Back to Journals » Infection and Drug Resistance » Volume 16
Potential Anti-Toxoplasmosis Efficiency of Phoenix dactylifera Extracts Loaded on Selenium Nanoparticles
Authors Wakid MH , Alsulami MN, Farid M , El Kholy WA
Received 4 October 2023
Accepted for publication 13 December 2023
Published 20 December 2023 Volume 2023:16 Pages 7743—7758
DOI https://doi.org/10.2147/IDR.S443047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Sandip Patil
Majed H Wakid,1,2 Muslimah N Alsulami,3 Mohamed Farid,4 Walaa A El Kholy5
1Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, 21589, Saudi Arabia; 2Special Infectious Agents Unit, King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia; 3Department of Biology, College of Science, University of Jeddah, Jeddah, Saudi Arabia; 4Sciences Academy of Experimental Researches, Special Scientific Foundation, Mansoura, Egypt; 5Department of Parasitology, Faculty of Medicine for Girls, Al-Azhar University, Cairo, Egypt
Correspondence: Majed H Wakid, Department of Medical Laboratory Sciences, Faculty of Applied Medical Sciences, King Abdulaziz University, P. O. Box 80324, Jeddah, 21589, Saudi Arabia, Tel +9662-6952000 ext. 25264, Email [email protected]
Background: Toxoplasmosis is a parasitic disease caused by Toxoplasma gondii that infects humans and many types of mammals and birds.
Objective: To investigate the effect of selenium nanoparticles (SeNPs) and Phoenix dactylifera (Pd) extracts loaded on SeNPs as a new agent to combat chronic T. gondii infections in murine model as an alternative method to standard Spiramycin drug therapy.
Methods: A total of 64 female mice were randomly divided into eight groups: GI: Normal control, GII: Positive control, GIII: infected and treated with Spiramycin, GIV: infected and treated with SeNPs, GV: infected and treated with aqueous extract of Pd, GVI: infected and treated with methanolic extract of Pd, GVII: infected and treated with aqueous extract of Pd loaded on SeNPs, GVIII: infected and treated with methanolic extract of Pd loaded on SeNPs. Date palm (P. dactylifera) fruits were identified and collected from the farms of Saudi Arabia. Preparation and characterization of SeNPs were done. The parasitological, histopathological examinations and biochemical changes were evaluated in all groups.
Results: Parasitological results showed significant differences in GVII in comparison to GII while GVIII showed significant differences in comparison to GII and GIII. The histopathological section of the cerebral cortex showed obvious alterations in the infected compared with untreated control groups. Aqueous and methanolic extracts of P. dactylifera loaded on SeNPs treatment showed improvement that indicated by few perivascular cuffing with few inflammatory cell infiltrations. Few granule cells with mild intracellular vacuolation and edema few deformed neurons with deep pyknotic nuclei. Microglia cells expression of Iba-1 and inflammatory cytokines (IL-4, IL-10 and INF-γ) in serum of all groups was higher in GII and lowest in GVIII followed by GVII.
Conclusion: SeNPs and P. dactylifera extracts loaded on SeNPs could be a potent agent to combat T. gondii infections.
Plain Language Summary: Toxoplasma gondii is a parasite, causing toxoplasmosis, has a sexual life cycle, which takes place in the small intestine of cats, and an asexual cycle that takes place in warmed animals and humans. It is a neurotropic obligate intracellular parasite. The pyrimethamine and sulfadiazine are the preferred treatment for toxoplasmosis. Recent studies have indicated adverse side effects such as osteoporosis and teratogenic effects, mostly in immunocompromised individuals. The authors hypothesized there is an urgent need to discover new natural products or biosynthetic agents with fewer side effects and higher efficacy. Dates are rich in dietary fiber, vitamins, fatty acids, and amino acids and play a key role in the neutralization of free radicals, which inhibits the onset and progression of several disorders. As selenium is essential for both innate and adaptive immune responses against bacterial and parasite infections. Moreover, nanoparticles have a large surface-volume ratio and numerous biological benefits such as their convenient entry into the cell compared to other particles. So, the authors reported that selenium nanoparticles and palm dates extracts loaded on them could be a potent agent to combat Toxoplasma infections and verifications were performed in the study.
Keywords: Phoenix dactylifera, selenium, nanoparticles, Toxoplasma gondii, spiramycin
Introduction
Toxoplasmosis is a parasite illness produced by Toxoplasma gondii that affects humans and many types of mammals and birds. This parasite has a sexual life cycle, which takes place in the small intestine of cats, and an asexual cycle that takes place in warmed animals and humans.1,2 Humans become infected by eating raw and undercooked meat and through the ingestion of contaminated vegetables and fruits. The other possible routes of transmission can be from the placenta to the embryo during pregnancy in humans, which can cause serious health problems.3 The symptoms of toxoplasmosis are diverse, although immunocompetent patients may have light symptoms such as fever, headache, and muscle pain or be asymptomatic. However, serious clinical manifestations involving the central nervous system (CNS) may also be observed in immunocompromised patients.4,5 T. gondii is a neurotropic obligate intracellular parasite.6 By infiltrating, reproducing in, and lysing endothelial cells, T. gondii tachyzoites are able to cross the blood-brain barrier (BBB) and get access to the brain.7 The BBB failure is then further exacerbated by the activated microglia emit pro-inflammatory cytokines.8
Ionized calcium-binding adapter molecule 1 (Iba1) is a 147-amino acid calcium-binding protein whose expression is restricted to macrophages/microglia. It is involved in the rearrangement of the actin cytoskeleton (cytoplasmic actin cross-linking activity) during cell movement and phagocytosis; in membrane ruffling and in the building of phagocytic cups.9
Pierezan et al (2014)10 demonstrated that Iba1 is marker is well conserved between different species, and have used the Iba1 antibody to detect normal, inflammatory, and neoplastic cells of the monocyte/macrophage lineage in different tissues from different species, including horses, sheep, cattle, mice, hamsters, and kangaroos. This immunohistochemical is a useful marker for the identification of inflammatory, proliferative, and neoplastic disorders. Previous studies reported that Iba1 is a specific cytoplasmic protein marker to microglia and macrophages.11,12
Also, Ide et al (2011)13 found that normal epidermal LCs and dermal histiocytes also express Iba1. In contrast, all melanomas, plasmacytomas, cutaneous lymphomas and mast cell tumors did not express Iba1, highlighting its specificity for cells of the monocyte/macrophage lineage. Iba1 has been previously recognized as a pan-macrophage marker’, being expressed by almost all subpopulations of cells of the monocyte/macrophage lineage in several organs of mice including the spleen, lymph nodes, thymus, bone marrow, heart, lung, kidney, intestines, skin, and histiocytic sarcomas in the central nervous system.
The prescribing concurrently of pyrimethamine and sulfadiazine is the preferred treatment for toxoplasmosis. Recent studies have indicated adverse side effects such as osteoporosis and teratogenic effects, mostly in immunocompromised individuals, when treated with this combination.14 Therefore, there is a need for further research aiming to discover new agents from natural products or biosynthetic agents with fewer side effects and higher efficacy for the treatment of toxoplasmosis. Dates are rich in dietary fiber, vitamins, fatty acids, and amino acids. They also include large concentrations of carbohydrates, salts, and minerals. Finally, they also play a key role in the neutralization of free radicals, which inhibits the onset and progression of several disorders.15 According to Al-Qarawi et al (2004),16 the palm date is very effective in neutralizing free radicals.
Selenium is an essential element for human health. In the case of deficiency of this element in the human body, some severe complications, such as insufficiency and impairment of the immune system, can occur.2,17 This element also exists in some beneficial proteins that have anticancer, antioxidant, and antimicrobial properties.2,18 Moreover, nanoparticles (NPs) have numerous biological benefits due to having a large surface-volume ratio. One such biological benefit is their convenient entry into the cell compared to other particles.2,19 Recent research has shown that selenium nanoparticles (SeNPs) may prevent growth of several pathogens, including Staphylococcus aureus, Escherichia coli and Leishmania spp.2,20 Although the precise antimicrobial mechanisms of this element have not yet been determined, some studies reported that the inorganic types of this element are able to affect membrane peroxidases to produce oxygen-free radicals, including superoxide anion. Nevertheless, other studies have revealed that SeNPs can stimulate apoptosis in various eukaryotic cells.21
As selenium is essential for both innate and adaptive immune responses against bacterial and parasite infections, selenium deficiency has been shown to impair both types of immunity and increase susceptibility to a broad variety of parasitic illnesses (Trypanosoma cruzi, Nippostrongylus brasiliensis, Heligmosomoides bakeri infection), bacterial (Listeria monocytogenes, Mycobacterium tuberculosis infection) and viral (Hepatitis C virus and HIV infection) infections. Recent studies have shown that treatment with SeNPs increases the cellular immunomodulatory cytokines such as IL-12, IFN-γ, TNF-α, IL-2 and some inflammatory mediators such as nitric oxide (NO). Many intracellular bacteria, including T. gondii, elicit a Th1 immune response via the synthesis of IL-12 and IFN-, and rats lacking either IL-12 or IFN- are unable to clear an infection with T. gondii.22
The ideal treatment for toxoplasmosis would be safe for human use, effective against the parasite, and able to eliminate even the dormant illness.23 According to some studies, NPs for biomedical reasons might make up most future treatment solutions for various ailments since there is growing interest in utilizing nanotechnology.24 NPs are now used in a wide range of biological applications because of their nanoscale dimensions and other advantageous surface reactivity. Additionally, due to their small size and ability to cross membrane barriers, NPs may form free radicals that have the ability to destroy infectious pathogen.25 Additionally, NPs may accumulate in tissues, providing cysts in host tissues with a powerful platform.26 Antimicrobial metal NPes such as silver and gold are of relevance for this purpose,15,27 anti-parasitic24 as well as other bioactivities and specific suppression of certain enzyme activities. Thus, the adaptability of metal NPs makes them an attractive option for future examination as anti-parasitic drugs, particularly against toxoplasmosis.28
Based on this knowledge and the documented antimicrobial and immune-modulating effect of selenium, we hypothesize that SeNPs could be a potent agent to combat T. gondii infections. In the present study, SeNPs and Phoenix dactylifera loaded on SeNPs were screened in a mouse model infected with T. gondii. The therapeutic efficacy provided by the different designs against the chronic strain of T. gondii in a mouse model were evaluated.
Materials and Methods
It is a prospective analytical comparative study. The experimental animals were managed in accordance with the guidelines of the Declaration of Helsinki after approval by the Research and Ethics Committee of the Faculty of Medicine for girls in Al Azhar University (FMG-IRB), number 202107933. Animal care followed the National Institute of Health (NIH) Guide for care and use of laboratory animals.
Animals
Sixty-four female mice (six to eight weeks old) weighing 20–25 g were supplied by Schistosome Biological Supply Center (Theodor Bilharz Research Institute (TBRI)). They were maintained in conditioned rooms (24±2°C) in a good-ventilated plastic cage, freely access to ad libitum (water and food). The investigation was carried out at the TBRI at Parasitology Department.
Animal Infection
Me49 strain of T. gondii was obtained from TBRI`s animal house. It was regularly maintained by repeated oral inoculation through an esophageal tube of Swiss albino mouse with 100 µL of brain homogenate of formerly infected mice containing ≈1×102 tissue cysts/mouse every 8 weeks.29
Drugs
Spiramycin (3 M.I.U) as a positive control drug was manufactured and provided by Pharaonia Pharmaceuticals, Egypt in the form of 1.5 mg tablet that was crushed and dissolved in one mL distilled water. It was given orally to mice (dose 200 mg/kg/body weight/mouse daily) for 7 consequent days.14
The Date Fruits (P. dactylifera) Preparation
The date palm (P. dactylifera) was identified and gathered from Saudi Arabian fields. In the date and palm center at Jeddah University in Saudi Arabia, all the date samples were taken in the edible stage and recognized by specialists and taxonomists. Specimens were then placed in the herbarium of the same institution. The powder was divided into a methanolic and aqueous extract.
Following a thorough washing to remove any clinging debris, 500 g of samples were dried by air before being further dried in a 60 °C air oven. To create date palm fruit powder, the dried date palm fruits were broken into small pieces and put through a heavy-duty grinder with 1–2 mm screens. This powder was then stored at −20°C until needed. 100 mL of boiling distilled water was used to soak 500 g of date fruit powder overnight. To get the first extract, fruit powder was boiled in distilled water at a temperature between 80 and 100°C. After the extract had reached room temperature, it was filtered. According to,30 the extract was then lyophilized and stored at −20°C until future usage. Powdered fruits (500gm) were extracted for methanolic extracts by soaking them in methanol 98% (10L) six times at room temperature, followed by a further extraction using methanol. The extract was collected and then condensed to dryness using a rotary evaporator at 45°C under vacuum and decreased pressure to produce the dried methanol extract,31 which was maintained at 4°C until further studies.
Preparation of SeNPs
With a few changes, the modified method of Li et al, (2010),32 was used to create and characterize SeNPs. Sodium selenite and ascorbic acid were generated by reacting them in a 1:3 sodium selenite to ascorbic acid ratio from a stock solution. Dropwise additions of ascorbic acid were made during a 30-min period with magnetic stirring at 600 rpm at room temperature. The mixture then allowed to interact with one another in concentrated form until a color shift from colorless to orange was seen. After the color shift is visible, the mixture was diluted to a dextrin solution concentration of 20 mL, and finally a further 10 min stirring.
Characterization of SeNPs
SeNPs average size (Z-average) besides polydispersity index (PDI) were evaluated using Zetasizer Nano ZS analyzer (Malvern Instruments, Malvern, UK) and evaluated by dynamic light scattering (DLS) technique.
SeNPs surface charge, being expressed as zeta potential (ZP) which detects the vesicles’ electrophoretic mobility in an electric field, was estimated in the same apparatus besides the same dilution in DW.
Morphology of SeNPs was conceived on transmission electron microscope (TEM) (JEOL JEM-2100, Tokyo, Japan). After adequate dilution with DW besides sonication, the carbon-coated copper grid was evenly covered with SeNPs dispersion, air-dried at room temperature and finally examined at 160 kV. Digital Micrograph (V 2.11.1404.0) and Soft Imaging Spectator software were utilized to complete the image capture, analysis process, besides particle sizing.
Study Design
Sixty-four female mice were split into eight different groups. GI: normal control; GII: positive control; GIII: infected and treated with Spiramycin (Spirex treated); GIV: infected and treated with SeNPs (SeNPs treated); GV: infected and treated with aqueous extract of P. dactylifera (P-Aque treated); GVI: infected and treated with methanolic extract of P. dactylifera (P-Meth treated); GVII: infected and treated with aqueous extract of P. dactylifera loaded on SeNPs (P-Aque with SeNPs treated); GVIII: infected and treated with methanolic extract of P. dactylifera loaded on SeNPs (P-Meth with SeNPs treated).
The Effect of Herbal Extracts on T. gondii Infection in vivo
Serum was collected for blood tests and biochemical changes. Brains were collected for parasitological, histological, and immunohistochemical assay.
Parasitological Examination
The sacrificed-mice brains were homogenized individually in one mL saline and sieved. The tissue cysts number/mouse brain was counted in brain homogenate (10 μL) utilizing light microscopy ×40 objective. The 3 counts average was multiplied by 100 to get the tissue cysts number per one mL of brain suspension.29 In each group, the cysts number mean/brain was estimated.
Serum Preparation
After the animals’ scarification, with and without the use of an anticoagulant, serum samples were collected in sterile vacuum tubes (EDTA). After centrifuging the blood at 3000 rpm for 20 min at 4°C, the serum was collected and kept at −20°C.
Immunological Analysis for IL-4, IL-10, and IFN-γ
Quantitative sandwich enzyme immunoassay measurements of IL-4, IL-10 and IFN-γ, using enzyme-linked immunosorbent assay (ELISA) kits designed for mice (Cat. No: MBS0430070, MBS704754 48T and MBS2500105 96T/48T/24T respectively), following the procedure supplied with each kit. A reader for ELISA that can measure absorbance at 450 nm.33
Histological Section Assay
Different groups’ samples of brain tissue were initially fixed in buffered formalin at a 10% (v/v) concentration before being processed to become paraffin blocks. Hematoxylin and eosin (H & E) stains were used to stain tissue, which were cut into sections of around 5-m thickness.34 The stained sections were then examined by light microscopy.
Immunohistochemical Staining and Analysis
This was performed according to the procedures and recommendations of the manufacturer. Antigen retrieved after treating brain tissue slices with hydrogen peroxide (H2O2, 0.3%) for 15 min to inactivate endogenous peroxidase. After that, the slices were incubated with the anti-Iba1 antibody (ab108539 - Abcam - 1:100) overnight at 4°C, then washed with PBS, incubated with the secondary antibody labelled with horseradish peroxidase (HRP) (DAKO EnVision kit) for 20 min. After PBS washing, the slices were incubated with diaminobenzidine tetrahydrochloride (DAB) for 10 min. Finally, tissue slices underwent dehydration and xylene cleaning before being counter-stained with hematoxylin, then examined under a light microscope.
Immunohistochemical Quantitative Analysis
Leica Application system modules for histological analysis (Leica Microsystems GmbH, Wetzlar, Germany), was used.35 Each immunostained tissue slice was scanned, segmented, and processed to determine the average positive area percentage of IbA-1++ reactive microglia in the cerebral cortices.
Iba-1 immunohistochemistry staining was performed on cortical brain tissue slices from frozen mouse brains. Eight thick sections were fixed with pre-cooled absolute methanol for 20 min and then blocked for 1 h at room temperature with 5% BSA and 0.3% Triton X-100 in PBS. The sections were treated overnight at 4°C with Iba-1-specific primary antibodies, rinsed with PBS, and incubated for 1 h at room temperature with Alexa Fluor® 488 donkey anti-goat IgG (H+L) to localize Iba-1. The paraffin was removed, and the sections were boiled in 10 mM citrate buffer (Lab Vision), pH 6.0, for 4 min to remove the antigen masking. Non-specific binding sites were treated with 10% natural goat serum for 60 min after treatment with 15% H2O2 for 10 min. Then came a series of PBS washes. In addition to the primary antibodies, polyclonal rabbit anti-human Ib2a (DAKO, Glostrup, Denmark), we also used HRP-linked antibodies against rabbit immunoglobulin (each diluted 1:2000). Semi-quantitative assessment-of immunoreactive cells from each brain. Light microscopy with a grading system of 0 = no cells, 1 = few cells (low densities), and 2 = numerous cells (high densities), were used to examine the brain slices.36
Statistical Analysis
The independent samples t-test was used to evaluate the data, indicates that there is a significant difference between each treatment group and the control group. This data was examined using SPSS 16.0 for Windows (SPSS Inc.). Depending on the results of the One-Way ANOVA test, the p-value compared across groups is substantially different. Based on a post hoc test, the p-value is substantially different across the groups (Tukey HSD). Initial p-values of less than 0.05 and less than 0.01 are both significant.
Results
Characterization of SeNPs
The mean size of SeNPs was 31–51 nm, and it is noted that the size of the most frequent particles ranges between 40–42.5 nm (Table 1).
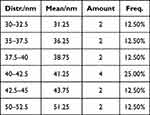 |
Table 1 SeNPs Size |
The values of Z-average, PDI, and ZP of SeNPs were found to be 65.18 nm, 0.293, and −22.0 mV, respectively. Imaging of the freshly prepared delicate NLPs dispersions were obtained using an ultra-high resolution electron microscope of the JEOL TEM microscope. Such images depicted the spherical morphology of the SeNPs with no aggregation observed (Figure 1).
 |
Figure 1 (A) SeNPs characterized by TEM (type JEOL-JEM 2100); (B) Hectogram of mean/nm particles size distribution; (C) Size distribution by intensity; (D) Zeta potential distribution (mV). |
Parasitological Results
Parasitological results (Table 2) showed that the number of Toxoplasma cysts in 0.01 gram of mice brain were significantly reduced in GIII, GV, GVI, VII and GVIII in comparison to GII with efficacy of 70%, 60%, 65%, 71%, and 83% respectively.
 |
Table 2 ME49 T. gondii Strain Brain Cysts Count in Different Groups |
Histopathological Results
Histopathological sections of the cerebral cortex of GII (infected non treated) showed obvious alterations compared with normal control group, the molecular layer of the cerebral cortex showed T. gondii cysts embedded in the tissue and massive deformed neurons surrounded by haloes. The external granular layer of the cerebral cortex of GIII (Spiramycin treated) showed few granule cells with mild intracellular vacuolation and edema associated with proliferation of glia cells and few neuropils appeared vacuolated. While, aqueous and methanolic extracts of P. dactylifera loaded on SeNPs treated groups showed improvement that indicated by few perivascular cuffing with few inflammatory cell infiltrations. Also, few granule cells with mild intracellular vacuolation and edema few deformed neurons with deep pyknotic nuclei were noticed. Most of the neurons appear with normal with central vesicular nucleus and few deformed neurons with deep pyknotic nuclei (Figure 2).
Immunological Results
The levels of IL-4, IL-10 and INF-γ were assessed in serum of all mice groups where GVIII mice exhibited lowest levels of detectable serum cytokines followed by GVII, whereas GII mice exhibited highest production of both cytokines (Table 3 and Figure 3).
 |
Table 3 Serum Level of IL-4, IL-10, and INF-γ of Different Groups |
 |
Figure 3 Serum level of IL-4, IL-10, and INF-γ of different groups. |
Immunohistochemical Results
Normal control (GI) has low expression of Iba1 level, while GII and GIV revealed an increased expression of Iba1 level. The remaining treated groups revealed reduced expression of Iba1 level (Table 4, Figures 4 and 5).
 |
Table 4 The Area Percent of Immunohistochemical Expression of Iba1 in Brain Tissues of the Studied Groups |
 |
Figure 5 The area percent of immunohistochemical expression of Iba1 in brain tissues of the studied groups. |
Discussion
The apicomplexan parasite T. gondii causes toxoplasmosis, which is often asymptomatic. Until now, it can be severe and even fatal in immunocompromised people and fetuses. There is a tremendous incentive to discover innovative therapies because there are not many therapy choices currently available. T. gondii is highly resistant to a variety of inhibitors, even those that patients use the most frequently.37 Moreover, the development of drug-resistant parasite results in clinical failure from prolonged medication therapy.38 Adversely, the high cost and limited availability of drugs for treatment of Toxoplasma, particularly in developing nations, results in a rise in the prescription of conventional drugs. Using in vivo and in vitro assays, researchers have recently started to systematically examine the effectiveness of herbal medicines and their mechanisms of action. Both conditions have been utilized in several studies.39
Although normal metabolic processes continuously produce free radicals, their rate of generation rises during some parasitic diseases. Hence, the transition of Toxoplasma between its tachyzoite and bradyzoite stages is accompanied by morphological and molecular biological variations, such as changes in host metabolism and the expression of stage-specific antigens.40 Chemicals that alter redox status can decrease parasite viability and, as a result, have potential as anti-Toxoplasma medications. On the other hand, host cells may suffer certain negative effects from the oxidative stress brought on by the activation of the inflammatory response. In this regard, the prospective utilization of natural antioxidants as potential cutting-edge anti-Toxoplasma treatments is worthwhile to examine. Animal research and in vitro results are encouraging. However, it was shown that supplementing with specific antioxidants encouraged a rise in parasitism, and the disease took a milder course as a result. Without a doubt, research in this field may have a big impact on how to treat toxoplasmosis in the future.41 It was reported that medical plants’ demonstrated anti-Toxoplasma effects, including survival rates, reduced cell damage, parasite loads, and burdens of brain cysts.42 Because they have fewer side effects, are more readily available, and are more widely accepted by society than the chemical drugs currently in use, natural compounds would help researchers develop new and more effective therapeutic applications for toxoplasmosis. Authors also stated that type I (RH strain) tachyzoite was the most frequently used strain to assess the effects of medicinal herbs.
Several studies indicated that endogenous and dietary antioxidants both demonstrated a key role as preventative agents through the neutralization of the reactive oxygen species (ROS), and that P. dactylifera fruits employed in the management of diseases actively promote the role of ROS in cancer.43–46 The effectiveness of plant phenolic chemicals, particularly flavonoids, as antioxidants with documented antimutagenic and anticarcinogenic activities was subsequently demonstrated.47 In addition, flavonoids have a wide range of desirable qualities, including antibacterial, antiparasitic, antifungal, antiviral, antiapoptotic and anti-inflammatory activity.48–50
The concentration of components is significantly affected by the extraction technique. The primary hydrophobic component of plant root extracts is alkamides, and ethanol extraction increases alkamide concentrations while retaining the more hydrophilic polysaccharides in aqueous extracts.51 Alcoholic and aqueous extracts were employed more frequently than other kinds of extracts.42
The black seed oil from Nigella sativa was able to considerably protect mice infected with the ME49 Toxoplasma strain against death and brain cyst loads as compared to the infected untreated animals.52 The presence of various phenolics and alkaloids in this plant may be responsible for its antiprotozoal properties. Also, Zingiber officinale (ginger) was employed as a natural remedy.53 The primary antioxidant components in ginger are its volatile oil, phenolic derivatives (zingerone), and oleoresin (gingerols and shogaols). In vitro and in vivo tests were conducted to assess the antiparasitic activity of ginger root extract against T. gondii.54 The researchers demonstrated that anti-Toxoplasma effects are due to inactivation of apoptotic proteins in infected host cells.
The cerebral cortex of GII (infected non-treated) was examined using histological sections, which revealed notable differences from the normal control group. The molecular layer of the cerebral cortex revealed embedded T. gondii cysts as well as massively damaged neurons surrounded by haloes. Also, the exterior granular layer of the GIII (Spiramycin treated) cerebral cortex revealed few granule cells with minor intracellular vacuolation and edema linked to glia cell growth, and few neuropils seemed vacuolated. Although P. dactylifera aqueous and methanolic extracts put on SeNPs treated groups demonstrated improvement as evidenced by a reduction in perivascular cuffing and inflammatory cell infiltration. Moreover, a few malformed neurons with deeply pyknotic nuclei and a few granule cells with minor internal vacuolation and edema.
Our findings are consistent with Alajmi et al (2019),15 who examined the anti-Toxoplasma effects of silver nanoparticles (AgNPs) synthesized in combination with extracts of natural plants (P. dactylifera and Ziziphus spina-christi) as a substitute for conventional sulfadiazine drug therapy and demonstrated distinct alterations of histological examinations in the infected compared with untreated control groups. On the other hand, pretreatment with NPs improved the histological traits represented by minimal pleomorphism, reduced hepatocyte degradation, and mild infiltration and fibrosis.
Additionally, the findings of the current study are consistent with GabAllah et al (2021),55 who demonstrated that the inflammatory responses of the brain, liver, and spleen tissues were reduced in the AuNPs-treated group in comparison to the Spiramycin-treated group and the non-treated mice with statistically significant decreases in the mean cyst count and size in the brain, liver.
Previous study found varying degrees of perivascular and interstitial inflammatory infiltrates, astrocytosis, degenerating neurons, and meningitis.56 Microglia and occasional encysted bradyzoites were observed pathologically. Parenchymal infiltrates of inflammatory cells, primarily lymphocytes, were found to be mild, moderate, and intense and to progress with time. The majority of Toxoplasma cysts in infected mouse brains were found in the cortex and striatum regions, which were segregated from perivascular and intra-parenchymal inflammation and far from areas of inflammation. On the other hand, the brains of the infected mice displayed generalized inflammation and perivascular cuffing, lesions with vascular dilatation, congestion, and lymphocytes concentrated around small blood vessels.
Wang et al (2019) found that infected mice’s brain sections revealed varying degrees of perivascular and interstitial inflammatory infiltrates, astrocytosis, degenerating neurons, and meningitis, as well as mild parenchymal infiltrates of inflammatory cells that grew more severe over time with a focus on lymphocytes as the main inflammatory cell type.57 The majority of Toxoplasma cysts in infected mouse brains were found in the cortex and striatum regions, which were segregated from perivascular and intra-parenchymal inflammation and far from areas of inflammation. On the other hand, the brains of the infected mice displayed generalized inflammation and perivascular cuffing, lesions with vascular dilatation, congestion, and lymphocytes concentrated around small blood vessels.
The brain parenchyma has historically been thought of as an immune privileged organ due to the absence of a traditional lymphatic system that drains the CNS antigens, the close capillary junctions of the BBB that limit immune cell entry, and the astrocyte end-feet systems that connect the cells to the outside of capillary walls.58 As a result, microglia are more crucial to the host’s defense than other cell types like astrocytes.59
The primary immune cells or resident macrophages in CNS are microglia generated from the monocyte/macrophage lineage.60 Toxoplasma parasite prefers neurons over local glial cells, where bradyzoites encysted in CNS and creates a latent or persistent infection.61 Using primary cells from murine and human fetal brains, a study found that as Toxoplasma infection progressed, astrocytes and microglia were able to successfully reduce the multiplication and the proliferation of the parasite, while bradyzoite were found in parasitophorous vacuoles in neuronal cells.62
There is some debate regarding certain brain cells’ capacity to contract cysts and catch infections, although it is generally agreed that neurons are the most important host cell,63,64 infection of microglia and astrocytes can also contribute to the development of tissue cysts.65 According to studies on the neurochemistry of chronic toxoplasmosis, the parasite alters the amounts, precursors, and metabolites of neurotransmitters.66 The injection of Toxoplasma rhoptries effector proteins into both parasite-invaded and uninvaded cells are one of the potential ways for influencing neuronal activity.67 T. gondii therefore has complete control over not just the cells it infects, but also the surrounding cells.68
In addition, T. gondii -infected microglial cells can regulate aminobutyric acid levels to alter neuronal activity in addition to inducing hypermigration to aid parasite dispersion in the brain parenchyma.69
According to previous studies, aberrant inflammatory processes and higher levels of microglial activation are present in patients with depression. These factors may be important in the pathophysiology of depression.70,71 The morphology, phenotype, and function of microglia are considerably altered by parenchymal injury, degeneration, or infection,72 changing from ramified to amoeboid forms and exhibiting greater expression levels of the particular microglial marker Iba-1.73
Due to the fact that prior research has shown that after 5 days of infection with the RH strain of T. gondii, the brain’s cortical tissue exhibits a severe inflammatory infiltration and an infiltration of pseudocysts populated by tachyzoites.74 Therefore, limiting T. gondii development in microglia is a more urgent concern than limiting growth in other brain cells.75
We further studied the status of microglia cells (expression of Iba-1) and the content of inflammatory cytokines (IL-4, IL-10 and INF-γ) in serum of all mice groups and found that GVIII mice exhibited lowest levels of detectable serum cytokines followed by GVII, whereas GII mice exhibited highest production of both cytokines. While expression of Iba-1 was higher in GII and lowest level in GVIII followed by GVII.
In agreement with Alajmi et al (2019),15 the results showed significant increase for the effect of AgNPS on hepatic TNF-α and IFN-γ in the level of the untreated infected group while showing a significant decrease in sulfadiazine groups compared with the control group. But this further was inhibited in AgNPs groups. Moreover, AgNPs in combination with extracts of natural plants (P. dactylifera L and Z. spina-christi) were more effective than the sulfadiazine groups.
Our findings are in line with those of Hwang et al (2018),76 who demonstrated that acute T. gondii infection similarly boosted the expression of pro-inflammatory cytokines (IFN- and TNF-), in addition to Iba-1, in microglia. Intriguingly, a more recent study found that T. gondii can activate microglia to induce neuronal death in an in vitro co-culture of microglia and neurons.75 Treatment with sertraline, a microglia inhibitor, was able to undo this change and lessen the neuronal damage that Toxoplasma-infected animals experienced in their cortex and hippocampus. The increased microglial activation, neuronal injury, and inflammatory reactions brought on by acute toxoplasmosis are thought to be mitigated by sertraline.
The combination of numerous medicinal compounds in the plant extracts (like amino acids, polysaccharides, proteins, alkaloids, phenolics, enzymes and vitamins) affect the reduction and stabilization of silver ions.77 In line with that, our study showed that plant extracts loaded on SeNPs revealed better results than NPs alone. Plant extracts can be employed in the production of metallic nanomaterials in addition to microbiological species.78
Conclusions
Our data reported the anti-toxoplasmosis effect of P. dactylifera fruits extracts loaded on SeNPs that could be due to its immunomodulatory and anti-inflammatory effects against T. gondii. However, future research is needed to detect the effective phyto-constituents of P. dactylifera fruits and its potential therapeutic capacities on various parasitic infections.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia for funding this project under grant no (G: 289-142-1443). The authors would like as well to thank the University of Jeddah, Jeddah, Saudi Arabia, and Sciences Academy of Experimental Researches, Mansoura, Egypt, and Al-Azhar University, Cairo, Egypt, for their cooperation during this study.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU), Jeddah, Saudi Arabia has funded this Project under grant no (G: 289-142-1443).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dubey JP. Toxoplasmosis - a waterborne zoonosis. Vet Parasitol. 2004;126(1–2):57–72. doi:10.1016/j.vetpar.2004.09.005
2. Keyhani A, Ziaali N, Shakibaie M, et al. Biogenic selenium nanoparticles target chronic toxoplasmosis with minimal cytotoxicity in a mouse model. J Med Microbiol. 2019;68(1):1–7. doi:10.1099/jmm.0.001111
3. Khryanin AA, Reshetnikov OV, Kuvshinova IN. Toxoplasmosis: epidemiology, diagnosis, treatment. Antibiot Khimioter. 2015;60(5–6):16–21.
4. Antczak M, Dzitko K, Długońska H. Human toxoplasmosis searching for novel chemotherapeutics. Biomed Pharmacother. 2016;82:677–684. doi:10.1016/j.biopha.2016.05.041
5. Alday PH, Doggett JS. Drugs in development for toxoplasmosis: advances, challenges, and current status. Drug Des Devel Ther. 2017;11:273–293. doi:10.2147/DDDT.S60973
6. Kochanowsky JA, Koshy AA. Toxoplasma gondii. Curr Biol. 2018;28(14):R770–R771. doi:10.1016/j.cub.2018.05.035
7. Konradt C, Ueno N, Christian DA, et al. Endothelial cells are a replicative niche for entry of Toxoplasma gondii to the central nervous system. Nat Microbiol. 2016;1(3):16001. doi:10.1038/nmicrobiol.2016.1
8. Elsheikha HM, Marra CM, Zhu XQ. Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin Microbiol Rev. 2020;34:e00115–e00119. doi:10.1128/CMR.00115-19
9. Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophages/microglia. J Cell Sci. 2000;113(17):3073–3084. doi:10.1242/jcs.113.17.3073
10. Pierezan F, Mansell J, Ambrus A, Rodrigues Hoffmann A. Immunohistochemical expression of ionized calcium binding adapter molecule 1 in cutaneous histiocytic proliferative, neoplastic and inflammatory disorders of dogs and cats. J Comp Pathol. 2014;151(4):347–351. doi:10.1016/j.jcpa.2014.07.003
11. Lier J, Streit WJ, Bechmann I. Beyond activation: characterizing microglial functional phenotypes. Cells. 2021;10(9):2236. doi:10.3390/cells10092236
12. Schwabenland M, Brück W, Priller J, Stadelmann C, Lassmann H, Prinz M. Analyzing microglial phenotypes across neuropathologies: a practical guide. Acta Neuropathol. 2021;142(6):923–936. doi:10.1007/s00401-021-02370-8
13. Ide T, Uchida K, Kagawa Y, Suzuki K, Nakayama H. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. J Vet Diagn Invest. 2011;23(1):127–132. doi:10.1177/104063871102300123
14. Montazeri M, Sharif M, Sarvi S, Mehrzadi S, Ahmadpour E, Daryani A. A systematic review of in vitro and in vivo activities of anti-Toxoplasma drugs and compounds (2006-2016). Front Microbiol. 2017;8:25. doi:10.3389/fmicb.2017.00025
15. Alajmi RA, Al-Megrin WA, Metwally D, et al. Anti- Toxoplasma activity of silver nanoparticles green synthesized with Phoenix dactylifera and Ziziphus spina-christi extracts which inhibits inflammation through liver regulation of cytokines in Balb/c mice. Biosci Rep. 2019;39(5):BSR20190379. doi:10.1042/BSR20190379
16. Al-Qarawi A, Mousa HM, Ali B, Abdel-Rahman H, El-Mougy SA. Protective effect of extracts from dates (Phoenix dactylifera L.) on carbon tetrachloride-induced hepatotoxicity in rats. Intern J Appl Res Vet Med. 2004;2:176–180.
17. Shakibaie M, Khorramizadeh MR, Faramarzi MA, Sabzevari O, Shahverdi AR. Biosynthesis and recovery of selenium nanoparticles and the effects on matrix metalloproteinase-2 expression. Biotechnol Appl Biochem. 2010;56(1):7–15. doi:10.1042/BA20100042
18. Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64(4):527–542. doi:10.1079/pns2005467
19. Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91(1):11–28. doi:10.1079/bjn20031015
20. Kojouri GA, Jahanabadi S, Shakibaie M, Ahadi AM, Shahverdi AR. Effect of selenium supplementation with sodium selenite and selenium nanoparticles on iron homeostasis and transferrin gene expression in sheep: a preliminary study. Res Vet Sci. 2012;93(1):275–278. doi:10.1016/j.rvsc.2011.07.029
21. Beheshti N, Soflaei S, Shakibaie M, et al. Efficacy of biogenic selenium nanoparticles against Leishmania major: in vitro and in vivo studies. J Trace Elem Med Biol. 2013;27(3):203–207. doi:10.1016/j.jtemb.2012.11.002
22. Yazdi MH, Mahdavi M, Faghfuri E, et al. Th1 immune response induction by biogenic selenium nanoparticles in mice with breast cancer: preliminary vaccine model. Iran J Biotechnol. 2015;13(2):1–9. doi:10.15171/ijb.1056
23. Das S, Bhattacharya A, Debnath N, Datta A, Goswami A. Nanoparticle-induced morphological transition of Bombyx mori nucleopolyhedrovirus: a novel method to treat silkworm grasserie disease. Appl Microbiol Biotechnol. 2013;97(13):6019–6030. doi:10.1007/s00253-013-4868-z
24. El-Khadragy M, Alolayan EM, Metwally DM, et al. Clinical efficacy associated with enhanced antioxidant enzyme activities of silver nanoparticles biosynthesized using Moringa oleifera leaf extract, against cutaneous leishmaniasis in a murine model of Leishmania major. Int J Environ Res Public Health. 2018;15(5):1037. doi:10.3390/ijerph15051037
25. Adeyemi OS, Murata Y, Sugi T, Kato K. Inorganic nanoparticles kill Toxoplasma gondii via changes in redox status and mitochondrial membrane potential. Int J Nanomed. 2017;12:1647–1661. doi:10.2147/IJN.S122178
26. Adeyemi OS, Sulaiman FA. Evaluation of metal nanoparticles for drug delivery systems. J Biomed Res. 2015;29(2):145–149. doi:10.7555/JBR.28.20130096
27. Vazini H, Esboei BR. The investigation of nanoparticles of gold’s fatality effect on Toxoplasma gondii parasite in an in vitro study. J Antimicrob Agents. 2018;4:2. doi:10.4172/2472-1212.1000172
28. Rahul S, Chandrashekhar P, Hemant B, et al. In vitro antiparasitic activity of microbial pigments and their combination with phytosynthesized metal nanoparticles. Parasitol Int. 2015;64(5):353–356. doi:10.1016/j.parint.2015.05.004
29. Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J. Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii. J Antimicrob Chemother. 2002;50(6):981–987. doi:10.1093/jac/dkf251
30. Kim IS, Yang MR, Lee OH, Kang SN. Antioxidant activities of hot water extracts from various spices. Int J Mol Sci. 2011;12(6):4120–4131. doi:10.3390/ijms12064120
31. Erian NS, Hamed HB, El-Khateeb AY, Farid M. Total polyphenols, flavonoids content and antioxidant activity of crude methanolic and aqueous extracts for some medicinal plant flowers. Arab J Sci Res Publ. 2016;2:53–61.
32. Li Q, Chen T, Yang F, Liu J, Zheng W. Facile and controllable one-step fabrication of selenium nanoparticles assisted by L-cysteine. Mater Lett. 2010;64(5):614–617. doi:10.1016/j.matlet.2009.12.019
33. El Aswad BW, Sadek GS. Parasitological, pathological and immunological effects of hesperidin treatment on murine schistosomiasis. Mansoni Life Sci J. 2014;11:840–855.
34. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:prot4986. doi:10.1101/pdb.prot4986
35. El-Deeb NK, El-Tanbouly DM, Khattab MA, El-Yamany MF, Mohamed AF. Crosstalk between PI3K/AKT/KLF4 signaling and microglia M1/M2 polarization as a novel mechanistic approach towards flibanserin repositioning in Parkinson’s disease. Int Immunopharmacol. 2022;112:109191. doi:10.1016/j.intimp.2022.109191
36. Bancroft JD, Gamble M. Theory and Practice of Histological Techniques.
37. McFadden DC, Camps M, Boothroyd JC. Resistance as a tool in the study of old and new drug targets in Toxoplasma. Drug Resist Updat. 2001;4(2):79–84. doi:10.1054/drup.2001.0184
38. Reynolds MG, Oh J, Roos DS. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 2001;45(4):1271–1277. doi:10.1128/AAC.45.4.1271-1277.2001
39. Benoit-Vical F, Santillana-Hayat M, Kone-Bamba D, Mallie M, Derouin F. Anti- Toxoplasma activity of vegetal extracts used in West African traditional medicine. Parasite. 2000;7(1):3–7. doi:10.1051/parasite/2000071003
40. Yan C, Liang LJ, Zheng KY, Zhu XQ. Impact of environmental factors on the emergence, transmission and distribution of Toxoplasma gondii. Parasit Vectors. 2016;9(1):137. doi:10.1186/s13071-016-1432-6
41. Szewczyk-Golec K, Pawłowska M, Wesołowski R, Wróblewski M, Mila-Kierzenkowska C. Oxidative stress as a possible target in the treatment of toxoplasmosis: perspectives and ambiguities. Int J Mol Sci. 2021;22(11):5705. doi:10.3390/ijms22115705
42. Sharif M, Sarvi S, Pagheh AS, et al. The efficacy of herbal medicines against Toxoplasma gondii during the last 3 decades: a systematic review. Can J Physiol Pharmacol. 2016;94(12):1237–1248. doi:10.1139/cjpp-2016-0039
43. Hameed MF, Mkashaf IA, Al-Shawi AAA, Hussein KA. antioxidant and anticancer activities of heart components extracted from Iraqi Phoneix dactylifera chick. Asian Pac J Cancer Prev. 2021;22(11):3533–3541. doi:10.31557/APJCP.2021.22.11.3533
44. Essa MM, Singh V, Guizani N, et al. Phoenix dactylifera L. fruits date fruit ameliorate oxidative stress in 3-np intoxicated PC12 cells. Int J Nutr Pharmacol Neurol Dis. 2019;9:41–47. doi:10.4103/ijnpnd.ijnpnd_51_18
45. Salem GA, Shaban A, Diab HA, et al. Phoenix dactylifera protects against oxidative stress and hepatic injury induced by paracetamol intoxication in rats. Biomed Pharmacother. 2018;104:366–374. doi:10.1016/j.biopha.2018.05.049
46. Khan F, Khan TJ, Kalamegam G, et al. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement Altern Med. 2017;17(1):418. doi:10.1186/s12906-017-1926-6
47. Metwaly MS, Dkhil MA, Al-Quraishy S. Anti-coccidial and anti-apoptotic activities of palm pollen grains on Eimeria papillata-induced infection in mice. Biologia. 2014;69(2):254–259. doi:10.2478/s11756-013-0297-9
48. Ullah A, Munir S, Badshah SL, et al. Important flavonoids and their role as a therapeutic agent. Molecules. 2020;25(22):5243. doi:10.3390/molecules25225243
49. Daoud A, Malika D, Bakari S, et al. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of date palm pollen (DPP) from two Tunisian cultivars. Arabian J Chem. 2015;12(8):3075–3086. doi:10.1016/j.arabjc.2015.07.014
50. Biglari F, AlKarkhi AF, Easa AM. Antioxidant activity and phenolic content of various date palm (Phoenix dactylifera) fruits from Iran. Food Chem. 2008;107(4):1636–1641. doi:10.1016/j.foodchem.2007.10.033
51. Hall C. Echinacea as a functional food ingredient. Adv Food Nutr Res. 2003;47:113–173. doi:10.1016/s1043-4526(03)47003-4
52. Rayan HZ, Wagih HM, Atwa MM. Efficacy of black seed oil from Nigella sativa against murine infection with cysts of Me49 strain of Toxoplasma gondii. Parasitol United J. 2011;4:165–176.
53. Rashidian A, Mehrzadi S, Ghannadi AR, Mahzooni P, Sadr S, Minaiyan M. Protective effect of ginger volatile oil against acetic acid-induced colitis in rats: a light microscopic evaluation. J Integr Med. 2014;12(2):115–120. doi:10.1016/S2095-4964(14)60011-X
54. Choi W, Jiang M, Chu J. Antiparasitic effects of Zingiber officinale (Ginger) extract against Toxoplasma gondii. J Appl Biomed. 2013;11(1):15–26. doi:10.2478/v10136-012-0014-y
55. GabAllah MR, Barakat AMA, Ahmed NS, El-Nadi NA. Histopathological and biochemical assessment of the therapeutic effect of gold nanoparticles on experimental chronic toxoplasmosis Parasitol United. J. 2021;14:171–177. doi:10.21608/puj.2021.73358.1117
56. Etewa S, Sarhan M, Moawad H, et al. Behavior and neuropsychiatric changes in experimental chronic toxoplasmosis: histopathological and immunohistochemical studies. Parasitol United J. 2021;14(2):183–192. doi:10.21608/PUJ.2021.75319.1120
57. Wang T, Sun X, Qin W, et al. From inflammatory reactions to neurotransmitter changes: implications for understanding the neurobehavioral changes in mice chronically infected with Toxoplasma gondii. Behav Brain Res. 2019;359:737–748. doi:10.1016/j.bbr.2018.09.011
58. Carson MJ, Doose JM, Melchior B, Schmid CD, Ploix CC. CNS immune privilege: hiding in plain sight. Immunol Rev. 2006;213(1):48–65. doi:10.1111/j.1600-065X.2006.00441.x
59. Peterson PK, Gekker G, Hu S, Chao CC. Intracellular survival and multiplication of Toxoplasma gondii in astrocytes. J Infect Dis. 1993;168(6):1472–1478. doi:10.1093/infdis/168.6.1472
60. Vyas A. Mechanisms of host behavioral change in Toxoplasma gondii rodent association. PLoS Pathog. 2015;
61. Contreras-Ochoa CO, Lagunas-Martínez A, Belkind-Gerson J, Díaz-Chávez J, Correa D. Toxoplasma gondii invasion and replication within neonate mouse astrocytes and changes in apoptosis related molecules. Exp Parasitol. 2013;134(2):256–265. doi:10.1016/j.exppara.2013.03.010
62. Lüder CG, Giraldo-Velåsquez M, Sendtner M, Gross U. Toxoplasma gondii in primary rat CNS cells: differential contribution of neurons, astrocytes, and microglial cells for the intracerebral development and stage differentiation. Exp Parasitol. 1999;93(1):23–32. doi:10.1006/expr.1999.4421
63. Cabral CM, Tuladhar S, Dietrich HK, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12(2):e1005447. doi:10.1371/journal.ppat.1005447
64. Dubey JP, Ferreira LR, Alsaad M, et al. Experimental toxoplasmosis in rats induced orally with eleven strains of Toxoplasma gondii of seven genotypes: tissue tropism, tissue cyst size, neural lesions, tissue cyst rupture without reactivation, and ocular lesions. PLoS One. 2016;11(5):e0156255. doi:10.1371/journal.pone.0156255
65. Hermes G, Ajioka JW, Kelly KA, et al. Neurological and behavioral abnormalities, ventricular dilatation, altered cellular functions, inflammation, and neuronal injury in brains of mice due to common, persistent, parasitic infection. J Neuroinflam. 2008;5(1):48. doi:10.1186/1742-2094-5-48
66. Sinai AP, Watts EA, Dhara A, Murphy RD, Gentry MS, Patwardhan A. Reexamining chronic Toxoplasma gondii infection: surprising activity for a “dormant” parasite. Curr Clin Microbiol Rep. 2016;3(4):175–185. doi:10.1007/s40588-016-0045-3
67. Koshy AA, Dietrich HK, Christian DA, et al. Toxoplasma co-opts host cells it does not invade. PLoS Pathog. 2012;8(7):e1002825. doi:10.1371/journal.ppat
68. Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6(1):79–88. doi:10.1038/nrmicro1800
69. Bhandage AK, Kanatani S, Barragan A. Toxoplasma-induced hypermigration of primary cortical microglia implicates GABAergic signaling. Front Cell Infect Microbiol. 2019;9:73. doi:10.3389/fcimb.2019.00073
70. Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. 2015;9:476. doi:10.3389/fncel.2015.00476
71. Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi:10.1016/j.biopsych.2008.11.029
72. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32(1):367–402. doi:10.1146/annurev-immunol-032713-120240
73. Meneses CS, Müller HY, Herzberg DE, Uberti B, Bustamante HA, Werner MP. Immunofluorescence characterization of spinal cord dorsal horn microglia and astrocytes in horses. PeerJ. 2017;5:e3965. doi:10.7717/peerj.3965
74. El Temsahy MM, El Kerdany ED, Eissa MM, Shalaby TI, Talaat IM, Mogahed NM. The effect of chitosan nanospheres on the immunogenicity of Toxoplasma lysate vaccine in mice. J Parasit Dis. 2016;40(3):611–626. doi:10.1007/s12639-014-0546-z
75. Lan HW, Lu YN, Zhao XD, et al. New role of sertraline against Toxoplasma gondii -induced depression-like behaviours in mice. Parasite Immunol. 2021;43(12):e12893. doi:10.1111/pim.12893
76. Hwang YS, Shin JH, Yang JP, Jung BK, Lee SH, Shin EH. Characteristics of infection immunity regulated by Toxoplasma gondii to maintain chronic infection in the brain. Front Immunol. 2018;9:158. doi:10.3389/fimmu.2018.00158
77. Keat CL, Aziz A, Eid AM, Elmarzugi NA. Biosynthesis of nanoparticles and silver nanoparticles. Bioresources Bioprocess. 2015;2(1):47. doi:10.1186/s40643-015-0076-2
78. Said DE, Elsamad LM, Gohar YM. Validity of silver, chitosan, and curcumin nanoparticles as anti-Giardia agents. Parasitol Res. 2012;111(2):545–554. doi:10.1007/s00436-012-2866-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


