins from Annona muricata as potential inhibitors of antiapoptotic proteins: a molecular modeling study. Drug Des Devel Ther. 2016;10:1399–1410. doi:10.2147/DDDT.S103216
33. Julianto TS. Fitokimia Tinjauan Metabolit Sekunder dan Skrining fitokimia [Phytochemicals Review of Secondary Metabolites and Phytochemical Screening]. In: Julianto TS, editor. Jakarta Penerbit Buku Kedokteran EGC. Vol. 53,
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
