Back to Journals » International Journal of Nanomedicine » Volume 18
Potential Efficacy of Herbal Medicine-Derived Carbon Dots in the Treatment of Diseases: From Mechanism to Clinic
Authors Zeng M, Wang Y, Liu M, Wei Y , Wen J, Zhang Y, Chen T, He N, Fan P, Dai X
Received 18 July 2023
Accepted for publication 24 October 2023
Published 9 November 2023 Volume 2023:18 Pages 6503—6525
DOI https://doi.org/10.2147/IJN.S431061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Mingtang Zeng,1 Yao Wang,1 Maozhu Liu,1 Yuxun Wei,2 Jie Wen,3 Yuchen Zhang,1 Tao Chen,4 Nianyu He,1 Ping Fan,1 Xinhua Dai5
1Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Department of Pharmacy, Zhongjiang County People’s Hospital, Deyang, 618000, People’s Republic of China; 3Department of Pharmacy, Shehong Municipal Hospital of Traditional Chinese Medicine, Shehong, 629600, People’s Republic of China; 4Key Laboratory of Medical Electrophysiology, Ministry of Education, School of Pharmacy of Southwest Medical University, Luzhou, 646000, People’s Republic of China; 5Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Xinhua Dai, Department of Laboratory Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Email [email protected] Ping Fan, Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Email [email protected]
Abstract: Carbon dots (CDs), a crucial component of nanomaterials, are zero-dimensional nanomaterials with carbon as the backbone structure and smaller than 10 nm. Due to their beneficial characteristics, they are widely used in biomedical fields such as biosensors, drug delivery, bio-imaging, and interactions with DNA. Interestingly, a novel type of carbon dot, generated by using herbal medicines as synthetic raw materials, has emerged as the most recent incomer in the family of CDs with the extensive growth in the number of materials selected for carbon dots synthesis. Herbal medicine-derived carbon dots (HM-CDs) have been employed in the biomedical industry, and are rapidly emerging as “modern nanomaterials” due to their unique structures and exceptional capabilities. Emerging trends suggest that their specific properties can be used in bleeding disorders, gastrointestinal disorders, inflammation-related diseases, and other common intractable diseases including cancer, menopausal syndrome, central nervous system disorders, and pain of various forms and causes. In addition, HM-CDs have been found to have organ-protective and antioxidant properties, as evidenced by extensive studies. This research provides a more comprehensive understanding of the biomedical applications of HM-CDs for the aforementioned disorders and investigates the intrinsic pharmacological activities and mechanisms of these HM-CDs to further advance their clinical applications.
Keywords: herbal medicine, carbon dots, disease treatment, nanomaterials, potential mechanism
Graphical Abstract:

Introduction
As an emerging carbon material after carbon nanotubes, graphene, fullerenes, and nano-diamonds, carbon dots (CDs) have superior properties than other nanomaterials, including ultra-fine size, favorable photoluminescence performance, low toxicity, strong biocompatibility, and excellent electron transfer ability.1,2 The bioactivity of CDs has also been investigated and discovered recently, which were used as mitochondrial oxidative stress amplifiers for targeted therapy of cancer, as well as for fluorescent biosensing and imaging through additional properties.3,4 Moreover, researchers from RMIT University stated that CDs can be utilized as a therapeutic platform to study drug delivery, distribution, metabolism, excretion, and toxicity.5 These investigations have established a solid foundation for the application of nano carbon dots in numerous research fields. However, the research on carbon dots (CDs) is mostly focused on the optimization of preparation methods and the expansion of application fields. The exploration of chemical and natural substances in synthetic raw materials also deserves considerable attention.
The use of “green” substances as raw materials is turning into a hot topic in production research as the notion of green chemistry steadily gains acceptance with societal development. For the synthesis of CDs, numerous green precursors, which have significant advantages such as abundant and renewable raw materials, free of chemical pollution as well as environmentally benign, can be employed as natural carbon sources.6 Various green carbon precursors have been studied and applied, including fruits, vegetables, and a variety of food and beverage products, to achieve the synthesis of materials with excellent properties, high cost-effectiveness, economic, and environmental protection.7 Herbal medicine (HM), one of these green precursors, has gained substantial attention due to its distinctive medical efficacy. Numerous clinical studies have demonstrated that herbal medicine has shown exceptional efficacy on various specific diseases such as SARS and COVID-19.8 However, the medicinal efficacy of herbal medicines is failing to be adequately expressed due to their complex composition. As a result, researchers have initiated attempts to prepare various nanoscale substances of herbal medicines. Interestingly, as one of the most distinctive drugs in clinical applications, herbal medicines can be used as raw materials under elevated temperature conditions to produce novel nanomaterials, which are called herbal medicine-derived carbon dots (HM-CDs), with a diameter of less than 10 nm.
Compared to general carbon dots, HM-CDs are synthesized using the medicinal components of herbs, thereby retaining the medicinal value and biological activities of the herbs. HM-CDs may contain active ingredients and chemical substances derived from herbs, such as polysaccharides, phenolic compounds, and alkaloids.9,10 These components confer specific medicinal properties and biological activities to HM-CDs. Due to the preservation of medicinal value and biological activities of herbs, HM-CDs find wider applications in areas such as herbal extraction, drug delivery, and targeted therapy.11 The structural characteristics, physicochemical qualities, and biological functions of HM-CDs vary amongst different herbal medicines. At present, multiple pharmacological experiments have demonstrated the biological effect of carbon dots in herbal medicines, which revealed the pharmacodynamic basis of herbal medicines in various diseases from a fresh perspective. The purpose of this paper is to exhibit a comparative summary of the synthesis strategies and the main properties of HM-CDs. Special attention is also given to the latest trends in the management of multiple human diseases (including bleeding disorders, inflammatory diseases, cancer, pain of various forms and causes, gastrointestinal disorders, etc.) based on HM-CDs. The intrinsic pharmacological activities and mechanisms of these HM-CDs are also discussed to further advance their clinical applications.
Synthesis Strategy of HM-CDs
To date, numerous techniques have been developed for the synthesis of CDs, such as pyrolysis, microwave-mediated synthesis, chemical oxidation, and hydrothermal treatment.12 For the preparation of different types of HM-CDs, each of these methods has distinct properties in terms of particle size, quantum yield, and pharmacological activity (Table 1). However, most of these methods are not environmentally friendly, which require copious volumes of strong acids, harsh synthesis conditions, and complex processes, making large-scale manufacturing challenging. From the perspective of environmental protection, this raises concerns about the toxicity and environmental impact of CDs, necessitating the urgent need for green, eco-friendly, and straightforward synthesis processes.13 The hydrothermal and calcination methods, which are the most commonly used methods for the preparation of HM-CDs, have the advantages of inexpensive instrumentation, environmental friendliness, and ease of operation, and will be a crucial strategy for further research on the bioactivity of HM-CDs.
 |
Table 1 The Comparative Summary of Synthesis Strategies for HM-CDs |
Hydrothermal Method
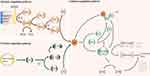
Hydrothermal synthesis is environmentally friendly without the addition of organic solvents.14 The surface of CDs does not require additional passivation to maximize safety and minimize toxicity. Prior to preparation, the dried herbs are cut into tiny pieces or ground into a powder in purified water. After the ultrasonic treatment, the mixture is transferred to a PTFE-lined stainless steel autoclave and heated at a specific temperature. To purify the CDs, the suspension needed to be further filtered through a 0.22 μm cellulose membrane and dialyzed for several days using a dialysis bag (Figure 1A).
 |
Figure 1 Preparation methods of HM-CDs. (A) hydrothermal synthesis process; (B) pyrolysis synthesis process; (C) microwave carbonization synthesis process. |
The reaction temperature would affect the properties of HM-CDs. The hydrothermal reaction temperature is generally 100–200°C.15–17 Li et al synthesized nitrogen-doped CDs of ginkgo fruits (H-N-CDs) at different temperatures.18 These CDs had the best fluorescence intensity and maximum quantum yield (QY) when the temperature was set to 200°C. In a separate study, the corresponding carbon dots were prepared based on Mentha haplocalyx Briq.19 When the temperature was below 120°C, electron microscopy revealed a significant amount of polymers, suggesting that the carbonization was incomplete and the CDs were difficult to form. However, no polymers were found by electron microscopy when the temperature reached 180°C, indicating that all compounds were carbonized. This phenomenon has also been observed in coix seed-CDs. The fluorescence intensity of coix seed-CDs decreased with the increase in temperature.20 In addition, Li et al used Salvia miltiorrhiza Bunge as a carbon source and synthesized three CDs (CDs-100, CDs-150, and CDs-180) by hydrothermal method at different temperatures (100, 150, and 180°C) for 6 h.21 The average diameters were 16.94, 1.53, and 2.03 nm, respectively.
Similarly, the reaction time affects the performance of the CDs. The QY of CDs generated from orange peel (Citrus reticulata Blanco.) at different time points at the same temperature decreases with increasing reaction time, while the particle size increases slightly.22,23 In another study, honey-CDs prepared by hydrothermal heating at 100°C for 2 h were only stable at 4°C for 3 months.24 The difference in fluorescence intensity of honey-CDs ceased to be significant when the synthesis time increased to 12 or even 16 hours, suggesting that the fluorescence intensity may have reached saturation. This phenomenon was also observed in aloe vera (Aloe L.) CDs, where the fluorescence intensity progressively increased with reaction time up to 11 h but decreased afterward.25
Therefore, the synthesis of CDs by the hydrothermal method needs to be adequately considered and validated with respect to the reaction temperature and time. The hydrothermal temperature should preferably be higher than 100°C. The required time can be determined by the color shift in the precursor solution, which is commonly yellow, orange, or brown.
Pyrolysis Method
High-temperature pyrolysis is a typical process in addition to hydrothermal synthesis. Natural organic materials are gradually transformed into CDs under vacuum or inert gas by high-temperature processes such as heating, dehydration, degradation, and carbonization.26 The process is easy to operate, solvent-free, inexpensive, and appropriate for mass manufacturing.27 The herbs are first placed in a crucible and heated at a specific temperature in a muffle furnace until they are charred. The charred medicine is then crushed and boiled in ultrapure water, and the upper liquid layer is collected. The solution was filtered through a 0.22 μm microporous membrane and dialyzed for several days using dialysis bags to purify the CDs (Figure 1B).
Carbonization is one of the main elements that affect the success rate of the preparation process through high-temperature calcination. There exist two main traditional methods of carbonation: carbonizing by stir-frying and carbonizing by calcining (also known as wok-covering calcining). Both techniques are applicable to drugs in general. Carbonizing by stir-frying means heating the drug in a preheated vessel over high or moderate heat until the drug turns reddish-brown inside and burns black on the outside, mainly used for root drugs such as carbonized rhubarb, carbonized ginger, and carbonized cortical peony. Carbonization by calcination implies heating and carbonizing the drug under high temperature and anoxic conditions. It is appropriate for loose or light medications that can be easily carbonized (such as Juncus efsus, Radix Rehmanniae, and Nodus Nelumbinis Rhizomatis).28 Unfortunately, the limitations of these conventional charring techniques make it challenging to regulate the charring of charcoal-based pharmaceuticals. For example, (i) non-uniform heating can lead to ashing, carbonization, or raw blanks; (ii) it is difficult to control the duration and degree of heating for light-weight drugs, resulting in excessive waste rate; (iii) the root drug is not dry inside, so it cannot be entirely charred; (iv) the operation is cumbersome, time-consuming, and smoke-filled. It is worth noting that the two most popular instrumental means for the production of HM-CDs are calcination in a muffle furnace and carbonization in a drying oven. This type of carbonization can solve the problem of uncontrollable temperature and time.
Compared to hydrothermal synthesis, high-temperature pyrolysis generally requires higher reaction temperatures (300°C-400°C) and shorter heating times. The optimal pyrolysis temperature varies when different carbon sources are used to synthesize CDs. Dager et al prepared fennel seed-CDs at 500°C for 3 h, which is the maximum calcination temperature recorded in the current study.29 These fennel seed-CDs with excellent properties can be preserved for up to 15 months. In contrast, the present HM-CDs made by high-temperature pyrolysis have heating temperatures as low as 220°C. At this temperature, Blue-light CDs were prepared using watermelon peel as the carbon source.30
However, the reality is that the reaction mechanism for the synthesis of HM-CDs is extremely complex and is affected by various factors such as the temperature, time, and pH of the reaction system. Zhang et al prepared PCC-CDs based on Phellodendri Chinensis Cortex (PCC) under different conditions. PCC-CDs produced at 400°C were a novel carbon-based nanomaterial with exceptional bioactivity, and their antipsoriatic activity was superior to those prepared at alternative temperatures (325°C and 475°C).31 Another study used pyrolysis to prepare Zingiberis rhizoma-based carbon dots (ZR-CDs) and examined their analgesic activity when carbonized at different temperatures (300, 350, and 400°C) for 1 h or at 350°C for varying periods of time (0.5, 1, and 1.5 h). Ultimately, ZR-CDs were found to have the optimum outcome when prepared at 350°C for 1 h.32 One hypothesis suggests that the multiple properties of CDs, such as rich chemical groups, size, and solubility, play an important role in their biological applications,33,34 which would lead to small or significant changes in the physicochemical properties of PCC-CDs prepared at different temperatures, resulting in large changes in the biological activity of the obtained CDs.
Alternative Methods
In view of the drawbacks of the aforementioned techniques, two novel carbonization techniques have been developed: heating with sand and microwave carbonization (Figure 1C). Due to its excellent thermal conductivity, highly heated sand prevents inhomogeneous heating of the drug, quickly achieves the desired energy, and is low-cost and simple to manufacture. The majority of charcoal-based medicines, including Nodus Nelumbinis Rhizomatis, Sanguisorba officinalis, and Fructus Crataegi, can be prepared using this approach, while light, friable, or non-separable medicines are not applicable. Yet, the idea behind microwave carbonization is to use energy transmission to cause the breaking of chemical bonds.35 The response time is drastically decreased and preparation efficacy is increased because of its simpler operation.36–38 This method has the advantages of high processing accuracy, minimum contamination, and a wide range of applications for light-textured charcoal herbs. Moreover, a substitute for traditional hydrothermal synthesis has been reported: microwave-assisted hydrothermal synthesis.39 Li et al prepared two ginkgo fruit-CDs (H-CDs/M-CDs) by hydrothermal (H) and microwave methods (M), respectively.18 The time required for M-CDs was 5–15 min, which was significantly shorter than that of the hydrothermal method and the particle size was relatively smaller. However, the performance of H-CDs is significantly superior to that of M-CDs. This is due, in part, to the more regular and homogeneous morphology of H-CDs, as well as to the fact that their quantum yields and lifetimes are larger than those of M-CDs and their fluorescence intensity is higher. Interestingly, the microwave technique can even prepare orange peel-CDs in 1 min with a yield of up to 16.20%.40 In terms of reflection time and efficiency, the microwave synthesis method is undoubtedly superior to hydrothermal and pyrolysis methods, and its time-saving, inexpensive, and easy-to-operate features are particularly attractive for environmentally friendly synthesis of HM-CDs from renewable herbs. Currently, more and more HM-CDs are being prepared by applying microwave carbonization (Table 2), but it is still far from being a completely developed technique.
 |
Table 2 HM-CDs Synthesized by Microwave Carbonization |
Another technique for creating HM-CDs, in addition to the ones mentioned above, is heating extraction using different solvents.47,48 For example, Wang et al prepared ethanol-papaya CDs (E-CDs) using 90% ethanol.49 Although the size of the E-CDs increased with the amount of organic macromolecules in 90% ethanol, alternative solvents may produce the best preparation of CDs when corresponding to various herbal species. Sugarcane (Saccharum sinensis Roxb.) has also been employed as a carbon source for the synthesis of herbal CDs via the solvothermal method.50,51 The use of organic solvents facilitated the carbonization process during the synthesis of CDs, which significantly altered the photophysical characteristics of the carbon nanoparticles. In another study, environmentally friendly CDs of the Codonopsis pilosula were prepared at room temperature using a one-step solvothermal method.52 The obtained codonopsis pilosula-derived CDs (CP-CDs) exhibited excellent fluorescence properties (QY up to 12.8%) and strong photostability without any passivation or functionalization on the CP-CDs surface.
Therefore, the preparation methods for HM-CDs are diverse, sharing similarities with general carbon dot synthesis methods. The common feature of these methods is that they mainly control the carbon source and reaction conditions to achieve the preparation of carbon dots. However, the distinctive aspect lies in the incorporation of herbal materials as the carbon source in the preparation of HM-CDs. Herbal materials contain abundant organic substances, such as polysaccharides, proteins, and polyphenols, which can be decomposed into carbon dots at high temperatures. The preparation methods of HM-CDs also consider the characteristics and medicinal effects of herbal materials. This includes selecting appropriate extraction methods, solvents, and reaction conditions to retain the effective components of herbal materials and convert them into carbon dots. Furthermore, the preparation methods of HM-CDs can be combined with traditional herbal processing techniques, such as decocting and frying, to further regulate the morphology and properties of carbon dots. These special preparation methods can endow HM-CDs with enhanced biocompatibility and drug release performance, making them suitable for applications in the field of biomedicine.
Overall, hydrothermal and pyrolysis technologies are the most popular methods for producing HM-CDs due to their practicality, economy, simplicity of usage, and environmental friendliness. However, the synthesis of HM-CDs by the hydrothermal method is normally considered as a time-consuming process. Although microwave-assisted methods are not as frequently used as hydrothermal and pyrolysis methods, their time-saving, low-cost, easy-to-operate, and efficient features are ideal for the synthesis of HM-CDs.
Main Factors Regulating the Pharmacological Activity of HM-CDs
Particle Size
It is well known that nanoscale HM-CDs have received considerable attention worldwide. The bulk of HM-CDs have an average particle size of less than 10 nm,53 while the average diameter of the smallest one is 1.12 nm.54 Pyrolysis can produce lower particle sizes, despite the fact that hydrothermal synthesis is more effective at achieving narrow particle size distributions of CDs.55 The particle size of HM-CDs prepared by pyrolysis was approximately 5 nm under the current synthesis conditions, which seems to represent no discernible difference in the particle sizes of HM-CDs synthesized by the two methods and merits additional exploration. Wang et al isolated a novel carbon dots (PT-CDs) derived from Pollen Typhae by pyrolysis to ameliorate acute kidney injury.56 The particle size of PT-CDs prepared at different temperatures of 250, 300, 350, and 400°C was less than 20 nm, and the average particle size tended to increase and then decrease, with the lowest value at 400°C (4.85 ± 2.06 nm). The BUN index (250°C, 32.46 ± 2.93 mmol/L; 300°C, 31.98 ± 3.29 mmol/L; 350°C, 31.13 ± 3.11 mmol/L; and 400°C, 31.05 ± 2.70 mmol/L) and CRE level (250°C, 241.95 ± 21.56 μmol/L; 300°C, 242.85 ± 18.79 μmol/L; 350°C, 231.75 ± 19.58 μmol/L; and 400°C, 223.42 ± 17.90 μmol/L) of rats decreased with the increase in preparation temperature after PT-CDs prepared under various conditions were used for treatment, and the best anti-AKI effect was observed at 400°C, according to the results of the renal function evaluation. It is thus speculated that particle size is also one of the elements that regulate the pharmacological activity of HM-CDs.
Nanoscale HM-CDs significantly improved membrane permeability and exerted a greater effect than herbal medicines (Figure 2). Pn-CDs can cross the blood-brain barrier (BBB), which may be due to the ultra-tiny size of Pn-CDs, the abundance of functional groups on the surface, and the strong affinity for the BBB endothelial cell membrane.17 Drugs are also covalently attached to CDs, which facilitates carrier-mediated macromolecular transport. These features enable CDs to enhance BBB permeability through passive transport.57 Ashrafizadeh et al summarized innovative drug delivery systems using functionalized CDs as carriers for the treatment of various neurological diseases.58 However, the costly modified ligands constrain their wide range of applications. Although some herbal drugs have been utilized for a long time for the treatment of neurological diseases,59 the BBB hinders the infiltration of herbal macromolecules. HM-CDs can enhance the BBB permeability of certain macromolecules under non-functionalized conditions. Therefore, this strategy has the potential to be a current breakthrough for herbal medicines to overcome biological barriers.
Quantum Yield
The quantum yield (QY) of pyrolytic synthesis was discovered to be lower than that of hydrothermal synthesis as a result of the diversity of carbon sources. Most CDs synthesized by pyrolysis had an average QY of less than 10%.53 However, two investigations produced different results for the synthesis of Schizonepetae Herba Carbonisata-CDs (SHC-CDs) under the same circumstances. One of the SHC-CDs had an average particle size of 0.8–4.0 nm and a QY of 2.26%,60 whereas those from the another had an average particle size of 1.29–6.87 nm and a QY of 6.31%.12 These findings illustrate the instability of the method. Nevertheless, Zhang et al created hair CDs with a higher QY (86.06%), which was significantly greater than that of citric acid CDs (19.73%), by combining pyrolysis and microwave.61 The fusion of the two synthetic techniques could offer potential benefits in addition to the differences in carbon sources. They also produced skin CDs with higher QY (51.35%), indicating that protein-rich materials are more suitable as precursors for CDs preparation.23 Thus, animal-derived herbs may be the most promising high-yielding drugs for future synthetic of CDs.
The carbon dots originating from the same part of different herbs have different properties. Researchers developed CDs from 14 different orange peels under the same preparation conditions with significant differences in QY,62 possibly related to the volatile oil content. In addition, there are differences in the properties of CDs extracted from different parts of the same herb. For instance, Jiang et al prepared ginkgo leaves-CDs with a high QY (22.80%) using a hydrothermal synthesis technique.18 However, ginkgo fruit-CDs had a QY of only 3.33%. Evidence suggests that herbs from various portions of the same plant produce distinct HM-CDs, presumably due to compositional variations.
Characterization Techniques for Herbal Medicine-Derived Carbon Dots
As a novel constituent of the “nanoparticle universe”, HM-CDs have garnered significant attention, prompting extensive investigations into their inherent characteristics using diverse analytical techniques. Distinct spectroscopic methodologies, such as FTIR and UV-Vis spectroscopy, have been judiciously employed to probe the nuanced attributes of herbal CDs. Furthermore, the crystal structure, elemental composition, morphology, and sundry properties of CDs extracted from natural products have been meticulously elucidated via electron microscopy, zeta potential analysis, and X-ray techniques.
Spectrographic Techniques
Spectroscopic techniques such as UV-Vis absorption spectroscopy, fluorescence spectroscopy, and Raman spectroscopy are employed to analyze the optical properties and electronic structure of HM-CDs. Interestingly, UV-Vis spectroscopy is commonly recommended to evaluate the optical properties of HM-CDs as they typically exhibit strong UV absorption, although the absorption peaks may vary. High-performance liquid chromatography and gel electrophoresis are utilized to separate HM-CDs, allowing for the isolation of CDs with different sizes and shapes. It has been confirmed that CDs with sizes of 1.2, 1.5–3, and 3.8 nm emit light in the visible (400–700 nm), UV (350 nm), and near-infrared (NIR) regions, respectively.63 Therefore, the absorption band peak centered around the UV region of 250–300 nm is often referred to as the typical π-π* transition peak in most CDs. For instance, Lycii fructus CDs synthesized through hydrothermal treatment exhibit a strong absorption peak at 271 nm in the UV region.64 CDs derived from Borassus flabellifer flower via thermal decomposition exhibit a UV absorption peak at 282 nm, which is attributed to the π-π* transition of aromatic C=C bonds.65 Moreover, the surface of HM-CDs is typically composed of various functional groups, such as hydroxyl, carboxyl, carbonyl, ether, or epoxy groups, depending on the synthesis techniques used.63 Fourier-transform infrared spectroscopy (FTIR) can be utilized to determine the surface functional groups of HM-CDs.66 For instance, the peaks appearing in FTIR spectra of CDs prepared by ultrasonication irradiation of crab shells were at 3398 cm−1, 2930 cm−1, 1640 cm−1, 1563 cm−1 and 1415 cm−1, which correspond to the stretching vibration of -H stretching, N-H stretching, C-H stretching, C=O stretching, N-H bending and C=C stretching.67 The advantage of FTIR in characterizing the surface functionalization of carbon dots lies in its affordability, ease of sample preparation, and rapidity.
Electron Microscopy Techniques
Electron microscopy techniques play a crucial role in the characterization of nanoparticles. Researchers have widely employed scanning electron microscopy (SEM) and transmission electron microscopy (TEM) to visualize HM-CDs and gain insights into their morphology, size, and formation mechanism.68 SEM involves scanning the surface of HM-CDs with a focused electron beam to generate images. However, since the particle size of HM-CDs is typically smaller than 10 nm, TEM, which utilizes high-energy electron beams to obtain images through the herbal CD samples, offers higher resolution and is more suitable for identifying small-sized particles compared to SEM.69 For instance, Dager et al utilized TEM to determine the size of HM-CDs synthesized from microwave irradiation of Fenugreek seeds, revealing an average diameter of 4.25 ± 0.56 nm.70 Moreover, high-resolution transmission electron microscopy (HRTEM) has proven effective in the structural analysis and detection of lattice defects in HM-CDs.68 HRTEM analysis demonstrated that green CDs derived from tomatoes exhibit a spherical shape, with a size distribution ranging from 5 to 10 nm.71
X-Ray Techniques
X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) are valuable techniques for analyzing the crystal structure and elemental composition of HM-CDs. XRD, as an important structural tool, is commonly used for effective characterization of CDs as it provides crucial information about their size and purity.63 The obtained XRD patterns are unique and serve as fingerprints of the periodic atomic arrangement, which can be determined by analyzing the distribution of atoms within the lattice.72 For instance, XRD analysis of CDs isolated from Actinidia deliciosa (kiwi) fruit extract through hydrothermal treatment revealed strong broad peaks around 2θ = 28.5° and a weak peak at 2θ = 40.3°, which can be attributed to the diffraction patterns of graphite carbon (002) and (001).73 The crystal size can then be calculated using the Scherer formula (D = kλ/βcos) by selecting the highest peak displayed in the XRD pattern. However, it is important to note that XRD is not suitable for characterizing amorphous CDs, as it is primarily used for determining key features of CDs with a crystalline structure.
Zeta Potential
Zeta potential, an essential measurement for evaluating the effective surface charge and quantifying the charge of nanoparticles, plays a crucial role in analyzing the stability of colloidal systems and the surface effects of nanoparticles. This measurement method is particularly important in assessing the toxicity of nanoparticles and their initial absorption by cell membranes.74 The magnitude of the zeta potential provides valuable insights into the electrical stability of the colloidal system. Research has shown that higher values of zeta potential indicate system stability, while the positive or negative sign of the zeta potential represents the surface charge of the nanoparticles. Nanoparticles with low zeta potential values tend to aggregate together.75 In a study conducted by the Ramanan group, carbon dots (CDs) were synthesized from algal blooms using microwave irradiation. The researchers successfully obtained highly negative zeta potential values (−22.3±8.39 mV), indicating that the synthesized CDs are negatively charged and rich in carboxyl functional groups.76 Thus, the measurement of zeta potential provides valuable insights into the stability and aggregation of HM-CDs.
Therefore, the characterization of HM-CDs is essential for a deeper understanding of their distinctive properties and behavior. Through analysis of the structural characteristics, one can elucidate their optical, electronic, and chemical properties. The optical properties and surface characteristics of HM-CDs play a crucial role in determining their efficacy in biological systems. Moreover, the morphology and size information of HM-CDs hold significant importance in comprehending their dispersibility and stability in herbal formulations.
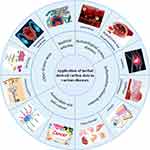
Application of Herbal Medicine-Derived Carbon Dots in Various Diseases
Existing CDs typically can only be used to cure diseases by loading pharmacophores or as drug delivery vehicles, requiring expensive chemical materials and sophisticated modification techniques. Interestingly, the ability of herbal medicines as precursors to overcome these limitations through their specific efficacy has naturally attracted the attention of researchers. The medical therapeutic effects of HM-CDs and their specific functional mechanisms are mainly reflected in the following aspects (Figure 3).
Hematological System
Hemostasis
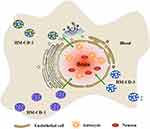
For the treatment of hemorrhagic disorders, the carbonization of herbal medicines has a lengthy history and a wealth of clinical evidence. Through the study of CDs in herbal medicines such as Schizonepetae Spica Carbonisata,12 Cirsii Japonici Herba Carbonisata,77 and Pollen Typhae Carbonisata,78 it was found that CDs were present in most herbal medicines with low toxicity, excellent water-solubility, and biocompatibility. Most extracted pure CDs showed positive hemostatic effects (Table 3). However, hemostasis is a complex system involving the interaction of endothelial cells, platelets, coagulation, and fibrinolytic systems (Figure 4). Routinely used coagulation parameters include activated partial thromboplastin time (APTT), prothrombin time (PT), thrombin time (TT), and fibrinogen (FIB). The PT value is related to the overall efficiency of the extrinsic coagulation pathway, while the APTT is tied to the intrinsic coagulation pathway. The common coagulation pathway and the activities that promote the conversion of FIB to fibrin in plasma are associated with TT and FIB levels.
 |
Table 3 HM-CDs as Potential Therapeutic Agents for Hematological Disorders |
Extrinsic Coagulation Pathway and Activation of the FIB System
Cirsium Setosum Carbonisata (CSC) has cooling, pain-relieving, and heat-clearing properties in traditional medicine. CSC-CDs synthesized based on CSC exhibited moderate hemostatic activity in a mouse model of tail amputation and liver scratch. The hemostatic effect of CSC-CDs may be related to the stimulation of extrinsic coagulation activity and activation of the FIB system, according to the researchers who evaluated coagulation parameters in mice and observed that mice treated with the CSC-CDs group had lower PT values and higher FIB values.79 Another study created novel water-soluble CDs using Schizonepetae Herba Carbonisata (SHC) as the sole precursor, and pharmacodynamic experiments revealed that SHC-CDs significantly inhibited hemorrhaging in a rat model of tail amputation and liver scratch.60 Based on the assessment of coagulation parameters in mice, these effects may be related to extrinsic coagulation activity and activation of the FIB system. Junci Medulla Carbonisata (JMC), a herbal medicine for the treatment of bleeding disorders, has not yet been identified as to its potential bioactive components or its mechanisms of action. Cheng’s group first identified novel CDs (JMC-CDs) in JMC and explored the hemostatic mechanism of JMC-CDs by measuring coagulation parameters in rats.80 JMC-CDs exhibited excellent hemostatic effects through extrinsic coagulation pathways and activation of the FIB system.
Intrinsic Coagulation Pathway and Activation of the FIB System
Ptollen Typhae Carbonisata (PTC) has been used as a hemostatic drug. To investigate its hemostatic pharmacological effects and mechanism, Yan et al identified and isolated novel water-soluble CDs (PTC-CDs) from the aqueous solution of PTC.78 The mouse model of tail amputation and liver scratch demonstrated that PTC-CDs exerted hemostatic effects by stimulating intrinsic coagulation pathways and activating the FIB system. After therapy, the high (15.05 s) and low (16.25 s) dose of PTC-CDs decreased APTT significantly (P < 0.01). The PTC-CDs (high, medium, and low concentration) and hemocoagulase groups showed a significant increase (P < 0.01) in the FIB (2.35, 2.30, 2.18, and 2.35 g/L, respectively) compared with that of the control group (2.08 g/L). Meanwhile, all doses of PTC-CDs and hemocoagulase increased PLT significantly to 1201, 1137, 1140, and 1040 × 109/L, which is in agreement with the results of the bleeding times. Zhao et al extracted a novel chemical from Egg yolk oil (EYO) and obtained EYO-CDs through pyrolysis in another study.81 EYO alone has no hemostatic effect and is regularly used in clinics to treat both acute and chronic eczema, as well as a variety of burns. Nonetheless, the experimental results point to the stimulation and activation of the intrinsic coagulation and FIB systems as the primary hemostatic mechanisms of the novel drug EYO-CDs. The reason for this phenomenon may be the enhanced absorbency and astringency of the HM-CDs generated after the carbonization of herbal medicines. Numerous loose pores are generated in their structure, which can cause accelerated hemostasis by physical adsorption. In addition, due to the peculiar structure of the carbon surface, it can activate plasma clotting factors and split platelets, releasing platelet factors to promote clotting.
Intrinsic and Extrinsic Coagulation Pathways
To compare the pharmacodynamic basis of plant and animal materials, PHT-CDs, OJT-CDs, DJT-CDs, and XYT-CDs (prepared from the dried pollen of T. angustifolia L., dry rhizome node of N. nucifera Gaertn., dry rhizome node of Cirsium japonicum Fisch. ex DC., and healthy human hair) were investigated for their hemostatic and anti-inflammatory activities in the pre-trauma phase.82 The four CDs were found to exhibit similar hemostatic effects and mechanisms. By enhancing the activity of pertinent coagulation components in the plasma via extrinsic coagulation routes, the different concentrations of CDs can drastically reduce the PT values. Meanwhile, different concentrations of CDs also shortened the APTT values, indicating that CDs can also affect the coagulation factors to transform the blood into a hypercoagulable state via the intrinsic coagulation pathway. This study serves as a reference for the development of current hemostatic materials and hemostatic drugs. Notably, the anti-inflammatory effect of XYT-CD prepared from human hair was stronger than the remaining three CDs.
Common Coagulation Pathway and Activation of the FIB System
Although Cirsii Japonici’s (CJ) hemostatic activity is obvious, its active ingredients and underlying mechanisms are yet unknown. Wang et al synthesized novel Cirsii Japonici-derived CDs (CJ-CDs) and evaluated their pharmacological activity and coagulation parameters in rats.77 The findings demonstrated that CJ-CDs dramatically reduced bleeding in mice caused by liver scratch or tail amputation, and suggested that the hemostatic effect may involve the common coagulation pathway, the FIB system. Similarly, scholars identified the presence of a different substance, Phellodendri Cortex-derived carbon dots (PCC-CDs), from the aqueous extract of Phellodendri Cortex and investigated the hemostatic activity of PCC-CDs by mouse models of tail amputation and liver scratch.83 The PCC-CDs group-treated mice exhibited satisfactory hemostatic effects (comparable to hemostatic agents), and for the first time, the hemostatic mechanism was found to be through the activation of the FIB system, thus exerting hemostatic efficacy. Acute trauma hemorrhage may benefit from synthetic PCC-CDs due to their outstanding stability, suitability for long-term storage, and potential as a complementary and alternative therapy.
Other
In addition to the aforementioned coagulation pathways, CDs can increase drug solubility, thereby facilitating drug absorption to indirectly improve the hemostatic effect. For instance, CDs in charcoal-based medications can increase the solubility of glycosides in water by affecting glycosidic acid. Luo et al investigated the effect of novel water-soluble CDs on baicalin, the main component of Radix Puerariae Carbonisata (RPC), and discovered that pure CDs considerably increased the solubility of baicalin in water.84 The oral bioavailability of RPC-CD was confirmed to be 1.7 times higher than that of pure baicalin. Furthermore, baicalin in Scutellaria baicalensis undergoes carbonization to become easily absorbed charcoal baicalin, which has a potent hemostatic effect. CDs obtained by high-temperature charring are among the key substances that play the role of hemostats and can be directly applied in the treatment of hemorrhagic symptoms of blood fever. It can also promote the absorption of glycosides and indirectly enhance the hemostatic effect.
Tail amputation and liver scratch models are common tools to study the hemostatic activity of drugs. However, Sun et al prepared Schizonepetae Spica Carbonisata (SSC)-derived CDs using an improved pyrolysis method and noted that the original SSC-CDs exhibited favorable hemostatic properties via PLT enhancement. More importantly, this is the first evaluation of the hemostatic bioactivity of SSC using the Deinagkistrodon acutus (D. acutus) venom model.12
Scholars have investigated the pharmacodynamic basis of the hemostatic effect of charcoal-based drugs by introducing transdisciplinary characterization techniques to herbal medicines. It was found that CDs, present in numerous herbal medicines, hold specific structural characteristics, physicochemical properties, and biological activities. These CDs are derived from a variety of natural products with different biological activities and deserve further investigation.
Hypoglycemic and Blood Enrichment
In addition to hemostasis, HM-CDs are also suitable for additional hematological diseases. Sun et al developed Jiaosanxian-derived CDs (JSX-CDs) with an average diameter of 4.4–6.4 nm by pyrolysis. JSX-CDs have a large number of surface groups,85 which contributes to their strong solubility and biological activity. The pharmacodynamic findings indicated that JSX-CDs, a promising new type of hypoglycemic agents, have excellent hypoglycemic efficacy and safe hypoglycemic activity. For hemopoietic effects, Xu et al successfully prepared Jujube-CDs (J-CDs) with excellent anemia therapeutic effects.86 In both in vitro and in vivo experiments, the synthesized J-CDs were able to promote the self-renewal of erythroid progenitor cells. They also specifically increased the proliferation of erythroid cells by modulating the hypoxia response pathway and increasing the phosphorylation levels of STAT5. Therefore, they have great potential as therapeutic agents for cancer-related anemia.
Bacterial Infection
Infections caused by fungi, bacteria, parasites, or viruses can cause numerous serious diseases. The identification and inactivation of several bacterial species in photosensitizers (PS) has been done using CDs as a possible fluorescent nanomaterial.87,88 HM-CDs also exhibit powerful photodynamic effects due to their optical properties and have been utilized to destroy bacteria under visible light irradiation.89 Yoon et al prepared mushroom CDs (MCDs) with intense blue fluorescence under the excitation of 360 nm UV light.90 Under LED visible light illumination, MCDs can produce ROS (such as OH- and O2-) that can adhere directly to the surface of Escherichia coli (E. coli) and induce cell membrane damage. Lin et al prepared four fluorescent CDs using various herbs (onion, ginger, garlic) and additional natural products (fish) as carbon sources.91 Onion CDs (O-CDs) demonstrated the strongest antibacterial efficacy against Pseudomonas fragilis of all of them. Persistent endodontic infections (PEIs) associated with Enterococcus faecalis (E. faecalis) biofilms are one of the most common dental lesions, and a study was conducted to prepare Fucoidan (FD)-derived CDs for the treatment of PEIs. By causing the development of both intracellular and extracellular reactive oxygen species and modifying the permeability of the bacteria, in vitro tests have shown that FD-CDs have a favorable inhibitory impact on Enterococcus faecalis and its biofilms. Importantly, FD-CDs penetrated root canals and dentin tubules, and removed E. faecalis biofilms, which has great potential for the treatment of PEIs.15 In addition, some of the HM-CDs alone could not significantly inhibit bacterial growth. However, as drug delivery systems, when loaded with herbal monomers that likewise failed to appreciably reduce bacterial growth, they demonstrated remarkable dose-dependent antibacterial activity against Gram-negative E. coli and Gram-positive S. aureus pathogens.45
Inflammation-Related Diseases
HM-CDs have also gained extensive research attention in the treatment of inflammatory diseases due to their distinctive advantages, such as great biocompatibility, photostability, and inherent targeting of functional groups. Wang et al synthesized a novel Mulberry Silkworm Cocoon-CDs (MSC- CDs) based on MSC.92 To assess the anti-inflammatory bioactivity of MSC-CDs, the authors of this work creatively applied three conventional experimental models of inflammation. The results showed that MSC-CDs possess significant anti-inflammatory activity, which may be related to the inhibition of inflammatory factors IL-6 and TNF-α expression, providing a reference for further investigation of the potential pharmacodynamic basis of MSC-CDs. In addition to anti-inflammation, the main aspects of HM-CDs for the treatment of inflammation-related diseases are as follows.
Arthritis
Arthritis is broadly defined as an inflammatory disease that occurs in the human joints and their surrounding tissues. The charcoal-processed drug AFIC of Aurantii fructus ymulturus (AFI) has long been used to treat inflammatory and metabolic diseases. However, the pharmacodynamic basis and action mechanism of AFIC remain unclear. Wang’s group produced a novel type of carbon dots (AFIC-CDs) through pyrolysis and carbonization.93 AFIC-CDs effectively attenuate the monosodium urate (MSU) crystal-induced inflammatory response by inhibiting the production of inflammatory factors (IL-1β and TNF-α), playing an influential role in the pathophysiology of acute gouty arthritis. Meanwhile, PLR-CDs reduced IL-1 and TNF levels in a dose-dependent manner, which reduced the severity of joint swelling in gouty arthritis.94
Epidermal Inflammation
The emergence of HM-CDs offers hope for the treatment of psoriasis, a chronic inflammatory skin disease. Zhang et al prepared novel non-toxic Phellodendri Cortex CDs (PCC-CDs).31 The considerable anti-psoriatic action of PCC-CDs was first demonstrated using a mouse model of psoriasis-like skin. The underlying mechanism may be related to the suppression of M1 polarization of macrophages and the relative promotion of M2 polarization. Systemic inflammatory reactions are generally accompanied by fever or hypothermia, and lipopolysaccharide (LPS)-induced fever is caused by inflammation. Therefore, Wu et al explored the effects of synthetic Lonicerae japonicae Flos (LJF) Carbonisata-CDs on LPS-induced fever and hypothermia models in rats.95 The experimental results showed that LJFC-CDs significantly attenuated the LPS-induced inflammatory response, as evidenced by the expression of TNF-α, IL-1β, IL-6 and the restoration of normal body temperature. Consequently, LJFC-CDs may have some anti-inflammatory properties and alleviate inflammation-induced fever and hypothermia.
Frostbite induced by cold conditions triggers varying degrees of tissue damage, but interventions are lacking.96 To bridge this gap, Kong et al synthesized Artemisiae Argyi Folium (AAF) Carbonisata-CDs (AAFC-CDs) by pyrolysis.97 AAFC-CDs ameliorate local inflammation by mediating IL-1β and TNF-α and provide the body with energy to alleviate the fall in blood glucose level caused by frostbite, so as to achieve anti-frostbite effects. In contrast to conventional AAF, isochlorogenic acid is no longer present in AAFC-CDs, but its specific composition has not been identified. The conventional AAF is not suitable for treating frostbite. Therefore, the emergence of AAFC-CDs may extend the practical applications of AAF.
Allergic Inflammation
Allergies are also frequently linked to inflammation. Scutellariae Radix Carbonisata (SRC) is a traditional medicine that can be used to treat allergic diseases. To elucidate the function and mechanism of the carbonized fraction in SRC, Kong et al isolated novel water-soluble SRC-CDs with particle sizes ranging from 2 to 9 nm from aqueous extracts of Scutellariae Radix Carbonisata.98 Their anti-inflammatory effects are directly related to their stabilization of mast cell agonism, which may be associated with the reduction of mast cell functional agonism, inhibition of RBL-2H3 cell degranulation, and reduction of histamine and inflammatory factor levels. SRC-CDs are therefore effective in reducing allergic responses. By demonstrating the anti-allergic action of SRC-CDs and the associated mechanisms, researchers have filled a research void and laid the groundwork for future innovative drug development. SRC-CDs may then be used as possible medications to treat allergic conditions.
Organ Damage Inflammation
The organ damage is accompanied by infiltration of inflammatory factors. Zhao et al found that ASAC-CDs synthesized by Armeniacae Semen Amarum could effectively inhibit the expression levels of inflammatory factors (IL-6, IL-1β, and TNF-α) and exhibited satisfactory anti-inflammatory effects, particularly the high-dose group.99 Compared to the model group (20.56 ± 1.41 pg/mL, 21.07 ± 2.26 pg/mL, and 69.49 ± 9.62 pg/mL, respectively), treatment with high concentrations of ASAC-CDs (8.13 ± 1.40 pg/mL, 8.53 ± 0.82 pg/mL, and 32.03 ± 5.20 pg/mL) significantly reduced the levels of IL-6, IL-1β, and TNF-α (p < 0.01). To a certain degree, they are able to reduce the increase of neutrophils in the blood and decrease the chemotaxis of neutrophils to inflammatory sites, thereby reducing the release of inflammatory mediators and inhibiting LPS-induced damage and deterioration of lung tissue. In a model of acute kidney injury, another study found that PCC-CDs had a direct renoprotective impact by reversing the rise in serum creatinine (SCR), blood urea nitrogen (BUN), urinary total protein (UTP), and microalbuminuria (MALB).100 PCC-CDs also attenuated the inflammatory response and thrombocytopenia associated with acute kidney injury, thus exerting a multifaceted effect. Inspired by the above, Wang et al isolated a novel carbon dots (PT-CDs) from Pollen Typhae.56 Using a rat model of rhabdomyolysis (RM)-induced acute kidney injury (AKI), the authors demonstrated that PT-CDs had significant activity in improving BUN and CRE levels, urine volume, renal index, and histopathological morphology in rats with RM-induced AKI. The intervention of PT-CDs dramatically reduced the degree of inflammatory response and oxidative stress, which may be related to the basal potential mechanism of anti-AKI activity. Additionally, cytotoxicity assays and biosafety assessments demonstrated the high biocompatibility of PT-CDs.
Herbal medicines are normally considered to be only for chronic diseases but slow to respond or ineffective for acute injuries.101 However, in addition to achieving protection of organs such as liver, kidney and lung through anti-inflammation, HM-CDs have confirmed the therapeutic effects of herbs on acute injuries. In a recent study,102 Paeoniae Radix Alba-derived CDs (PRAC-CDs) can inhibit alanine transaminase (ALT) and acetone transaminase (AST) levels and have a mitigating effect on the rise in TBA and TBIL in a mouse model of acute liver injury. By eliminating free oxygen, preventing lipid peroxidation of hepatocytes, controlling bile acid metabolism, reducing malondialdehyde (MDA) levels and increasing superoxide dismutase (SOD) levels, PRAC-CDs exhibit excellent hepatoprotective effects. The Junci Medulla Carbonisata carbon dots (JMC-CDs) also achieved a similar hepatoprotective effect.80 In animal models of trauma hemorrhagic and internal hemorrhage caused by Deinagkistrodon acutus venom, the researchers showed that JMC-CDs not only had significant hemostatic effects, but also prevented hemorrhagic liver injury with reduced levels of biochemical indicators of liver injury such as aspartate aminotransferase, alanine amino transferase, alkaline phosphatase, total bilirubin, and direct bilirubin.
Inflammation of the Gastrointestinal Tract
The pharmacological effects of HM-CDs are also involved in the gastrointestinal system for the treatment of various ulcers, which may also be associated with the inflammatory responses. Recently, Hu et al showed that Radix Sophorae Flavescentis carbonisata (RSFC)-CDs could inhibit ethanol-induced acute gastric ulcers in rats by suppressing the release of TNF-α and IL-6 through downregulation of the NF-κB pathway.103 Most notably, RSFC has been widely used for the treatment of systemic ulcerative diseases. The authors hypothesized that HM-CDs produced by high-temperature pyrolysis may have inherent biological activity, though the active ingredients were not disclosed. Another study synthesized GRR-CDs using Glycyrrhizae Radix et Rhizoma (GRR) as precursors by an environment-friendly one-step pyrolysis process.104 GRR-CDs significantly reduced the oxidative damage to the gastric mucosa and tissues caused by alcohol, as well as restored the expression of malondialdehyde, superoxide dismutase, and nitric oxide in the serum and tissues of mice. This suggests that the explicit anti-ulcer activity of GRR-CDs, which provides a fresh perspective on how to investigate the pharmacodynamic basis of GRR.
Cancer
Herbal medicines offer more potent and distinctive anti-tumor effects, and some of them can be combined with radiotherapy to lessen toxicity and boost efficacy. Similarly, CDs prepared by herbal medicine have great potential for oncology treatments. The strategy of combining herbal medicine and CDs is also expected to reduce anti-cancer side effects, increase tumor accumulation, and enhance therapeutic effectiveness. Inspired by curcumin, Li et al prepared novel CDs (G-CDs) based on Ginger and found that G-CDs could have an extremely strong inhibitory effect on the growth of HepG2 cells by up-regulating the expression of the p53 gene in cancer cells and inducing the level of intracellular ROS.105 G-CDs also exhibited significant anti-hepatocellular carcinoma activity in vivo, which was able to accumulate at the tumor site through enhanced permeation retention (EPR) effect in solid tumors. Ginsenoside Re-based carbon dots (Re-CDs) with a particle size of 4.6 nm were created in another investigation.106 Re-CDs have demonstrated reduced toxicity to normal cells and higher efficacy in preventing cancer cell proliferation when compared to APIs. Their cancer-fighting effects were coupled with high levels of ROS and the creation of apoptosis associated with caspase-3. Furthermore, Arul et al prepared nitrogen-doped CDs (N-CDs) by a simple hydrothermal method using Actinidia deliciosa (A. deliciosa) fruit extract as a carbonized precursor and aqueous ammonia as a nitrogen dopant.73 When tested on mouse fibroblast (L-929) cells and human breast cancer (MCF7) cells, the N-CDs also exhibited some anticancer activity.
Diseases Related to Oxidative Stress
Normal levels of ROS play a decisive role in cell signaling and homeostasis, but excessive ROS accumulation may lead to oxidative damage, inflammation, various diseases, and cancer. Some HM-CDs also have potent antioxidant activities. Among them, natural gynostemma fluorescent CDs can protect zebrafish from oxidative stress by increasing ROS-related enzymes, thus reducing ROS levels through a compensatory mechanism. As a result, as antioxidants, they are effective in reducing ROS damage in Hela cells and zebrafish.107 Additionally, utilizing Salvia miltiorrhiza Bunge as a carbon source, Li et al created multifunctional antioxidant CDs.21 Compared to natural Salvia extracts, the resulting CDs had higher antioxidant capacity and greater ability to scavenge ROS, attenuating abiotic stress in plants and opening up a wide range of potential applications in botany. Subsequently, the group synthesized a Salvia miltiorrhiza Bunge-derived CDs.108 Under conditions of salt and nutrient deficiency, the abundance of functional groups (-OH and -COOH) on the surface encourages Ca2+ signaling and environmental adaptation in plants, which in turn causes ROS-independent Ca2+ activation in the root system. As such, the CDs can be utilized for crop enhancement as both a ROS scavenger and a simultaneous Ca2+ signaling amplifier.
Additional Diseases
Antinociceptive Effects
Ginger has been used as an analgesic with notable results for more than a thousand years, while its material basis is still unknown. With Zingiber officinale Roscoe (ZR) as the raw material, Zhang et al prepared a revolutionary environmentally friendly CDs (ZR-CDs) utilizing direct pyrolysis.32 The authors confirmed the significant analgesic activity of ZR-CDs using classical hot-plate, tail-immersion, and acetic acid writhing methods, and demonstrated for the first time that the analgesic effect of ZR-CDs was mediated by an opioid-like mechanism and the regulation of 5-hydroxytryptamine levels in serum. In addition to ZR, a study has prepared non-toxic nanocarrier GRR-CDs using Glycyrrhizae Radix et Rhizoma as the only material and an environmentally friendly, simple and low-cost calcination method, which increased the glycyrrhizic acid (GA) solubility significantly by 27-fold.109 In both the hot-plate model and the acetic acid-induced writhing model, the GRR-CDs-GA complex showed significantly higher antinociceptive activity compared to the unprocessed GRR-CDs and GA. These results support the promising application of GRR-CDs as a technique to improve the solubility and antinociceptive properties of poorly water-soluble drugs (such as GA).
Menopausal Syndrome
Glycyrrhizae Radix et Rhizoma (GRR) is frequently used in the treatment of menopausal syndrome (MPS) and other gynecological disorders in addition to the antinociceptive activity. Zhang et al successfully synthesized GRR into GRR-CDs by pyrolysis.110 The study is the first to demonstrate that GRR-CDs can alleviate MPS by elevating the estradiol (E2) level, decreasing follicle stimulating hormone (FSH) and luteinizing hormone (LH) levels, and raising the degree of uterine atrophy. This not only indicates the potential of GRR-CDs as a drug to alleviate menopausal syndrome and its associated symptoms, but also provides possibility for nanomedicines to treat hormonal disorders.
Anxiolytic Effects
People are susceptible to developing depression and anxiety disorders in response to stress. Os Draconis (OD) has gained recognition as a medication that has been used for a long time to treat neurological diseases. In order to elucidate the biological basis of the anxiolytic effects of OD, a study isolated the novel OD-CDs obtained from Os Draconis.111 Interestingly, OD-CDs significantly reduced anxiety in four behavioral tests, including the Open Field Test (OFT), Light/Dark Box Test (LDT), Elevated Plus Maze Test (EPMT), and Novelty-Suppressed Feeding Test (NSFT). The results also imply that OD-CDs mediate the modulation of monoaminergic neurotransmitters and the HPA axis to a certain extent, although additional research is required to pinpoint the precise processes. Given that OD-CDs exhibit observable anxiolytic effects, this supports their development as novel anxiolytic agents that merit additional study.
In summary, HM-CDs, as an emerging nanomaterial, have been widely used in the medical field for their remarkable therapeutic effects due to their excellent photoluminescence capabilities, superior chemical stability and low toxicity, water dispersibility, and biocompatibility. It is noteworthy that the study of the auto-biological activity of HM-CDs has received increasing attention, which is anticipated to reveal their various pharmacological and active effects. However, the therapeutic mechanisms of HM-CDs have not been thoroughly investigated yet, which need to be further explored. In addition, as nanomaterials, elucidating the metabolic processes of HM-CDs in vivo is another major challenge.
Conclusion and Outlook
CDs, as a novel class of fluorescent carbon nanomaterials, have made numerous significant breakthroughs from their fundamental optical features to potential applications. As a fresh branch of CDs, HM-CDs have been extensively applied in disease treatment. The potential therapeutic efficacy and fluorescence properties are significant markers to distinguish HM-CDs from ordinary CDs. HM-CDs have a higher pharmacological activity than raw material products, which may lead to altered therapeutic efficacy. Their removal of the requirement for drug loading can successfully prevent negative effects, promising significant advances in the near future. Despite the rapid advancement of CDs in herbal medicine, there are still numerous issues that remain to be resolved.
First, although CDs show low toxicity, their potential effects on humans are unknown. The tiny molecule compounds produced by the photodegradation of CDs cause some toxicity.112 On the other hand, some CDs have novel cytotoxic properties, including ROS-generating toxicity and dose-dependent toxicity. Therefore, the risks of HM-CDs in clinical therapeutic applications still require further attention and research. Second, the preparation and processing of CDs are not subject to a unified objective quantitative assessment. It is challenging to guarantee the stable and uniform quality of CDs under different preparation circumstances (temperature, time, etc.), which raises the possibility of variations in the composition and pharmacological properties of HM-CDs. The most popular procedures for preparation, hydrothermal synthesis and high-temperature pyrolysis, produce CDs with unstable QY, particle size, and fluorescence intensity. Third, the intrinsic active ingredient of HM-CDs remains uncertain, while the active ingredient is crucial for the treatment of diseases. The identification of active substances is direct evidence to elucidate the mechanism of action of HM-CDs. Under high temperatures, current synthesis techniques may lead to the decomposition or even disappearance of some actual components of herbal medicines. Therefore, the remaining fraction of active compounds in HM-CDs under different synthesis methods and conditions is an important direction for future research. High performance liquid chromatography and tandem mass spectrometry (HPLC-MS) may be able to offer some answers. Fourth, further research is needed on the in vivo distribution and metabolism of HM-CDs. In contrast to classical medicine, the circulation of HM-CDs in living bodies and organs is unclear and the interaction with living molecules is complicated, leading to the limitations of CDs in clinical applications. Last but not least, the mechanism of the luminescence of HM-CDs is still unclear. The lack of a universally applicable luminescence mechanism, generally due to the difficulty in determining the structure of the synthesized HM-CDs, has limited the structural modification of HM-CDs and the improvement of luminescence properties to meet clinical needs. As a consequence, the structural composition of CDs synthesized based on herbal medicine and the luminescence mechanisms associated with them remain to be further explored.
In order to expedite the translation of HM-CDs from the laboratory to clinical applications, there are currently challenges such as complexity of composition, quality control, individual variations, clinical validation, and ethical and regulatory issues. HM-CDs are prepared from herbal extracts, which contain multiple complex components. Understanding the effects and interactions of these components on the human body is a difficult task that requires detailed analysis and research. Ensuring consistent quality of HM-CDs is another challenge due to the diversity and variability of herbal sources. To overcome this, it is important to establish standardized preparation methods and implement quality control measures. Moreover, the response and effects of HM-CDs may vary among different patients, influenced by factors such as genetic background and metabolic differences. Therefore, individualized studies and evaluations are necessary to determine the appropriate application methods and dosages for specific patient populations. Clinical trials are essential to validate the efficacy and safety of HM-CDs. These trials will provide the necessary evidence to support their use in clinical settings. However, it is crucial to comply with ethical principles and legal regulations in order to ensure patient safety and privacy during these trials and in the eventual market adoption of HM-CDs. Considering HM-CDs as a novel nanomaterial, it is important to subject them to additional ethical and regulatory scrutiny to address any potential risks or concerns. The integration of modern technologies such as proteomics, genomics, and metabolomics is conducive to promoting the industrial development of HM-CDs. The primary focus of our future research will be on systematic studies of the toxicity and metabolic pathways of HM-CDs in animal models, optimization of HM-CDs preparation methods, biodistribution and active ingredient analysis of HM-CDs, and the precise mechanism of interaction between the human system and HM-CDs.
Abbreviation
CDs, Carbon dots; HM, Herbal medicine; HM-CDs, Herbal medicine-derived carbon dots; SARS, Severe acute respiratory syndrome; COVID-19, Coronavirus disease 2019; PTFE, Polytetrafluoroethylene; QY, Quantum yield; PCC-CDs, Phellodendri Chinensis Cortex-based carbon dots; ZR-CDs, Zingiberis rhizoma-based carbon dots; E-CDs, ethanol-papaya CDs; CP-CDs, codonopsis pilosula-derived CDs; PT-CDs, Pollen Typhae-based carbon dots; BUN, Blood urea nitrogen; CRE, Creatinine; AKI, Acute kidney injury; BBB, Blood-brain barrier; SHC-CDs, Schizonepetae Herba Carbonisata-CDs; NIR, near-infrared; SEM, scanning electron microscopy; TEM, transmission electron microscopy; HRTEM, high-resolution transmission electron microscopy; XRD, X-ray diffraction; XPS, X-ray photoelectron spectroscopy; APTT, activated partial thromboplastin time; PT, prothrombin time; TT, thrombin time; FIB, fibrinogen; TF, tissue factor; HMWK, high molecular weight kininogen; KLK, kallikrein; PKLK, prekallikrein; CSC, Cirsium Setosum Carbonisata; SHC, Schizonepetae Herba Carbonisata; JMC, Junci Medulla Carbonisata; PTC, Ptollen Typhae Carbonisata; EYO, Egg yolk oil; CJ, Cirsii Japonici’s; RPC, Radix Puerariae Carbonisata; SSC, Schizonepetae Spica Carbonisata; JSX, Jiaosanxian; PS, photosensitizers; MCDs, mushroom CDs; ROS, Reactive oxygen species; PEIs, Persistent endodontic infections; MSC-CDs, Mulberry Silkworm Cocoon-CDs; AFI, Aurantii fructus ymulturus; MSU, monosodium urate; LPS, lipopolysaccharide; LJF, Lonicerae japonicae Flos; AAF, Artemisiae Argyi Folium; SRC, Scutellariae Radix Carbonisata; SCR, serum creatinine; UTP, urinary total protein; MALB, microalbuminuria; RM, rhabdomyolysis; PRAC-CDs, Paeoniae Radix Alba-derived CDs; ALT, alanine transaminase; AST, acetone transaminase; MDA, malondialdehyde; SOD, superoxide dismutase; GRR, Glycyrrhizae Radix et Rhizoma; EPR, enhanced permeation retention; GA, glycyrrhizic acid; FSH, follicle stimulating hormone; LH, luteinizing hormone; OFT, Open Field Test; LDT, Light/Dark Box Test; EPMT, Elevated Plus Maze Test; NSFT, Novelty-Suppressed Feeding Test; HPLC-MS, High performance liquid chromatography and tandem mass spectrometry.
Data Sharing Statement
All data generated or analyzed during this study are included in this manuscript.
Funding
This research was supported by National Key Clinical Specialties Construction Program. This study was funded by the Key Research and Development Project of the Department of Science and Technology of Sichuan Province (No. 2021YFS0274).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Shen LM, Liu J. New development in carbon quantum dots technical applications. Talanta. 2016;156–157:245–256. doi:10.1016/j.talanta.2016.05.028
2. Wang K, Gao Z, Gao G, et al. Systematic safety evaluation on photoluminescent carbon dots. Nanoscale Res Lett. 2013;8(1):122. doi:10.1186/1556-276X-8-122
3. Gong N, Ma X, Ye X, et al.. Carbon-dot-supported atomically dispersed gold as a mitochondrial oxidative stress amplifier for cancer treatment. Nat Nanotechnol. 2019;14(4):379–387. doi:10.1038/s41565-019-0373-6
4. Lu L, Feng C, Xu J, et al.. Hydrophobic-carbon-dot-based dual-emission micelle for ratiometric fluorescence biosensing and imaging of Cu in liver cells. Biosens Bioelectron. 2017;92:101–108. doi:10.1016/j.bios.2017.01.066
5. Truskewycz A, Yin H, Halberg N, et al.. Carbon dot therapeutic platforms: administration, distribution, metabolism, excretion, toxicity, and therapeutic potential. Small. 2022;18(16):e2106342. doi:10.1002/smll.202106342
6. Egorova MN, Tomskaya AE, Kapitonov AN, Alekseev AA, Smagulova SA. Hydrothermal synthesis of luminescent carbon dots from glucose and birch bark soot. J Struct Chem. 2018;59:780.
7. Liu Y, Zhao Y, Zhang YS. One-step green synthesized fluorescent carbon nanodots from bamboo leaves for copper(II) ion detection. Actuators B Chem. 2014;196:647. doi:10.1016/j.snb.2014.02.053
8. Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N, Xu Y. Traditional herbal medicine candidates as complementary treatments for COVID-19: a review of their mechanisms, pros and cons. Evid Based Complement Alternat Med. 2020;2020:2560645. doi:10.1155/2020/2560645
9. Yi Y, Lamikanra O, Sun J, Wang LM, Min T, Wang HX. Activity diversity structure-activity relationship of polysaccharides from lotus root varieties. Carbohydr Polym. 2018;190:67–76. doi:10.1016/j.carbpol.2017.11.090
10. Dai J, Wang YJ, Research E. Nitrogen-doped carbon quantum dots with Pinellia ternata as carbon source for high sensitive determination of chromium (VI). Appl EcolEnviron Res. 2019;17:12139–12153.
11. Sun L, Zhang R, Zhang T, et al. Synthesis, applications and biosafety evaluation of carbon dots derived from herbal medicine. Biomed Mater. 2023;18(4):042004. doi:10.1088/1748-605X/acdeb8
12. Sun Z, Lu F, Cheng J, et al.. Haemostatic bioactivity of novel Schizonepetae Spica Carbonisata-derived carbon dots via platelet counts elevation. Artif Cells Nanomed Biotechnol. 2018;46(sup3):S308–S317. doi:10.1080/21691401.2018.1492419
13. Miao P, Han K, Tang Y, Wang B, Lin T, Cheng W. Recent advances in carbon nanodots: synthesis, properties and biomedical applications. Nanoscale. 2015;7(5):1586–1595. doi:10.1039/c4nr05712k
14. Li W, Wang S, Li Y, et al.. One-step hydrothermal synthesis of fluorescent nanocrystalline cellulose/carbon dot hydrogels. Carbohydr Polym. 2017;175:7–17. doi:10.1016/j.carbpol.2017.07.062
15. Tang S, Zhang H, Mei L, et al.. Fucoidan-derived carbon dots against Enterococcus faecalis biofilm and infected dentinal tubules for the treatment of persistent endodontic infections. J Nanobiotechnology. 2022;20(1):321. doi:10.1186/s12951-022-01501-x
16. Wang Y, Chen J, Tian J, et al.. Tryptophan-sorbitol based carbon quantum dots for theranostics against hepatocellular carcinoma. J Nanobiotechnology. 2022;20(1):78. doi:10.1186/s12951-022-01275-2
17. Zheng X, Qin K, He L, et al.. Novel fluorescent nitrogen-doped carbon dots derived from Panax notoginseng for bioimaging and high selectivity detection of Cr6. Analyst. 2021;146(3):911–919. doi:10.1039/d0an01599g
18. Li L, Li L, Chen C-P, Cui F. Green synthesis of nitrogen-doped carbon dots from ginkgo fruits and the application in cell imaging. Inorg Chem Commun. 2017;86:227–231. doi:10.1016/j.inoche.2017.10.006
19. Shen Y, Wu H, Wu W, Zhou L, Dai Z, Dong S. A facile hydrothermal method to synthesize fluorescent carbon dots for detecting iron. Mat Express. 2020;10(7):1135. doi:10.1166/mex.2020.1745
20. Zhang S, Wang Z, Pang Y, et al.. Highly fluorescent carbon dots from coix seed for the determination of furazolidone and temperature. Spectrochim Acta A Mol Biomol Spectrosc. 2021;260:119969. doi:10.1016/j.saa.2021.119969
21. Li Y, Li W, Yang X, et al.. Salvia Miltiorrhiza -derived carbon dots as scavengers of reactive oxygen species for reducing oxidative damage of plants. ACS Appl Nano Mater. 2021;4(1):113. doi:10.1021/acsanm.0c02419
22. Surendran P, Lakshmanan A, Vinitha G, Ramalingam G, Rameshkumar P. Facile preparation of high fluorescent carbon quantum dots from Orange waste peels for nonlinear optical applications. Luminescence. 2020;35(2):196–202. doi:10.1002/bio.3713
23. Wang M, Shi R, Gao M, et al.. Sensitivity fluorescent switching sensor for Cr (VI) and ascorbic acid detection based on Orange peels-derived carbon dots modified with EDTA. Food Chem. 2020;318:126506. doi:10.1016/j.foodchem.2020.126506
24. Yang X, Zhuo Y, Zhu S, Luo Y, Feng Y, Dou Y. Novel and green synthesis of high-fluorescent carbon dots originated from honey for sensing and imaging. Biosens Bioelectron. 2014;60:292–298. doi:10.1016/j.bios.2014.04.046
25. Xu H, Yang X, Li G, Zhao C, Liao X. Green synthesis of fluorescent carbon dots for selective detection of tartrazine in food samples. J Agric Food Chem. 2015;63(30):6707–6714. doi:10.1021/acs.jafc.5b02319
26. Kang C, Huang Y, Yang H, Yan XF, Chen ZP. A review of carbon dots produced from biomass wastes. Nanomaterials. 2020;10(11):2316. doi:10.3390/nano10112316
27. Wang R, Lu K-Q, Tang Z-R, Xu Y-J. Recent progress in carbon quantum dots: synthesis, properties and applications in photocatalysis. J Mater Chem A. 2017;5(8):3717–3734. doi:10.1039/C6TA08660H
28. Chen Z, Ye SY, Yang Y, Li ZY. A review on charred traditional Chinese herbs: carbonization to yield a haemostatic effect. Pharm Biol. 2019;57(1):498–506. doi:10.1080/13880209.2019.1645700
29. Dager A, Uchida T, Maekawa T, Tachibana M. Synthesis and characterization of mono-disperse carbon quantum dots from fennel seeds: photoluminescence analysis using machine learning. Sci Rep. 2019;9(1):14004. doi:10.1038/s41598-019-50397-5
30. Zhou J, Sheng Z, Han H, Zou M, Li C. Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source. Mater Lett. 2012;66(1):222–224. doi:10.1016/j.matlet.2011.08.081
31. Zhang M, Cheng J, Hu J, et al.. Green Phellodendri Chinensis Cortex-based carbon dots for ameliorating imiquimod-induced psoriasis-like inflammation in mice. J Nanobiotechnology. 2021;19(1):105. doi:10.1186/s12951-021-00847-y
32. Zhang M, Cheng J, Zhang Y, et al.. Green synthesis of Zingiberis rhizoma-based carbon dots attenuates chemical and thermal stimulus pain in mice. Nanomedicine. 2020;15(9):851–869. doi:10.2217/nnm-2019-0369
33. Jović D, Jaćević V, Kuča K, et al.. The puzzling potential of carbon nanomaterials: general properties, application, and toxicity. Nanomaterials. 2020;10(8):1508. doi:10.3390/nano10081508
34. Abu Rabe DI, Al Awak MM, Yang F, et al.. The dominant role of surface functionalization in carbon dots’ photo-activated antibacterial activity. Int J Nanomedicine. 2019;14:2655–2665. doi:10.2147/IJN.S200493
35. Ghosal K, Ghosh A. Carbon dots: the next generation platform for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2019;96:887–903. doi:10.1016/j.msec.2018.11.060
36. In I, Park SY, Lim D, et al. Correction to simple microwave-assisted synthesis of amphiphilic carbon quantum dots from A3/B2 polyamidation monomer set. ACS Appl Mater Interfaces. 2018;10(3):3153. doi:10.1021/acsami.7b18854
37. Liu H, He Z, Jiang LP, Zhu JJ. Microwave-assisted synthesis of wavelength-tunable photoluminescent carbon nanodots and their potential applications. ACS Appl Mater Interfaces. 2015;7(8):4913–4920. doi:10.1021/am508994w
38. Shen Z, Zhang C, Yu X, et al.. Microwave-assisted synthesis of cyclen functional carbon dots to construct a ratiometric fluorescent probe for tetracycline detection. J Mater Chem C. 2018;6(36):9636–9641. doi:10.1039/C8TC02982B
39. Chung S, Revia RA, Zhang M. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv Mater. 2021;33(22):e1904362. doi:10.1002/adma.201904362
40. Hu X, Li Y, Xu Y, et al.. Green one-step synthesis of carbon quantum dots from Orange peel for fluorescent detection of Escherichia coli in milk. Food Chem. 2021;339:127775. doi:10.1016/j.foodchem.2020.127775
41. Architha N, Ragupathi M, Shobana C, et al.. Microwave-assisted green synthesis of fluorescent carbon quantum dots from Mexican Mint extract for fe detection and bio-imaging applications. Environ Res. 2021;199:111263. doi:10.1016/j.envres.2021.111263
42. Feng J, Wang WJ, Hai X, Yu YL, Wang JH. Green preparation of nitrogen-doped carbon dots derived from silkworm chrysalis for cell imaging. J Mater Chem B. 2016;4(3):387–393. doi:10.1039/c5tb01999k
43. Isnaeni Rahmawati I, Intan R, Zakaria M. Photoluminescence study of carbon dots from ginger and galangal herbs using microwave technique. J Phys. 2018;985:012004.
44. Tejwan N, Sharma A, Thakur S, Das J. Green synthesis of a novel carbon dots from red Korean ginseng and its application for Fe sensing and preparation of nanocatalyst2+. Inorg Chem Commun. 2021;134:108985. doi:10.1016/j.inoche.2021.108985
45. Tejwan N, Kundu M, Sharma A, Das J. Preprint; 2020. Available from: https://www.researchsquare.com/article/rs-28922/v1.
46. Feng Y, Zhong D, Miao H, Yang X. Carbon dots derived from rose flowers for tetracycline sensing. Talanta. 2015;140:128–133. doi:10.1016/j.talanta.2015.03.038
47. Tejwan N, Saini AK, Sharma A, Singh TA, Kumar N, Das J. Metal-doped and hybrid carbon dots: a comprehensive review on their synthesis and biomedical applications. J Control Release. 2021;330:132–150. doi:10.1016/j.jconrel.2020.12.023
48. Agrawal A, Cho SH, Zandi O, Ghosh S, Johns RW, Milliron DJ. Localized surface plasmon resonance in semiconductor nanocrystals. Chem Rev. 2018;118(6):3121–3207. doi:10.1021/acs.chemrev.7b00613
49. Wang N, Wang Y, Guo T, Yang T, Chen M, Wang J. Green preparation of carbon dots with papaya as carbon source for effective fluorescent sensing of Iron (III) and Escherichia coli. Biosens Bioelectron. 2016;85:68–75. doi:10.1016/j.bios.2016.04.089
50. Moreira VA, Toito Suarez W, de Oliveira Krambeck Franco M, Gambarra Neto FF. Eco-friendly synthesis of cuprizone-functionalized luminescent carbon dots and application as a sensor for the determination of copper(ii) in wastewater. Anal Methods. 2018;10(37):4570. doi:10.1039/C8AY00928G
51. Sim LC, Wong JL, Hak CH, Tai JY, Leong KH, Saravanan P. Sugarcane juice derived carbon dot-graphitic carbon nitride composites for bisphenol A degradation under sunlight irradiation. Beilstein J Nanotechnol. 2018;9:353–363. doi:10.3762/bjnano.9.35
52. Qiu Y, Gao D, Yin H, et al.. Facile, green and energy-efficient preparation of fluorescent carbon dots from processed traditional Chinese medicine and their applications for on-site semi-quantitative visual detection of Cr(VI). Sens Actuators B Chem. 2020;324:128722. doi:10.1016/j.snb.2020.128722
53. Luo WK, Zhang LL, Yang ZY, et al.. Herbal medicine derived carbon dots: synthesis and applications in therapeutics, bioimaging and sensing. J Nanobiotechnology. 2021;19(1):320. doi:10.1186/s12951-021-01072-3
54. Xue M, Zou M, Zhao J, Zhan Z, Zhao S. Green preparation of fluorescent carbon dots from lychee seeds and their application for the selective detection of methylene blue and imaging in living cells. J Mater Chem B. 2015;3(33):6783–6789. doi:10.1039/c5tb01073j
55. Parvin N, Mandal TK. Synthesis of a highly fluorescence nitrogen-doped carbon quantum dots bioimaging probe and its in vivo clearance and printing applications. RSC Adv. 2016;6(22):18134–18140. doi:10.1039/C5RA25402G
56. Wang X, Wu T, Yang Y, et al.. Ultrasmall and highly biocompatible carbon dots derived from natural plant with amelioration against acute kidney injury. J Nanobiotechnology. 2023;21(1):63. doi:10.1186/s12951-023-01795-5
57. Zhou Y, Peng Z, Seven ES, Leblanc RM. Crossing the blood-brain barrier with nanoparticles. J Control Release. 2018;270:290–303. doi:10.1016/j.jconrel.2017.12.015
58. Ashrafizadeh M, Mohammadinejad R, Kailasa SK, Ahmadi Z, Afshar EG, Pardakhty A. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: a review. Adv Colloid Interface Sci. 2020;278:102123. doi:10.1016/j.cis.2020.102123
59. Li Y, Liu Y, Cui J, et al. Cohort studies on chronic non-communicable diseases treated with traditional Chinese medicine: a bibliometric analysis. Front Pharmacol. 2021;12:639860. doi:10.3389/fphar.2021.639860
60. Zhang M, Zhao Y, Cheng J, et al.. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif Cells Nanomed Biotechnol. 2018;46(8):1562–1571. doi:10.1080/21691401.2017.1379015
61. Zhang JH, Niu A, Li J, Fu JW, Xu Q, Pei DS. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci Rep. 2016;6:37860. doi:10.1038/srep37860
62. Jiang X, Qin D, Mo G, et al.. Ginkgo leaf-based synthesis of nitrogen-doped carbon quantum dots for highly sensitive detection of salazosulfapyridine in mouse plasma. J Pharm Biomed Anal. 2019;164:514–519. doi:10.1016/j.jpba.2018.11.025
63. Singh I, Arora R, Dhiman H, Pahwa R. Carbon quantum dots: synthesis, characterization and biomedical applications. Turk J Pharm Sci. 2018;15(2):219–230. doi:10.4274/tjps.63497
64. Sun X, He J, Yang S, et al. Green synthesis of carbon dots originated from Lycii Fructus for effective fluorescent sensing of ferric ion and multicolor cell imaging. J Photochem Photobiol B. 2017;175:219–225. doi:10.1016/j.jphotobiol.2017.08.035
65. Murugan N, Sundaramoorthy AK. Green synthesis of fluorescent carbon dots from Borassus flabellifer flowers for label-free highly selective and sensitive detection of Fe 3+ ions. New J Chem. 2018;42(16):13297–13307. doi:10.1039/C8NJ01894D
66. Roy P, Chen PC, Periasamy AP, Chen YN, Chang HT. Photoluminescent carbon nanodots: synthesis, physicochemical properties and analytical applications. Mater Today. 2015;18(8):447–458. doi:10.1016/j.mattod.2015.04.005
67. Dehvari K, Liu KY, Tseng PJ, Gedda G, Girma WM, Chang JY. Sonochemical-assisted green synthesis of nitrogen-doped carbon dots from crab shell as targeted nanoprobes for cell imaging. J Taiwan Inst Chem Eng. 2019;95:495–503. doi:10.1016/j.jtice.2018.08.037
68. Hu Q, Gong X, Liu L, Choi MM. Characterization and analytical separation of fluorescent carbon nanodots. J Nanomater. 2017;2017:1–23.
69. Parvin N, Kumar V, Joo SW, Park SS, Mandal TK. Recent advances in the characterized identification of mono-to-multi-layer graphene and its biomedical applications: a review. Electronics. 2022;11(20):3345. doi:10.3390/electronics11203345
70. Dager A, Baliyan A, Kurosu S, Maekawa T, Tachibana M. Ultrafast synthesis of carbon quantum dots from fenugreek seeds using microwave plasma enhanced decomposition: application of C-QDs to grow fluorescent protein crystals. Sci Rep. 2020;10(1):12333. doi:10.1038/s41598-020-69264-9
71. Kailasa SK, Ha S, Baek SH, et al. Tuning of carbon dots emission color for sensing of Fe3+ ion and bioimaging applications. Mater Sci Eng C Mater Biol Appl. 2019;98:834–842. doi:10.1016/j.msec.2019.01.002
72. Bunaciu AA, Udriştioiu EG, Aboul-Enein HY. X-ray diffraction: instrumentation and applications. Crit Rev Anal Chem. 2015;45(4):289–299. doi:10.1080/10408347.2014.949616
73. Arul V, Sethuraman MG. Facile green synthesis of fluorescent N-doped carbon dots from Actinidia deliciosa and their catalytic activity and cytotoxicity applications. Opt Mater. 2018;78:181–190. doi:10.1016/j.optmat.2018.02.029
74. Rasmussen MK, Pedersen JN, Marie R. Size and surface charge characterization of nanoparticles with a salt gradient. Nat Commun. 2020;11(1):2337. doi:10.1038/s41467-020-15889-3
75. Sivasankaran U, Jesny S, Jose AR, Girish Kumar K. Fluorescence determination of glutathione using tissue paper-derived carbon dots as fluorophores. Anal Sci. 2017;33(3):281–285. doi:10.2116/analsci.33.281
76. Ramanan V, Thiyagarajan SK, Raji K, Suresh R, Sekar R, Ramamurthy P. Outright green synthesis of fluorescent carbon dots from eutrophic algal blooms for in vitro imaging. ACS Sustain Chem Eng. 2016;4(9):4724–4731. doi:10.1021/acssuschemeng.6b00935
77. Wang Y, Kong H, Liu X, et al.. Novel carbon dots derived from cirsii japonici herba carbonisata and their haemostatic effect. J Biomed Nanotechnol. 2018;14(9):1635–1644. doi:10.1166/jbn.2018.2613
78. Yan X, Zhao Y, Luo J, et al.. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J Nanobiotechnology. 2017;15(1):60. doi:10.1186/s12951-017-0296-z
79. Luo J, Zhang M, Cheng J, et al.. Hemostatic effect of novel carbon dots derived from Cirsium setosum Carbonisata. RSC Adv. 2018;8(66):37707–37714. doi:10.1039/c8ra06340k
80. Cheng J, Zhang M, Sun Z, et al.. Hemostatic and hepatoprotective bioactivity of junci medulla carbonisata-derived carbon dots. Nanomedicine. 2019;14(4):431–446. doi:10.2217/nnm-2018-0285
81. Zhao Y, Zhang Y, Liu X, et al.. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci Rep. 2017;7(1):4452. doi:10.1038/s41598-017-04073-1
82. Han B, Shen L, Xie H, et al.. Synthesis of carbon dots with hemostatic effects using traditional Chinese medicine as a biomass carbon source. ACS Omega. 2023;8(3):3176–3183. doi:10.1021/acsomega.2c06600
83. Liu X, Wang Y, Yan X, et al.. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine. 2018;13(4):391–405. doi:10.2217/nnm-2017-0297
84. Luo J, Kong H, Zhang M, et al.. Novel carbon dots-derived from radix puerariae carbonisata significantly improve the solubility and bioavailability of baicalin. J Biomed Nanotechnol. 2019;15(1):151–161. doi:10.1166/jbn.2019.2675
85. Sun Z, Lu F, Cheng J, et al.. Hypoglycemic bioactivity of novel eco-friendly carbon dots derived from traditional Chinese medicine. J Biomed Nanotechnol. 2018;14(12):2146–2155. doi:10.1166/jbn.2018.2653
86. Xu Y, Wang B, Zhang M, et al. Carbon dots as a potential therapeutic agent for the treatment of cancer-related anemia. Adv Mater. 2022;34(19):e2200905. doi:10.1002/adma.202200905
87. Sun Y, Zhang M, Bhandari B, Yang C. Recent development of carbon quantum dots: biological toxicity, antibacterial properties and application in foods. Food Rev Int. 2022;38(7):1513. doi:10.1080/87559129.2020.1818255
88. Dong X, Liang W, Meziani MJ, Sun YP, Yang L. Carbon dots as potent antimicrobial agents. Theranostics. 2020;10(2):671–686. doi:10.7150/thno.39863
89. Wu Y, Li C, van der Mei HC, Busscher HJ, Ren Y. Carbon quantum dots derived from different carbon sources for antibacterial applications. Antibiotics. 2021;10(6):623. doi:10.3390/antibiotics10060623
90. Venkateswarlu S, Viswanath B, Reddy AS, Yoon M. Sens Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Actuators B Chem. 2018;258:172. doi:10.1016/j.snb.2017.11.044
91. Lin R, Cheng S, Tan M. Green synthesis of fluorescent carbon dots with antibacterial activity and their application in Atlantic mackerel (Scomber scombrus) storage. Food Funct. 2022;13(4):2098–2108. doi:10.1039/d1fo03426j
92. Wang X, Zhang Y, Kong H, et al.. Novel mulberry silkworm cocoon-derived carbon dots and their anti-inflammatory properties. Artif Cells Nanomed Biotechnol. 2020;48(1):68–76. doi:10.1080/21691401.2019.1699810
93. Wang S, Zhang Y, Kong H, et al.. Antihyperuricemic and anti-gouty arthritis activities of Aurantii fructus immaturus carbonisata-derived carbon dots. Nanomedicine. 2019;14(22):2925–2939. doi:10.2217/nnm-2019-0255
94. Wang X, Zhang Y, Zhang M, et al.. Novel carbon dots derived from Puerariae lobatae radix and their anti-gout effects. Molecules. 2019;24(22):4152. doi:10.3390/molecules24224152
95. Wu J, Zhang M, Cheng J, et al.. Effect of Lonicerae japonicae flos carbonisata-derived carbon dots on rat models of fever and hypothermia induced by lipopolysaccharide. Int J Nanomedicine. 2020;15:4139–4149. doi:10.2147/IJN.S248467
96. Lorentzen AK, Davis C, Penninga L. Interventions for frostbite injuries. Cochrane Database Syst Rev. 2020;12(12):CD012980. doi:10.1002/14651858.CD012980.pub2
97. Kong H, Zhao Y, Zhu Y, et al.. Carbon dots from artemisiae argyi folium carbonisata: strengthening the anti-frostbite ability. Artif Cells Nanomed Biotechnol. 2021;49(1):11–19. doi:10.1080/21691401.2020.1862134
98. Kong H, Zhao Y, Cao P, et al.. The bioactivity of scutellariae radix carbonisata-derived carbon dots: antiallergic effect. J Biomed Nanotechnol. 2021;17(12):2485–2494. doi:10.1166/jbn.2021.3200
99. Zhao Y, Zhang Y, Kong H, Cheng G, Qu H, Zhao Y. Protective effects of carbon dots derived from Armeniacae Semen Amarum Carbonisata against acute lung injury induced by lipopolysaccharides in rats. Int J Nanomedicine. 2022;17:1–14. doi:10.2147/IJN.S338886
100. Zhang M, Cheng J, Sun Z, et al.. Protective effects of carbon dots derived from phellodendri chinensis cortex carbonisata against deinagkistrodon acutus venom-induced acute kidney injury. Nanoscale Res Lett. 2019;14(1):377. doi:10.1186/s11671-019-3198-1
101. He J, Hou XY. The potential contributions of traditional Chinese medicine to emergency medicine. World J Emerg Med. 2013;4(2):92–97. doi:10.5847/wjem.j.issn.1920-8642.2013.02.002
102. Zhao Y, Zhang Y, Kong H, et al.. Carbon dots from paeoniae radix alba carbonisata: hepatoprotective effect. Int J Nanomedicine. 2020;15:9049–9059. doi:10.2147/IJN.S281976
103. Hu J, Luo J, Zhang M, et al.. Protective effects of radix sophorae flavescentis carbonisata-based carbon dots against ethanol-induced acute gastric ulcer in rats: anti-inflammatory and antioxidant activities. Int J Nanomedicine. 2021;16:2461–2475. doi:10.2147/IJN.S289515
104. Liu Y, Zhang M, Cheng J, et al.. Novel carbon dots derived from Glycyrrhizae Radix et Rhizoma and their anti-gastric ulcer effect. Molecules. 2021;26(6):1512. doi:10.3390/molecules26061512
105. Li CL, Ou CM, Huang CC, et al.. Carbon dots prepared from ginger exhibiting efficient inhibition of human hepatocellular carcinoma cells. J Mater Chem B. 2014;2(28):4564–4571. doi:10.1039/c4tb00216d
106. Yao H, Li J, Song Y, et al.. Synthesis of ginsenoside Re-based carbon dots applied for bioimaging and effective inhibition of cancer cells. Int J Nanomedicine. 2018;13:6249–6264. doi:10.2147/IJN.S176176
107. Wei X, Li L, Liu J, et al.. Green synthesis of fluorescent carbon dots from gynostemma for bioimaging and antioxidant in zebrafish. ACS Appl Mater Interfaces. 2019;11(10):9832–9840. doi:10.1021/acsami.9b00074
108. Li Y, Tang Z, Pan Z, et al.. Calcium-mobilizing properties of Salvia miltiorrhiza-derived carbon dots confer enhanced environmental adaptability in plants. ACS Nano. 2022;16(3):4357–4370. doi:10.1021/acsnano.1c10556
109. Zhang M, Cheng J, Luo J, et al.. Development of ecofriendly carbon dots for improving solubility and antinociceptive activity of glycyrrhizic acid. J Biomed Nanotechnol. 2021;17(4):640–651. doi:10.1166/jbn.2021.3058
110. Zhang Y, Chen Y, Bai X, et al.. Glycyrrhizae radix et rhizoma-derived carbon dots and their effect on menopause syndrome in ovariectomized mice. Molecules. 2023;28(4):1830. doi:10.3390/molecules28041830
111. Chen Y, Xiong W, Zhang Y, et al.. Carbon dots derived from os draconis and their anxiolytic effect. Int J Nanomedicine. 2022;17:4975–4988. doi:10.2147/IJN.S382112
112. Liu YY, Yu NY, Fang WD, et al.. Photodegradation of carbon dots cause cytotoxicity. Nat Commun. 2021;12(1):812. doi:10.1038/s41467-021-21080-z
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.