Back to Journals » OncoTargets and Therapy » Volume 13
Potential Influences of RNF6 on Prognosis and Metastasis of Colorectal Cancer: A Clinical Analysis
Received 3 September 2019
Accepted for publication 22 February 2020
Published 9 March 2020 Volume 2020:13 Pages 2031—2036
DOI https://doi.org/10.2147/OTT.S229772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
This paper has been retracted.
Huili Zhu, Chunhui Wang
Department of Gastroenterology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Chunhui Wang
Department of Gastroenterology, West China Hospital, Sichuan University, GuoXue Street 37, Chengdu 610041, People’s Republic of China
Tel +86 28-85422114
Email [email protected]
Introduction: Ring finger protein 6 (RNF6) locates on the chromatin 13q.12.13, where amplification is frequently occurred in colorectal cancer (CRC). Previous studies have reported the role of RNF6 to accelerate the progression and metastasis of CRC.
Methods: In this paper, we mainly analyzed the potential of RNF6 to predict the prognosis and metastasis of CRC. Based on the cut-off value of RNF6, enrolled CRC patients were assigned into high- and low-level group. Correlation between RNF6 level and survival of CRC patients was assessed.
Results: Our findings revealed that RNF6 was upregulated in CRC tissues. IHC staining demonstrated higher positive expression of RNF6 in CRC tissues. Nearly 61.2% CRC patients had a positive expression of RNF6. Moreover, RNF6 was closely linked to lymphovascular invasion (LV) (P=0.006), invasion depth (P=0.001), metastasis (P< 0.001) and TNM staging (P< 0.001). In CRC tissues, RNF6 level was negatively correlated to that of E-cadherin (r=− 0.7093, P< 0.0001). OS (overall survival) and RFS (recurrence-free survival) were worse in CRC patients with high-level RNF6, and tumor cell metastasis was believed to be the major reason.
Conclusion: Therefore, RNF6 was confirmed to be a hallmark predicting the prognosis and metastasis in CRC patients.
Keywords: RNF6, E-cadherin, CRC, metastasis, survival
Introduction
Globally, total cases of CRC rank the fourth in all types of malignancies.1 CRC is a fatal malignant tumor.2 The etiology and pathogenesis of CRC are complex, involving genetic variations and intestinal flora disorder.3,4 It is generally considered that unhealthy lifestyle is closely linked to the occurrence of CRC. For instance, high-fat diet would enhance the susceptibility to CRC.5 Development of CRC-associated hallmarks contributes to effective prevention of CRC.
RNF6 is RING domain E3 ubiquitin ligase belonging to RNF family.6 RNF6 locates on the chromosome 13q12.13, which is often overexpressed in CRC tissues.7 Initially, RNF6 was considered as a tumor-suppressor gene.8 Later, RNF6 is found to accelerate the progression of prostate cancer through abnormal ubiquitination of androgen receptor.9 In CRC cells, overexpression of RNF6 accelerates proliferative ability and suppresses apoptosis of tumor cells through ubiquitinating TLE3 to activate β-catenin.6 RNF6 can promote the metastasis of liver cancer cells through ubiquitination of FoxA1.10 As a result, RNF6 is now believed as an oncogene.
This paper aims to uncover the potential of RNF6 to predict the prognosis and metastasis of CRC by analyzing clinical outcomes OS and RFS. Since E-cadherin is an adhesion molecule associated with tumor metastasis, the underlying correlation between RNF6 and E-cadherin was examined as well.
Methods
CRC Patients
A total of 247 CRC patients undergoing surgery in our hospital from 2005 to 2010 were enrolled. Patients who received pre-operative treatments were excluded. Primary CRC was confirmed by two pathologists independently. TNM staging was assessed as previously reported.11 CRC tissues and paired adjacent normal tissues were collect for further analysis. This study was approved by the ethic committee of West China Hospital, Sichuan University. All patients provided written informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry (IHC)
CRC and adjacent normal tissues were immersed in 4% polyformaldehyde for 24 h. Paraffin-embedded tissues were treated with xylene, gradient concentrations of alcohol (100–70%) and reacted with antigen repair solution in a pressure cooker for 2 min. Subsequently, tissues were treated with 3% H2O2 for 20 min, 5% bovine serum albumin (BSA) for 10 min and incubated with anti-RNF6 (ab204506) and anti-E-cadherin (ab1416). On the other day, sections were dyed with diaminobenzidine (DAB), counterstained with haematoxylin and dehydrated in alcohol (100–70%). After xylene treatment, sections were sealed and observed.
RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted from frozen tissues using TRIzol (Invitrogen). After quantification by Nanodrop, 1ug of total RNA was applied for reverse transcription using High Capacity cDNA Reverse Transcriptase kit (Applied Biosystems) according to the instructions.RT‐PCR Detection Kit (Ambion) was used for RT-PCR according to the instructed protocol. Standard plasmids of RNF6 and GAPDH were made to calculate the absolute value of each gene and RNF6 expression is the ratio between RNF6 and GAPDH (RNF6 absolute value/GAPDH absolute value).
Statistical Analysis
SPSS 21.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The correlation between RNF6 and clinicopathologic parameters in CRC patients was analyzed by the Student’s t-test. Correlation between levels of RNF6 and E-cadherin was assessed by the Pearson correlation coefficient. To evaluate the statistical significance of RFS and OS, Kaplan-Meier method was introduced. Multivariate analysis with Cox regression analysis were applied for assessing potential factors influencing prognosis in CRC patients. Log-rank test was applied to evaluate the statistical correlation between the differences in survival distribution. P<0.05 considered statistically significant.
Results
Association between RNF6 mRNA expression and clinicopathologic characteristics in 247 CRC patients were analyzed and it showed that RNF6 was significantly up-regulated in CRC tissues compared to adjacent normal tissues (Figure 1A). IHC staining of the 247 paired tissues also confirmed higher positive expression of RNF6 in CRC tissues (Figure 1B). Based on the mRNA expression of RNF6, we set the mean value as the cut-off value, which defined high expression if the value is larger or equal than mean value or low expression if the value is lower than mean value. In 247 cases of CRC tissues, 127 CRC patients (51.4%) were enrolled in high-level group and 120 (48.6%) were in low-level group. Baseline characteristics of these CRC patients are listed in Table 1. The average age at the surgery was 64.7 years (32–91 years), and the male-female ratio was 140:107. The average tumor size was 5.5 cm (1.1–13.4 cm).
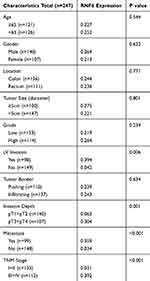 |
Table 1 Correlation Between RNF6 Expression and Clinical Characteristics in CRC Patients |
Subsequently, correlation between RNF6 level and clinicopathologic characteristics in CRC patients was determined. The data uncovered that LV (P=0.006), deep invasion depth (P=0.001), metastasis (P<0.001) and advanced TNM staging (P<0.001) were closely linked to high-level RNF6 in CRC patients.
RNF6 Was Reversely Correlated with E-Cadherin
E-cadherin is a protein closely related to tumor cell invasion and metastasis.12 To further confirm the relationship between RNF6 and tumor metastasis, potential correlation between expression levels of RNF6 and E-cadherin was investigated. By comparing the mRNA expression of RNF6 and E-cadherin of the 247 patients, we found a negative correlation between RNF6 and E-cadherin (r=−0.8116, P<0.001) (Figure 2). It is indicated that RNF6 is closely related to tumor metastasis.
 |
Figure 2 Correlation between RNF6 and E-cadherin analyzed by the Pearson’s Chi-square test. |
RNF6 Expression Was Correlated with RFS and OS
Subsequently, correlation between RNF6 level with RFS and OS in CRC patients was analyzed. Tumor recurrence during follow-up days occurred in 72 patients (29.1%). A total of 108 patients (43.7%) died during follow-up days, and 8 of them (3.2%) died of unknown reasons. There were 10 patients (4.0%) survived from CRC recurrence or metastasis. Besides, 129 patients (52.2%) survived during follow-up days without recurrence or metastasis. Kaplan-Meier analysis was applied to analyze the correlation between RNF6 and RFS and OS. Based on the categorized high and low RNF6 expression mentioned above, Kaplan-Meier curves revealed a close relationship between RNF6 level and RFS in CRC patients (P<0.001) (Figure 3A). Upregulation of RNF6 level in CRC markedly lowered RFS. The average RFS in high-level and low-level RNF6 groups was 52.8 months (95% CI=23.8–92.6) and 162.4 months (95% CI=68.5–187.4), respectively.
 |
Figure 3 Correlation between RNF6 level with RFS (A) and OS (B). |
In addition, RNF6 was linked to OS in CRC patients as well. Higher level of RNF6 predicted shorter OS (P<0.001) (Figure 3B). The average OS in high-level and low-level RNF6 groups was 64.5 months (95% CI=43.1–101.3) and 145.8 months (95% CI=89.4–193.2), respectively.
Multivariate cox regression analysis was conducted for identifying relevant factors influencing RFS and OS in CRC patients. It is found that RNF6 was an independent prognostic factor significantly associated with RFS and OS. The relative risk (RR) of tumor recurrence and OS for CRC patients with high RNF6 expression was 5.718 (2.349–10.133) and 3.448 (1.991–6.328), respectively. Other influencing factors were discovered as well, including invasive depth (P=0.015), metastasis (P<0.001) and TNM staging (P<0.001) (Table 2).
 |
Table 2 Multivariate Cox Regression Analysis on Factors Influencing Prognosis in CRC Patients |
Metastasis Is Responsible for the Death in RNF6 High Expressed Patients
To investigate whether tumor cell metastasis is responsible for the death of CRC patients with high RNF6 expression, we divided the 127 CRC patients with high-level RNF6 into two groups: patients with metastasis (84, 66.1%) and patients without metastases (43,33.9%). Kaplan-Meier curves unveiled a higher mortality in metastatic patients relative to those non-metastatic ones (p<0.001) (Figure 4). It is suggested that tumor cell metastasis is responsible for RNF6-induced OS worsen in CRC patients.
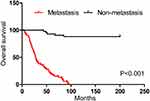 |
Figure 4 OS analysis in CRC patients with high-level RNF6. |
Discussion
Studies have shown that RNF6 amplification occurs in the early stage of tumor development and continues until the late stage. Moreover, diagnosis of RNF6 amplification in the early stage of tumor markedly elevates recurrent risks.6 However, the potential of RNF6 to be a clinical indicator for predicting the prognosis and metastasis of CRC remains to be explored. In this paper, 247 CRC patients undergoing surgery in our hospital from 2005 to 2010 were enrolled. It is well known that tumorigenesis is accompanied by a series of activated oncogenes and inactivated tumor-suppressor genes. Although RNF6 was initially considered to be a tumor-suppressor gene, later researches have uncovered its carcinogenic effect. RNF6 overexpression is able to accelerate CRC cells to proliferate and inhibits apoptosis.6,8,9 Our findings demonstrated that both mRNA and protein levels of RNF6 were upregulated in CRC tissues, suggesting its oncogenic effect.
About 90% of tumor-related death is resulted from tumor cell metastasis.13 In vitro experiments illustrated that overexpression of RNF6 accelerates CRC cells to migrate and invade.6 Moreover, RNF6 stimulates tumor metastasis and progression via inducing epithelial-mesenchymal transition (EMT), a reversible process in which epithelial cells are transformed into mesenchymal cells.12 The occurrence of ETM is often accompanied by changes in adhesion molecules between epithelial cells, including downregulation of E-cadherin and Claudin-1, and upregulation of mesenchymal markers SNAI1/2, TWIST1/2, and ZEB1/2. These changes further lead to metastatic phenotype changes in tumor cells.14–16 Our results revealed a negative correlation between expression levels of RNF6 and E-cadherin. In many epithelial-derived solid tumors, E-cadherin deficiency and acquisition of mesenchymal phenotypes are believed to be linked to poor prognosis and high rate of metastasis.17 Furthermore, RNF6 upregulation was verified to correlate to RFS and OS in CRC patients. Notably, mortality in metastatic CRC patients with high expression of RNF6 was much higher than those of non-metastatic patients, indicating that metastasis is a major reason for CRC mortality.
Conclusion
Therefore, RNF6 was confirmed to be a hallmark predicting the prognosis and metastasis in CRC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol Biomarkers Prev. 2016;25(1):16–27. doi:10.1158/1055-9965.EPI-15-0578
3. Tariq K, Ghias K. Colorectal cancer carcinogenesis: a review of mechanisms. Cancer Biol Med. 2016;13(1):120–135. doi:10.28092/j.issn.2095-3941.2015.0103
4. Wu N, Yang X, Zhang R, et al. Dysbiosis signature of fecal microbiota in colorectal cancer patients. Microb Ecol. 2013;66(2):462–470. doi:10.1007/s00248-013-0245-9
5. Zhang M, Yang XJ. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J Gastroenterol. 2016;22(40):8905–8909. doi:10.3748/wjg.v22.i40.8905
6. Liu L, Zhang Y, Wong CC, et al. RNF6 promotes colorectal cancer by activating the Wnt/beta-catenin pathway via ubiquitination of TLE3. Cancer Res. 2018;78(8):1958–1971. doi:10.1158/0008-5472.CAN-17-2683
7. Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi:10.1038/nature11252
8. Lo HS, Hu N, Gere S, et al. Identification of somatic mutations of the RNF6 gene in human esophageal squamous cell carcinoma. Cancer Res. 2002;62(15):4191–4193.
9. Xu K, Shimelis H, Linn DE, et al. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell. 2009;15(4):270–282. doi:10.1016/j.ccr.2009.02.021
10. Cai J, Xiong Q, Jiang X, Zhou S, Liu T. RNF6 facilitates metastasis and radioresistance in hepatocellular carcinoma through ubiquitination of FoxA1. Exp Cell Res. 2019;374(1):152–161. doi:10.1016/j.yexcr.2018.11.019
11. Stewart CJ, Hillery S, Plattell C. Protocol for the examination of specimens from patients with primary carcinomas of the colon and rectum. Arch Pathol Lab Med. 2009;133(9):
12. Jie D, Zhongmin Z, Guoqing L, et al. Positive expression of LSD1 and negative expression of E-cadherin correlate with metastasis and poor prognosis of colon cancer. Dig Dis Sci. 2013;58(6):1581–1589. doi:10.1007/s10620-012-2552-2
13. Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6(6):449–458. doi:10.1038/nrc1886
14. Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126(Pt 2):393–401. doi:10.1242/jcs.100115
15. Dhawan P, Singh AB, Deane NG, et al. Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J Clin Invest. 2005;115(7):1765–1776. doi:10.1172/JCI24543
16. Mani SA, Guo W, Liao MJ, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi:10.1016/j.cell.2008.03.027
17. Kim JH, Kim CN, Kang DW. Squalene epoxidase correlates E-Cadherin expression and overall survival in colorectal cancer patients: the impact on prognosis and correlation to clinicopathologic features. J Clin Med. 2019;8:5. doi:10.3390/jcm8050632
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

