Back to Journals » Journal of Pain Research » Volume 17
Postoperative MRI Findings Following PELD and Their Correlations with Clinical Prognosis are Investigated by Injecting Contrast into Annulus Fibrosus Intraoperatively
Authors Bu J, Wang Z, Ma C, Gao J, Liu G, Pang L, He B, Dong M, Zhang Q, Lei Y , Xu L, Huang S, Li Y , Liu G
Received 20 October 2023
Accepted for publication 30 December 2023
Published 30 January 2024 Volume 2024:17 Pages 381—392
DOI https://doi.org/10.2147/JPR.S442224
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Krishnan Chakravarthy
Jinhui Bu,1,2,* Zhenfei Wang,2,* Chao Ma,1,2 Juan Gao,2 Guangpu Liu,2 Libo Pang,2 Bo He,1 Minghui Dong,1 Quan Zhang,1 Yan Lei,1 Long Xu,2 Sen Huang,1 Yuming Li,3 Guangwang Liu1,2
1Xuzhou Clinical School of Xuzhou Medical University, Xuzhou, People’s Republic of China; 2Department of Orthopedic Surgery, Xuzhou Central Hospital, Xuzhou, People’s Republic of China; 3Department of Surgery, Boston Children’s Hospital, Harvard Medical School, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: Yuming Li, Tel +1-8573524988, Fax +1-8572188459, Email [email protected]; Guangwang Liu, Tel +86-18012018238, Fax +86-51683956400, Email [email protected]
Objective: To validate whether a residual mass demonstrated on early postoperative MR after percutaneous endoscopic lumbar discectomy (PELD) is indeed an intraoperatively retained annulus fibrosus, and explore the correlation between imaging changes in the residual mass and clinical prognosis of patients.
Methods: A prospective study of 118 patients were included. During surgery, a contrast medium, Gadopentetate Dimeglumine, was injected around the ruptured annulus fibrosus. The intensity of the T2 signal, the size of the remaining mass (SR), and the cross-sectional area of the spinal canal (SCSA), VAS, and ODI were assessed at preoperative, 1-h (7-day), 6-month, and 12-month postoperative intervals. Based on VAS at 7 days post-surgery, patients were classified into either a non-remission group (Group A, VAS > 3) or a remission group (Group B, VAS ≤ 3).
Results: Six patients who developed recurrent LDH were excluded. A residual mass was detected on MRI 1 h after surgery in 94.6% (106/112). During one year of follow-up, 90.1% (101/112) of the patients displayed fibrous annulus remodeling, although 68.7% (77/112) still exhibited herniation. Significant differences were found in the ODI between Groups A and B one week after surgery (p < 0.001). However, no significant differences were observed in T2 signal intensity, SR, and SCSA at 1-h, 6-month and 12-month post-surgery (p > 0.05) between the two groups. In a multiple linear regression analysis, early postoperative ODI changes were associated with T2 signal (B = − 10.22, sig < 0.05), long-term changes were associated with alterations in SR (B = 5.63, sig < 0.05) and SCSA (B = − 0.13, sig < 0.05).
Conclusion: The residual mass observed in early postoperative MR images after PELD was the retained annulus fibrosus intraoperatively. Short-term changes in clinical symptoms after PELD were linked to T2 signal intensity, while long-term changes were associated with changes in SR and SCSA.
Keywords: spinal endoscopy, residual mass, remodeling of fibrous annulus, contrast medium
Introduction
Lumbar disc herniation (LDH) is a prevalent degenerative disease of the spine that frequently occurs in older individuals and those engaged in heavy manual labor.1 This condition is generally caused by degeneration of the lumbar disc or a tear in the annulus fibrosus, which allows the nucleus pulposus to protrude and exert pressure on the dural sac or nerve roots, resulting in clinical symptoms such as lower back pain and sciatica.2 The frequency of cases of lumbar disc herniation is on the rise, and patients who have not experienced symptom relief after three months of nonsurgical interventions such as physical therapy and medication typically require surgical treatment.3 Since Kambin et al4 proposed and first used percutaneous spinal endoscopic lumbar discectomy (PELD) in 1973, this procedure has become increasingly advanced for treating LDH. The operator removes the hypertrophic ligamentum flavum and the protruding nucleus pulposus using miniature surgical instruments under direct endoscopic guidance, effectively alleviating pressure on the dural sac or nerve roots. The ruptured annulus fibrosus is preserved and repaired. Compared to traditional open lumbar discectomy and lumbar microdiscectomy, PELD offers benefits such as reduced tissue trauma, shorter hospital stays, and a lower infection rate.3,5
The utility of early post-surgical spinal MRI has proven to be limited, showing a poor correlation with the clinical outcomes of patients.6,7 Studies have indicated that nearly 90% of patients depict a residual mass and ipsilateral nerve root compression on immediate postoperative MRI scans following PELD. These imaging reports often describe phenomena such as “in a spinal protrusion”, “T2 high or intermediate signal”, “T1 intermediate signal”, and “mixed signal shadows”, which are considered to reflect epidural soft tissue edema and changes in spinal canal anatomy, such as residual annulus fibrosus or epidural hematoma.7,8 Postoperative imaging findings may be misinterpreted by some patients who do not experience symptomatic relief after PELD, assuming that the intraoperative retained annulus fibrosus was a protrusion that had not been completely removed during the initial procedure. Consequently, they may opt for additional, unnecessary surgeries, increasing the medical burden.9,10
Consequently, we used PELD as a treatment for LDH. We monitored the alterations in the residual mass within the spinal canal and evaluated its correlation with clinical outcomes. This was achieved by injecting a gadolinium-based contrast medium into the annulus fibrosus left in place during surgery. We then conducted MRI scans of the lumbar spine within 1 h post-surgery and compared these results with lumbar MRI scans taken at 6 and 12 months postoperatively.
Data and Methods
General Information
In the cohort of patients with lumbar disc herniation at the Department of Spine Surgery at our hospital between January 2021 and June 2022, 118 individuals met the predefined inclusion criteria and were subsequently enrolled in the study. This study was approved by the Biomedical Research Ethics Review Committee of our hospital. Inclusion criteria included the following: (1) low back pain with sciatica, (2) preoperative lumbar MRI can clearly display the site of single-segment disc herniation, (3) after three months of strict conservative treatment (medication, and physical therapy), (4) receive PELD surgery, (5) signed informed consent. The exclusion criteria were as follows: (1) with lumbar spinal stenosis, (2) asymptomatic LDH, (3) extremely lateral LDH, (4) active infection, (5) lumbar spondylolisthesis, (6) trauma; and (7) renal hypofunction.11 Clinical and imaging data were collected from all patients, such as age, gender, disease duration, surgical segment, surgical complications, laboratory values of renal function (BUN and Scr) before and within 24–48h after surgery, and MRI images of the lumbar spine (preoperatively, 1 h, 6 months, and 12 months postoperatively). The clinical indices (VAS and ODI) were assessed and recorded before the operation and at 1 week, 6 months, and 12 months postoperatively, and the clinical efficacy was assessed using the modified MacNab at the final follow-up. The patients were classified into a non-remission group (Group A, VAS > 3) and a remission group (Group B, VAS > 3) based on their VAS scores seven days postoperatively.
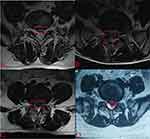
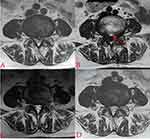
Qualitative and quantitative analyses of each patient’s MRI images were performed by two spine surgeons, such as T2 signal intensity and the size of the residual mass, and the cross-sectional area of the spinal canal. The predominant signal intensity of the residual mass on the T2-weighted image was compared with that of the paraspinal muscular tissue in the same image, if the signal intensity was higher than, equal to, or lower than the latter, the signal was rated as high, medium, or low, respectively (Figure 1A–C). The size of the residual mass (SR) was measured by drawing a line at the base of the disc at the protrusion, then drawing a vertical line from the first line to the most prominent point and measuring this distance (Figure 1D). Spinal canal cross-sectional area (SCSA): Spinal canal boundary measurements were performed in the plane of the residual mass, anteriorly by the posterior edge of the residual mass, posteriorly by the anterior edge of the ligamentum flavum, and bilaterally by the inner edge of the pedicle root, if the image of the spinal canal demonstrated opening at the sides, bilaterally by the outer edges of nerve roots (Figure 1D).
Surgery Procedure
The gadopentetate dimeglumine contrast solution (0.02 g/mL) was prepared by taking 1 mL of gadopentetate dimeglumine contrast medium (Magnevist; specification: 15 mL: 7.035 g) and 20 mL of sterile saline, mixing them uniformly, and placing them on the intraoperative sterile table for backup.
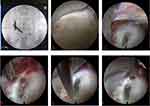
In this study, the patient was positioned prone, and the surgical site was subsequently exposed, sterilized, and draped in accordance with the standard surgical protocols. Local anesthesia was administered via injection of 0.5% lidocaine at the segment targeted for surgery. Under fluoroscopic guidance from a C-arm device, a guide needle was precisely inserted at the inferior margin of the lamina space and medial of the inferior articular process on the side of the herniated disc to the ligamentum flavum of the corresponding segment. An incision measuring approximately 0.8 cm was made on the skin. After confirming the appropriate depth and spacing via fluoroscopy, dilating, and working cannulas were inserted through the guide needle, followed by placing a spinal endoscope (Figure 2A). Subsequently, portions of the ligamentum flavum and extradural adipose tissue that obstructed the visual field were excised. Surgical instruments were carefully introduced into the vertebral canal, revealing the dural sac and nerve roots, which were gently displaced to the contralateral side for protection. Endoscopic surgical tools were then employed to excise the pathological nucleus pulposus, thereby fully decompressing the affected nerve root (Figure 2B and C). Once satisfactory pulsation of the nerve root was observed, bipolar radiofrequency ablation was performed to achieve hemostasis. Concurrently, potential nerve endings on the posterior annulus fibrosus surface were ablated using the same modality.
Under direct endoscopic surveillance, the working cannula was rotated in situ, the dural sac and nerve root were placed outside the cannula for protection, and the cannula was gently pressed down to adequately display annulus fibrosus breach and its periphery, when a long microscopic puncture needle pierced the periphery of the ruptured annulus fibrosus. A slight resistance was felt, the gadopentetate dimeglumine contrast solution was slowly pushed in, and the endoscopic display saw liquid outflow from the injection site, and the injection was stopped in time. The above maneuver was repeated, 3–5 mL of gadopentetate dextran contrast solution was administered into the annulus fibrosus periphery uniformly (Figure 2D–F). After continuous rinsing with sterile saline for 5–7 min, the working cannula was subsequently removed, and the surgical incision was closed with a single suture, followed by the insertion of a drainage tube.
Within 1h after surgery, the patient underwent lumbar MRI scan to show the distribution of gadolinium in the annulus fibrosus, compared with the preoperative MR (Figures 3 and 4).
Data Analysis
Data were statistically analyzed using SPSS statistical software (IBMSPSS 25.0, Chicago, USA). Measurement data were reported as the mean ± standard deviation. Counting data were reported as frequency number (%). Measurement information was tested using a paired t-test, an independent sample t-test, and repeated ANOVA. The Kruskal–Wallis H-test was used to test the counting data. The Pearson test was used for the correlation of parametric data. The Spearman test was used for correlation of non-parametric data. Multiple linear regression was utilized to analyze the correlation between short- and long-term postoperative changes in clinical indicators (ODI) and changes in imaging parameters (T2 signal intensity, SR, and SCSA). Statistical significance was set at P < 0.05.
Results
The study cohort consisted of 118 patients, including 50 females and 68 males, with a mean age of 52 years (range 27–77 years). Six patients who developed recurrent lumbar disc herniation at the 1-year postoperative follow-up and were excluded from the study. No significant abnormalities in BUN and Scr levels were observed in all patients before and after surgery (Table 1). MRI scans taken 1 h postoperatively revealed a residual mass in 94.6% (106/112) of the cases. The patients were classified into a non-remission group (Group A, 31 patients, 27.7%) and a remission group (Group B, 81 patients, 72.3%) based on their VAS scores at seven days postoperatively. There were no statistical differences between the two groups in terms of age, gender, disease duration, and surgical segments (Table 2). In both groups, no intraoperative adverse events were observed, including nerve damage or cerebrospinal fluid leakage. The patients in both groups displayed significant improvements in VAS, ODI, SR, and SCSA at 12 months after surgery compared with the preoperative period, and the difference was statistically significant (p < 0.001, Table 3, Figure 5A–D). There was a significant difference between patients in groups A and B regarding ODI at one week postoperatively, and the difference was statistically significant (p< 0.001, Table 3, Figure 5A and B). However, no statistical difference existed between the two groups in terms of SR and SCSA at 1 h postoperatively and in terms of VAS, ODI, SR, and SCSA at 6 and 12 months postoperatively (p > 0.05, Table 3, Figure 5A–D). There were no statistically significant differences between the T2 signal intensity of the residual mass in the two groups at 1 h, 6 months, and 12 months postoperatively (p > 0.05, Table 4). In the correlation analysis between clinical indicators and imaging parameters, a short change in ODI was correlated with the T2 signal of the residual mass, and a long change in ODI was linked to the T2 signal of the residual mass, changes in SR, and changes in SCSA (Table 5). In a multiple linear regression analysis with changes in ODI changes (one week postoperatively and one year after surgery) as a dependent variable, early postoperative ODI changes were associated with the T2 signal of the residual mass (B = –10.22, sig < 0.05), and long-term postoperative ODI changes were connected to changes in SR (B = 5.63, sig < 0.05, Table 6) and SCSA (B = –0.13, sig < 0.05, Table 7). About 83.0% (93/112) of the patients demonstrated excellent clinical outcomes, with an excellent rate of 80.6% (25/31) in Group A and 83.9% (68/81) in Group B. Furthermore, 90.1% (101/112) of the cohort exhibited fibrous annulus remodeling, although 68.7% (77/112) continued to exhibit herniation of the fibrous annulus (23 patients in Group A and 54 patients in Group B) (Figures 3A, C, D, 4A, C, and D).
 |
Table 1 Comparison of Laboratory Values of Renal Function (BUN, Scr) in All Patients Preoperatively and 24–48 h Postoperatively |
 |
Table 2 Clinical Data of Groups A and B (Age, Gender, Disease Duration, and Surgical Segment) |
 |
Table 3 Clinical Parameters VAS and ODI and Imaging Parameters SR and SCSA in Groups A and B at Preoperative and 1 h, 6 Months, 12 Months Postoperative |
 |
Table 4 The Number of the Residual Mass and the Intensity of the T2 Signal of the Residual Mass at 1 h, 6 Months, and 12 Months After Surgery in Groups A and B |
 |
Table 5 Correlation Analysis of ODI Changes (One Week Postoperatively and One Year Postoperatively) with Changes in Imaging Parameters |
 |
Table 6 Multiple Linear Regression Analysis of MRI Parameters with ODI Changes at One Week Postoperatively as a Dependent Variable |
 |
Table 7 Multiple Linear Regression Analysis of MRI Parameters with ODI Changes at One Year Postoperatively as a Dependent Variable |
Discussion
This study aimed to illustrate that “the residual mass in the operated segment shown on early MRI after PELD is indeed the intraoperatively retained annulus fibrosus”. Due to the inherent complexities and steep learning curve associated with PELD,12 spinal surgeons with limited experience can use the findings of this study to pre-emptively inform patients about postoperative MRI results and possible symptoms, such as lower limb numbness, acidic swelling, and aching.10 This proactive communication can alleviate patient anxiety concerning disease recurrence and minimize the likelihood of unwarranted reoperations.
PELD is a common minimally invasive technique for LDH treatment. Compared to open lumbar discectomy with fusion internal fixation or lumbar microdiscectomy, PELD offers distinct advantages. It circumvents extensive trauma, significant blood loss, post-surgical chronic lumbar pain, and diminished lumbar mobility. Furthermore, it preserves the height of the intervertebral disc, minimizes lamina and ligamentous complex damage, prevents posterior shift of axial loads, delays vertebral joint degeneration and modic changes, and reduces the surgical and hospitalization durations for patients.13–15 However, PELD is not devoid of potential complications, including dural injury, residual mass, recurrence, and harm to the cauda equina and spinal nerve roots.16 Notably, recurrent lumbar disc herniation following PELD has a reported incidence of 5.7%–15%, with the majority manifesting within six months post-surgery.17 Li et al18 indicated correlations between the severity of disc degeneration, the magnitude of post-surgical fibrous annulus ruptures, and herniation sites with early PELD recurrence. Expansive fibrous annulus ruptures and defects accelerate disc degeneration. In turn, this can lead to nerve root adhesion, aseptic inflammation, and subsequent chronic lumbar pain after PELD. To mitigate the annulus fibrosus defect and reduce the disc herniation recurrence rate, Mahatthanatrakul et al7 recommended that surgeons ablate granulation tissue inside the annulus fibrosus fissure and reduce the fissure with bipolar radiofrequency following the decompression of nerve roots or dural sac, while preserving the annulus fibrosus in the herniated disc.
MRI is a widely utilized diagnostic tool for LDH, renowned for its non-invasive nature, high-resolution imaging, and ability to differentiate tissue signals based on varying scanning sequences.19 This effectively delineates the relationship between the intervertebral disc, dural sac, and epidural soft tissue. Early postoperative MRIs in LDH patients frequently depict ipsilateral nerve roots compressed by residual masses and unusual disc signals in the operated segment.6,7,19 Weber et al6 conducted lumbar microdiscectomy on 30 patients, followed by 3.0 T MRI scans 24 h post-surgery. Interestingly, 80% of patients displayed epidural soft tissue modifications and intra-spinal protrusions reminiscent of preoperative images at the surgical site. In a study involving 31 PELD patients, Mahatthanatrakul et al7 revealed that all of them had intra-spinal protrusions in the operated segment on MRIs taken within 24 h post-surgery. The aforementioned protrusions demonstrated high and intermediate mixed signal shadows with nerve root compression and thickening on T2-weighted images. In this study, with moderate inter-rater consistency across all variables, 94.6% (106/112) of cases demonstrated a residual mass on MRIs taken 1 h post-surgery. The signal distribution on T2 was 64% high, 28% intermediate, and 8% low. Specifically, T2 high and intermediate signals typically indicated epidural edema or hematoma, while T2 low signals were associated with residual annulus fibrosus. As postoperative recovery progressed, the body absorbed epidural edema and hematoma, whereas the residual annulus fibrosus underwent structural alterations, distancing it from the nerve root. Wang et al8 deduced that post-PELD early MRIs typically displayed quicker subsidence of the T2 high residual mass signal than open lumbar discectomy, predominantly within three months post-surgery. This T2 signal was linked to short-term clinical outcomes (p = 0.004) but not to medium- and long-term results. Furthermore, a multifactorial regression analysis found that a smaller DCSA in the early postoperative phase was linked to radicular pain after lumbar decompression (sig < 0.05, OR: 1.26). This radicular pain tended to be alleviated as the dural sac expanded in most patients.9 In our findings, short-term ODI variations were correlated with the intensity of the T2 signal of the residual mass observed at 1 h post-surgery (B = −10.22, sig < 0.05). Meanwhile, long-term ODI alterations were associated with changes in the size of the residual mass (B = 5.63, sig < 0.05) and the spinal canal cross-sectional area (B = −0.13, sig < 0.05). In essence, early post-surgery MRIs with high T2 signals in the residual mass primarily forecasted short-term clinical improvement, whereas the decrease in residual mass and dural area expansion signified long-term clinical improvement.
Earlier research on the “residual mass” in the literature has posited, based on multiple postoperative MRI evaluations, that this mass primarily consisted of intraoperative remnants of the annulus fibrosus.7,8,19 Simultaneously, because lumbar MR enhancement is weak for intraoperative visualization of the retained annulus fibrosus, intuitive results cannot be achieved. Consequently, a distinctive aspect of our study is the intraoperative injection of 3–5 mL of gadopentetate dimeglumine contrast solution (0.02 g/mL) directly into the retained annulus fibrosus. This allows immediate postoperative MRI T1-weight scans to distinctly visualize the periphery of the annulus fibrosus rupture distinctly. Gadolinium, a water-soluble, non-iodine contrast agent, is dispersed in extracellular fluid. It generates a potent magnetic field that alters proton relaxation rates in water molecules. Due to the long T1 relaxation time of body tissue, low concentrations of gadopentetate dimeglumine contrast medium have a large effect on the T1 relaxation time, which can enhance tissue signal, and are often used as MRI-positive enhancers.11,20 The rate of adverse reactions to intravenously administered gadolinium-based medications was 0.06%.11 Vital signs were monitored throughout the operation to mitigate risks, such as allergic reactions. Renal function tests (BUN, Scr, etc.) were performed within 24–48 h postoperatively, and no renal impairment was observed in the laboratory results. In the postoperative MRI T1-weight scan, a pronounced high signal could be seen around the exterior of the residual mass. This signal, which coincides with the size and location of the preoperative disc herniation and is aligned with the endoscopically observed contrast medium injection site, solidified its identification as the retained annulus fibrosus (Figures 3 and 4).
Following PELD, the early postoperative stage usually involves nerve root compression by the residual mass without manifesting distinct clinical symptoms.21 Nonetheless, some patients report persistent symptoms postoperatively, including sensations of numbness, tingling, or localized burning in the lower limbs. Several factors may be responsible for these manifestations; the first of them is, preoperative nerve root fiber bundle injury. Wang et al22 identified that the length of preoperative symptoms and the extent of nerve root compression were independent risk factors for numbness and weakness in the lower extremities post-PELD (p < 0.05, OR: 1.015). Second, inadequate local anesthesia, compression from the endoscopic working cannula, and irritation from surgical instruments could induce inflammatory swelling in the nerve root, which can lead to temporary sensory and motor deficits.16 Third is the premature resumption of work and activities. Liang et al23 reported a lower VAS score for post-surgical lumbar pain in patients with limited mobility than in those without such restrictions within a month after the operation. Restricting mobility after PELD may significantly reduce LDH recurrence and alleviate lumbar pain. Finally, inflammatory edema of nerve roots during the recovery stage. Zhang et al24 found that rebound pain started within one month after PELD (10.4%, 15/114), typically with leg pain with or without lower back pain lasting less than one month, which was relieved after a short period of adequate rest or conservative treatment. In addition, the degree of neural decompression achieved during surgery is not always accurately reflected in early postoperative MRIs. In our study, the size of the residual mass and the spinal canal cross-sectional area demonstrated no significant differences between the groups at 1 h, 6 months, or 12 months post-surgery (p < 0.05). The only notable difference was in the clinical symptoms reported one-week post-operation (p > 0.05). In contrast to clinical outcomes based on the presence or absence of post-PELD residual disc herniation, Baek et al10 suggested that for asymptomatic patients with observable residual disc tissue, a “watchful waiting” approach could be more prudent than immediate follow-up surgery. For patients with lingering symptoms post-surgery, we advocate for symptomatic remedies, such as bed rest and rehabilitative physiotherapy. For those experiencing negligible postoperative improvements or exacerbation of pre-surgical symptoms, especially if imaging indicates “nerve root compression” and “herniation with T2 low signal”, we advise 6–12 weeks of symptomatic treatment. Surgical exploration should be considered if no substantial improvement is observed.
This study has several limitations. A notable limitation was the small sample size, which may have affected the study’s broader applicability. Furthermore, all surgeries were performed by a single surgeon using an identical procedure, which further limits generalizability. Although patients exhibited a range of symptoms one-week post-surgery, such as numbness in the lower extremities, soreness, and swelling, this study did not differentiate or delve into these varied symptoms. We relied solely on VAS to gauge the patient’s tolerance to these residual symptoms. Furthermore, MRI parameters during follow-up, such as cut level, thickness, and angle, may differ from the initial MRI scan. This discrepancy could introduce minor inaccuracies in SR and SCSA.
Conclusion
By injecting contrast into the annulus fibrosus during percutaneous spinal endoscopy, our team determined that the residual mass on the early post-PELD MRI images was the annulus fibrosus left during surgery. At 1-year postoperative follow-up, 90.1% (101/112) of the patients displayed remodeling of the fibrous annulus, although 68.7% (77/112) still demonstrated herniation of the fibrous annulus. Short-term variations in clinical symptoms after PELD were associated with the T2 signal intensity of the residual mass. Meanwhile, long-term changes were associated with alterations in the size of the residual mass and cross-sectional area of the spinal canal.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Xuzhou Central Hospital. The participants provided their written informed consent to participate in this study.
Acknowledgments
Thank you for all the support from the XuZhou Central Hospital and Xuzhou Medical University.
Author Contributions
Jinhui Bu is the first author and Zhenfei Wang is the first co-first author, their contributions are the same. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Projects of Medical Key and leading talents of Xuzhou (XWRCHT20220050, XWRCHT20210035), Xuzhou Plan of introducing a team of clinical medical experts(2019TD002), the Project of Health Innovation Teams of Xuzhou (XWCX201601), Xuzhou Medical Foundation for Youth Reserved Experts Fund (2014006), Jiangsu Provincial Medical Youth Talent (QNRC2016392), the Scientific Research Projects of Jiangsu Provincial Health Commission (M2022048, Z2022040), “Six one Projects” for High-level Health Talents in Jiangsu Province (LGY2018047), Jiangsu Province “333” talents Project, The Project of Science and Technology of Xuzhou (KC22161), Postdoctoral Project of China (314233).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Benzakour T, Igoumenou V, Mavrogenis AF, Benzakour A. Current concepts for lumbar disc herniation. Int Orthop. 2019;43(4):841–851. doi:10.1007/s00264-018-4247-6
2. Ge R, Liu Z, Huang W. Percutaneous transforaminal endoscopic discectomy is a safer approach for lumbar disc herniation. Am J Transl Res. 2022;14(9):6359–6367.
3. Jiang X, Zhou X, Xu N. Clinical effects of transforaminal and interlaminar percutaneous endoscopic discectomy for lumbar disc herniation: a retrospective study. Medicine. 2018;97(48):e13417. doi:10.1097/MD.0000000000013417
4. Mayer HM. A history of endoscopic lumbar spine surgery: what have we learnt? BioMed Res Int. 2019;2019:4583943. doi:10.1155/2019/4583943
5. Wang Z, Huang S, Xu L, et al. A retrospective study of the mid-term efficacy of full-endoscopic annulus fibrosus suture following lumbar discectomy. Front Surg. 2022;9:1011746. doi:10.3389/fsurg.2022.1011746
6. Weber C, Kvistad KA, Moholdt VA, Nygaard OP, Solheim O. Repeated 3.0 tesla magnetic resonance imaging after clinically successful lumbar disc surgery. Spine. 2016;41(3):239–245. doi:10.1097/BRS.0000000000001189
7. Mahatthanatrakul A, Kotheeranurak V, Lin GX, Hur JW, Chung HJ, Kim JS. Comparative analysis of the intervertebral disc signal and annulus changes between immediate and 1-year postoperative MRI after transforaminal endoscopic lumbar discectomy and annuloplasty. Neuroradiology. 2019;61(4):411–419. doi:10.1007/s00234-019-02174-4
8. Wang Y, Luo G, Wang J, Zhu M, Li C, Teng H. Early postoperative magnetic resonance imaging findings after percutaneous endoscopic lumbar discectomy and their correlations with clinical outcomes. World Neurosurg. 2018;111:e241–e249. doi:10.1016/j.wneu.2017.12.032
9. Futatsugi T, Takahashi J, Oba H, et al. Early postoperative magnetic resonance imaging in detecting radicular pain after lumbar decompression surgery: retrospective study of the relationship between dural sac cross-sectional area and postoperative radicular pain. Clin Spine Surg. 2017;30(6):E733–E737. doi:10.1097/BSD.0000000000000342
10. Baek J, Yang SH, Kim CH, et al. Postoperative longitudinal outcomes in patients with residual disc fragments after percutaneous endoscopic lumbar discectomy. Pain Physician. 2018;21(4):E457–E466.
11. Granata V, Cascella M, Fusco R, et al. Immediate adverse reactions to gadolinium-based MR contrast media: a retrospective analysis on 10,608 examinations. BioMed Res Int. 2016;2016:3918292. doi:10.1155/2016/3918292
12. Ahn Y, Lee S, Son S, Kim H, Kim JE. Learning curve for transforaminal percutaneous endoscopic lumbar discectomy: a systematic review. World Neurosurg. 2020;143:471–479. doi:10.1016/j.wneu.2020.08.044
13. Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine. 2008;33(9):931–939. doi:10.1097/BRS.0b013e31816c8af7
14. Heo JH, Kim CH, Chung CK, et al. Quantity of disc removal and radiological outcomes of percutaneous endoscopic lumbar discectomy. Pain Physician. 2017;20(5):E737–E746.
15. Ruan W, Feng F, Liu Z, Xie J, Cai L, Ping A. Comparison of percutaneous endoscopic lumbar discectomy versus open lumbar microdiscectomy for lumbar disc herniation: a meta-analysis. Int J Surg. 2016;31:86–92. doi:10.1016/j.ijsu.2016.05.061
16. Zhou C, Zhang G, Panchal RR, et al. Unique complications of percutaneous endoscopic lumbar discectomy and percutaneous endoscopic interlaminar discectomy. Pain Physician. 2018;21(2):E105–E112.
17. Kim CH, Chung CK, Choi Y, et al. The long-term reoperation rate following surgery for lumbar herniated intervertebral disc disease: a nationwide sample cohort study with a 10-year follow-up. Spine. 2019;44:1382–1389. doi:10.1097/BRS.0000000000003065
18. Li ZP, Liu LL, Liu H, et al. Radiologic analysis of causes of early recurrence after percutaneous endoscopic transforaminal discectomy. Global Spine J. 2022;1270932355. doi:10.1177/21925682221096061
19. Schenck C, van Susante J, van Gorp M, Belder R, Vleggeert-Lankamp C. Lumbar spinal canal dimensions measured intraoperatively after decompression are not properly rendered on early postoperative MRI. Acta Neurochir. 2016;158(5):981–988. doi:10.1007/s00701-016-2777-5
20. Davies J, Siebenhandl-Wolff P, Tranquart F, Jones P, Evans P. Gadolinium: pharmacokinetics and toxicity in humans and laboratory animals following contrast agent administration. Arch Toxicol. 2022;96(2):403–429. doi:10.1007/s00204-021-03189-8
21. Yan D, Zhang Z, Zhang Z. Residual leg numbness after endoscopic discectomy treatment of lumbar disc herniation. BMC Musculoskelet Disord. 2020;21(1):273. doi:10.1186/s12891-020-03302-5
22. Wang Y, Gao F, Zou H. Numbness and weakness recovered at a less extent in patients with lumbar disc herniation after percutaneous endoscopic lumbar discectomy. Pain Res Manag. 2019;2019:4642701. doi:10.1155/2019/4642701
23. Liang X, Wang Y, Yue Y, Li Y, Meng C. Whether Out-of-Bed activity restriction in the early postoperative period of PELD is beneficial to therapeutic efficacy or reduce recurrence. Front Surg. 2022;9:860140. doi:10.3389/fsurg.2022.860140
24. Zhang C, Li Z, Yu K, Wang Y. A postoperative phenomenon of percutaneous endoscopic lumbar discectomy: rebound pain. Orthop Surg. 2021;13(8):2196–2205. doi:10.1111/os.13088
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.