Back to Journals » Orthopedic Research and Reviews » Volume 14
Postoperative Fluid Collections in Total Joint Arthroplasty: A Narrative Review
Authors Smith D , Berdis G, Singh V, Caughran A, Bullock M
Received 11 November 2021
Accepted for publication 8 February 2022
Published 19 February 2022 Volume 2022:14 Pages 43—57
DOI https://doi.org/10.2147/ORR.S348919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Dylan Smith,1 Galen Berdis,2 Vishavpreet Singh,2 Alexander Caughran,2 Matthew Bullock2
1Marshall University, Joan C. Edwards School of Medicine, Huntington, WV, USA; 2Department of Orthopaedics, Marshall University, Joan C. Edwards School of Medicine, Huntington, WV, USA
Correspondence: Dylan Smith, Email [email protected]
Abstract: A post-operative fluid collection (POFC) represents a common finding in both primary and revision total joint arthroplasty (TJA). Fortunately, most resolve on their own, but in instances where they become symptomatic, prompt identification and management are paramount, especially when they occur adjacent to a joint arthroplasty because of the increased the risk of developing a periprosthetic joint infection. A strong clinical suspicion with appropriate clinical exam is required along with select imaging modalities to arrive at a diagnosis. Meticulous surgical technique is crucial to prevent POFC, but new emerging treatments continue to evolve. This article presents an updated overview of incidence, pathophysiology, diagnosis, and management of POFC in the setting of TJA. We review the role of select imaging modalities as well as summarize current literature regarding new treatments such as sclerotherapy agents, acellular dermal matrices, and negative pressure wound therapy. Future studies are necessary to explore the interplay of inflammatory mediators in POFC formation and to define their role in fluid collection resolution.
Keywords: total joint arthroplasty, periprosthetic joint infection, acellular dermal matrices
Introduction
Post-operative fluid collections (POFC) are common in both primary and revision total joint arthroplasty (TJA).1 Large symptomatic fluid collections represent an avenue for bacterial seeding of a joint replacement which may lead to a periprosthetic joint infection (PJI).2 Management of PJI carries significant morbidity and mortality for the patient and an immense burden on the healthcare system. Hematomas and seromas are two of the most common POFC within the literature, but lymphoceles have also been described.3 Large fluid collections prolong the recovery process because of the potential for wound breakdown and infection in the acute post-operative period.
In patients undergoing TJA who are at high risk of POFC, meticulous surgical technique is paramount. Risk factors for POFC include multiple fascial planes, revision surgery, persistent peri-wound pressure and tension, inadequate hemostasis, and trauma in the acute post-operative period. According to Sloan et al,4 the revision THA population is most at risk for POFC complications. THA has four times greater rates of comorbidity and nearly three times greater incidence of hospitalization mortality than revision TKA.4
The management of fluid collections is well studied in the general and plastic surgery literature. Recent attention has been focused on the management of post-operative wound drainage due to the increased risk of developing a PJI.5 Beyond case studies and retrospective cohort studies, there is little written about the identification and management of POFC and joint replacement. Therefore, the purpose of this article is to present an updated narrative review of the incidence, pathophysiology, diagnosis, and current management of POFC in the setting of TJA.
Incidence
The reported incidence of POFC after TJA varies widely, ranging from 0.5% to 52.6%.6,7 Many studies fail to differentiate a seroma from hematoma and instead report them together as a singular entity. Nichols et al6 reported the rates of seroma or hematoma after primary total knee arthroplasty (TKA), primary total hip arthroplasty (THA), revision TKA, and revision THA to be 0.5%, 0.6%, 1.5%, and 2.2%, respectively. Procedures removing tissue are more often accompanied by seroma formation due to the creation of dead space.8 This setting is more common in revision procedures or complex primary joint replacements requiring extensive soft tissue dissection. Through their analysis of data collected for the National Hospital Discharge Survey from 1990 to 2004, Memtsoudis et al9 observed the incidence of seroma/hematoma formation to be 1.1% for primary unilateral TKA, 2.5% for primary bilateral TKA, and 2% for revision TKA. This study suggests that patient co-morbidities and nutritional status play a role in seroma/hematoma formation.
Pachowsky et al7 reported that about half of their patients developed a post-operative seroma, but the majority resolved without surgical intervention. Biber et al10 failed to detect any significant differences between posterior and lateral approaches during hip hemiarthroplasty, suggesting that the surgical approach does not seem to influence the development of a subcutaneous fluid collection. Regardless of the location of the incision, proper surgical technique is paramount to preventing a successful outcome.
Pathogenesis
Most of the available knowledge of seromas comes from plastic and reconstructive surgery literature as seroma formation is more common after extensive soft tissue procedures. A seroma is classically described as a collection of exudative fluid within the subcutaneous tissues adjacent to an incision in potential space created by surgery or trauma.11 A seroma usually develops within two weeks after surgery or trauma.12 Seromas often present with a peri-incisional swelling with a palpable fluid wave sign.13
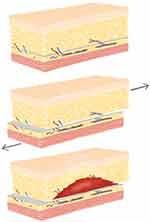
In orthopaedic literature, the Morel-Lavallee lesion is often described as a traumatic seroma that results from a shearing load applied to the soft tissue, which causes a separation of the hypodermis from the underlying fascia (Figure 1). As a result, the perforating blood vessels and lymphatic channels that traverse the zone of injury drain into a potential space leading to a fluid collection. This fluid can be composed of blood, lymph, and fat in various concentrations. Continued pressure, friction, or trauma can cause the seroma to enlarge and occupy larger areas along subcutaneous regions.14
On the contrary, a hematoma results from an arterial or venous insult that permits blood products to permeate the potential space within muscle, between fascial layers, or within subcutaneous tissues (Figure 2). Hematomas usually materialize within 48–72 hours of surgery or trauma to the affected area.15 Blood coagulation within muscle can give rise to heterotopic ossification, whereas continued bleeding within restrictive fascial layers can contribute to compartment syndrome. A mature hematoma often presents as a firm mass containing coagulated blood products.16 Unlike seromas, the blood products from hematomas include copious amounts of inflammatory mediators that can potentiate mass effect and inflammation around the area of surgery.17 When a hematoma occurs in the subcutaneous tissues it can present with characteristics consistent with a seroma. The superior and inferior lateral genicular arteries of the knee along with the medial and lateral femoral circumflex arteries of the hip have been implicated in hematoma formation within arthroplasty literature.18,19
 |
Figure 2 Illustration depicting possible POFC locations. (A) Superficial to the fascia and muscle. (B) Deep to the fascia and communicate with the joint. (C) Intramuscular. |
Incisions through large soft tissue envelopes are at higher risk for developing POFC due to the extent of vessel disruption during a surgical approach. Extensive dissection can damage nearby blood vessels and lymphatics, which can directly lead to seroma fluid accumulation.20 Several studies cited that the type of surgery and the extent of soft tissue dissection are statistically significant factors contributing to seroma formation. On the contrary, Loo et al20 found that age and obesity did not directly correlate with seroma formation, and meticulous hemostasis appears to be central to hematoma prevention.
Both seroma and hematoma formation are fueled by factors related to the acute inflammatory response after trauma.21 POFCs contain an inflammatory milieu comprised white blood cells, cytokines, growth factors, and proteinases capable of amplifying further inflammation.22 Certain factors, such as Interleukin-6 (IL-6) and Tumor Necrosis Factor-α (TNF-α), are necessary for post-operative wound healing, while Interleukin-4 (IL-4) and Interferon-ɤ have been found to accompany wound necrosis and an enlarging seroma.23 Continued fibrinolytic activity leads to further liquefaction of subcutaneous fat, contributing to enlarging fluid accumulation, which further propagates the inflammatory cycle.24 New research aimed at slowing expression of damaging mediators while promoting growth factors to encourage wound healing is ongoing.25
Diagnosis
Collecting a thorough patient history and performing a detailed physical exam are critical to differentiate a seroma from hematoma. The differential diagnosis of a POFC can be challenging because signs and symptoms can mimic seroma, hematoma, or infection. Therefore, an appropriate periprosthetic joint infection (PJI) work-up is paramount. The most recent 2018 Musculoskeletal Infection Society definition now incorporates biomarkers to assist in the diagnosis of PJI.26 Workup usually includes serum laboratory markers of inflammation, Erythrocyte Sedimentation Rate (ESR) and C-Reactive Protein (CRP), along with an aspiration of the fluid collection, whether intra- or extra-articular. During the acute postoperative period, laboratory markers for infection are often elevated and may not serve as definitive markers of infection. Therefore, a strong clinical suspicion combined with physical examination and laboratory results should be used to arrive at a diagnosis of infection.
Temporally, a seroma develops between two and four weeks after surgery and usually presents as a soft tissue swelling with accompanying soft tissue induration, warmth, and erythema in proximity to the incision. The fluid collection is usually superficial to muscular fascia and most prominent in the subcutaneous tissues.27 If drainage occurs, it is usually serous in nature and may contain scant blood products.
Acute hematomas usually present within days of surgery and display some of the same signs and symptoms of a seroma. Hematomas can occur in the subcutaneous tissues, but unlike seromas they have been known to organize in deeper aspects of fascia and muscle. Patients may complain of pain with the motion of the affected joint secondary to the mass effect of an intra-articular hematoma or develop heterotopic ossification from an intra-muscular hematoma. Evaluation of independent risk factors for hematoma formation in hip arthroplasty, as described by Mortazavi et al,28 include blood loss, administration of fresh frozen plasma and Vitamin K, perioperative anticoagulation, and hormonal therapy. All of these may increase suspicion of hematoma over seroma.
While there is low suspicion of infection, yet clinical concern about soft tissue fluid collection remains, further imaging studies or aspiration may assist the diagnosis.
Ultrasound
Ultrasound (US) is a noninvasive, relatively inexpensive, and easily accessible modality for evaluation of subcutaneous pathology without the risk of radiation. Reflected sound waves gathered by the collection probe provide an image based on the echogenicity of the surrounding tissues (Figure 3). US examination may reveal simple or complex fluid pockets and help classify the size, depth, and extension of the fluid collection.29 Quantifying fluid collections about the hip via US has excellent interobserver reliability with the intra-class correlation coefficient reported at 0.98 for bone-to-capsule distance and 0.99 for defining the fluid collection dimensions.30
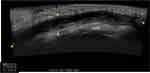 |
Figure 3 Sagittal view of right knee depicting a long seroma within the subcutaneous tissues located superficial to the knee extensor mechanism. |
Kong et al31 demonstrated that US can increase the accuracy in diagnosing hematomas after THA. In their study, only 53% of subcutaneous hematomas diagnosed via US could be detected on clinical examination. Białecki et al32 found that fluid collections can be easily recognized after THA as hypoechoic or anechoic areas in the soft tissues. Hoefnagels et al30 found that US is a highly reproducible method for assessment of fluid collection in superficial layer between the skin and the fascia lata and the deep layer between the fascia lata and the femoral cortex after THA.
Differentiation of seroma from hematoma based solely upon US alone may be difficult. Cho et al33 describe how the sonographic features of hematomas in the acute phase are hyperechoic with a poorly defined margin, which changes to progressively anechoic due to hemolysis within 96 hours after clotting. Hematomas that change to serosanguinous cysts over weeks to months usually demonstrate a better circumscribed appearance on US analysis.
Computed Tomography
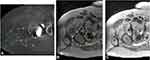
Computed Tomography (CT) is a valuable imaging modality in the evaluation of fluid collections. However, depending on the location and proximity of a fluid collection to the implants, the extent of a seroma/hematoma may be difficult to define due to the metal artifact created by the arthroplasty components. Artifact can be minimized by increasing peak voltage and tube charge, reducing collimation, extending the display CT scale, and decreasing the cross-sectional area of the implant that the radiation beam must traverse through patient positioning.34 Metal artifact reduction algorithms have been shown to improve the image quality35 (Figures 4 and 5).
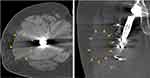 |
Figure 5 Axial (left) and coronal (right) CT scans depicting a hematoma (+42 Hounsfield Units). Note location deep to the Iliotibial band fascia. |
Hounsfield Units (HU) can be used to differentiate between different types of fluid collections based on CT. A seroma usually has a radiodensity value of less than 20 HU, while an uncoagulated hematoma is usually between 30 and 45 HU and a coagulated hematoma is between 60 and 100 HU. Roth et al36 described the appearance of a hematoma on axial contrast enhanced CT scan as a heterogeneous fluid without surrounding inflammation.
CT appearance of seromas can be more variable. Park et al37 describe seroma fluid collections with homogenous (5–38 HU) appearance but may have heterogenous (40–50 HU) attenuation, all with mild peripheral rim enhancement. Furthermore, these POFC may contain pockets of air, usually a sequela of recent surgery, but rarely may also represent an infection caused by gas-forming bacteria. If an acute seroma or hematoma is suspected, a CT angiogram can be ordered in attempts to locate the vessel(s) contributing to the POFC.
Magnetic Resonance Imaging
Magnetic Resonance Imaging (MRI) is a more expensive and time-consuming method for evaluating a POFC. Just as in CT imaging, there are a variety of methods to enhance image quality by minimizing artifact scatter associated with metal implants.38 Fast or turbo spin echo and inversion recovery pulse sequences have been shown to greatly improve image quality.39
Aliprandi et al40 found MRI to be a highly reliable and reproducible method to evaluate periprosthetic fluid collections. They defined serous fluid collections as hypointense on T1 and hyperintense on T2 and Short Tau Inversion Recovery (STIR) (Figures 6 and 7). Conversely, hematoma collections display hyperintense T1 and inhomogeneous hyperintense T2 and STIR. On MRI, an acute hematoma can present as a complex periarticular fluid collection, which may cause capsular distention if intraarticular or a soft tissue mass when extraarticular.38 MRI may also show the degree of soft tissue disruption or show a hematoma-related mass effect on surrounding nerves. Reports of motor deficits due to impingement of sciatic or femoral nerves secondary to a mass effect have been reported in the literature.41,42 As an additional benefit, MR angiography may demonstrate a source vessel as a cause of hematoma and can help guide endovascular treatment or arthroscopic or endovascular ablation management.
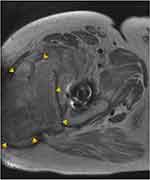 |
Figure 7 Note the intra-muscular location of the fluid collection. Heterogenous appearance and deep location are most consistent with hematoma based on this axial T1-sequence MRI. |
According to Davies et al,43 the majority of the seromas are homogenous and hypointense with respect to muscle on T1-weighted images and hyperintense on T2-weighted and STIR images. Some seromas may show hyperintensity with varying degrees of heterogeneity on T2-weighted images due to septations, fluid–fluid levels, and/or debris.
Management Strategies
Prevention
The best treatment for POFCs is prevention. There are several measures that can be exercised intraoperatively to help avoid POFC formation. Strategies consistent with quality surgical techniques including sharp soft tissue dissection, appropriate hemostasis, and avoiding multiple large subcutaneous fascial planes are paramount. Electrocautery should be used sparingly over sharp dissection due to the potential for more extensive soft tissue damage, which can increase potential space formation and subsequent infection.44 When in doubt, the incision should be carefully enlarged to relieve pressure and/or extraneous shear in subcutaneous tissues that can contribute to tissue necrosis. Although intra-articular post-operative drains have fallen out of favor for routine arthroplasty cases, a robust soft tissue envelope with a large incision can benefit from a temporary subcutaneous drain superficial to the fascia to further prevent POFC accumulation.
A recent study conducted by Pent et al45 retrospectively reviewed 4512 patients and highlighted the potential benefits of using topical vancomycin powder in the setting of TJA to decrease PJI. Interestingly, a retrospective study by Dial et al46 reviewed 265 patients undergoing THA and found that using vancomycin powder resulted in a statistically significant increase in seroma formation within the treatment group. With regard to POFCs, the vancomycin powder strategy may increase the possibility of seroma formation in high-risk individuals.
Negative Pressure Wound Therapy (NPWT)
Negative Pressure Wound Therapy (NPWT) has been gaining traction regarding POFC prevention and management. A vacuum-assisted closure dressing is applied to a closed incision with constant pressure (~125 mmHg) for several days after surgery.47 In a small prospective randomized control trial of 19 patients, Pachowsky et al7 demonstrated a statistically significant decrease in the rate of seroma formation with the use of NPWT. Meanwhile, Mortazavi et al28 found that of 109 patients with post-operative incisional drainage after THA, 76% had uneventful wound healing without the need for surgical intervention when NPWT was utilized. Despite the recent systematic review and meta-analysis by Kosins et al48 and concluded that there was no added benefit to using NPWT in the setting of orthopedic procedures, several studies have seen a perceived benefit in regard to decreasing POFC formation. Keeney et al49 concluded that NPWT improved short-term wound complications such as POFC’s, particularly in patients with a BMI above 35. This has also been shown in Matar et al,50 adding that NPWT is not indicated in all TJA, but can provide substantial benefits for high-risk patients.
NPWT was first believed to exert a continuous compressive force over the subcutaneous tissues immediately surrounding the incision to help decrease shearing forces and eliminate potential dead space.51 Studies have shown that subcutaneous tissue edema accompanying inflammation can lead to the development of a POFC.17,21 NPWT has been shown to enhance elimination of this fluid by aiding wound apposition, modulating soft tissue tension around the incision, and normalizing stress fields within soft tissues to allow neighboring lymphatic channels to evacuate residual exudate.52 NPWT has been shown to stimulate angiogenesis and the migration of keratinocytes at wound edges, which enhances wound healing in the acute stages.53 Increased angiogenin-1 and ratios of angiogenin-1 to angiogenin-2, as well as the level of phosphorylation of the tyrosine kinase 2 receptor, occur during the application of NPWT.54 These factors are important in the later stages of wound healing and in the role of promoting stabilization and maturation of wound angiogenesis.55
NPWT has been shown to be cost-effective for patients, decreasing post-operative superficial and deep infection rates and thus preventing the need for expensive revision procedures.56,57 A recent study projected the total hospital costs associated with PJI to be 1.85 billion dollars by the year 2030.58 Therefore, the utility of therapeutic and economic benefits of NPWT is worthy of further investigation. Well-designed studies on the effects of NPWT and prevention of POFC are ongoing.59
Aspiration
Aspiration can be helpful as both a diagnostic tool and a therapeutic modality to decompress the fluid collection and relieve pressure on surrounding structures. Prompt identification and management of a POFC is important to prevent deleterious and possible further surgical intervention. The various blood products in a POFC can further amplify the inflammation cascade, which makes evacuating the collection crucial to protecting the healing tissues.17
Seromas are comprised of serous exudate with minimal blood products in contrast to hematomas, which contain mostly blood products including red blood cells, fibrin, and platelets (Figure 8). The serous nature of the seroma makes aspiration with a large-bore needle more feasible when compared to an evolving hematoma, which may be difficult to aspiration given the coagulated components.
Reich et al60 employed a nonsurgical protocol to manage POFCs in knee and hip arthroplasties. An aspiration of the fluid collection enabled them to rule out infection as well as decompress the surrounding tissues. After aspiration, the incision was appropriately reinforced, compression wrap was applied, anticoagulants were paused, patient activity was modified, and antibiotics were prescribed for cloudy aspirate or surrounding cellulitis. This strategy is useful in small to moderate size POFCs.
Serial aspiration of a moderate sized POFC at regular intervals using sterile technique has been recommended in the past for symptomatic relief and to permit localized reabsorption of the fluid, which can take several weeks to months. Aspiration of a symptomatic mature or coagulated hematoma can be difficult, and a surgical decompression with irrigation and debridement may be required. Above all, the presence of purulence or laboratory fluid analysis concerning infection requires prompt intervention in attempts to salvage a TJA.
Surgical Strategies
Acellular Dermal Matrices
Acellular dermal matrices (ADM) have been utilized in soft tissue defects to provide a scaffold and stimulate epithelial growth and revascularization of the affected area.61 ADMs have been employed in a wide variety of plastic surgery procedures. In orthopedics, a urinary bladder matrix (UBM) has been used to successfully treat a persistent seroma in a patient with a TKA.11 The UBM provides chemotactic factors that endorse epithelial and nerve cell growth as well as fibroblast migration, which help eliminate the potential space responsible for the persistent seroma. The authors concluded that usage of the UBM in specific patients may be considered as a treatment option in the setting of a recurrent seroma in TKA. It is important to note that the patient was receiving concurrent NPWT. Therefore, it is difficult to evaluate the efficacy of using ADM alone in this patient. Larger studies across different arthroplasty procedures are needed to confirm the efficacy of ADM.
Sclerosing Agents
Sclerotherapy is another management strategy utilized for persistent seroma and fluid collection formation. This procedure involves placing an irritating agent within subcutaneous tissues to induce an inflammatory response to produce tissue fibrosis. Different sclerosing agents have been utilized to close the potential space and prevent recurrent fluid collection. Reports throughout orthopedic, spine, and plastic surgery literature demonstrate good to excellent rates of success in small underpowered case series and studies.62–64
A systematic review by Sood et al64 examined the outcomes of erythromycin, ethanol, fibrin glue, OK-432 (Streptococcus pyogenes with benzylpenicillin potassium), polidocanol, povidone-iodine, talc, and tetracycline antibiotics for management of a variety of seromas. Talc and tetracyclines were two of the more commonly used agents. However, all of the agents reviewed were “safe and effective” for recurrent seromas. The efficacy and safety of this treatment strategy in the total joint arthroplasty literature is sparse, and future randomized controlled trials are needed to assess the most effective and safe sclerosing agents.
Open Irrigation and Debridement
While less invasive options have failed, open irrigation and debridement (I&D) to evacuate the POFC is an option. Chronic fluid collections can develop a cystic capsule that has to be excised to prevent future recurrence. The role for I&D of symptomatic seromas without associated wound drainage or periprosthetic joint infection is less clear. If the fluid collection is superficial to the deep fascia, an argument can be made to only debride subcutaneous tissue. However, according to the recent MSIS criteria, if there is any suspicion on how the seroma/hematoma communicates with the arthroplasty, a formal deep I&D with synovectomy and polyethylene exchange must be executed. This procedure is then followed with at least six weeks of intravenous antibiotics to cover for a potential bacterial contamination. ESR and CRP values are typically monitored until resolution with the potential for long-term oral antibiotic suppression in high-risk populations. Future studies will be needed to elucidate the role for I&D with or without polyethylene exchange for symptomatic seromas without associated wound drainage or periprosthetic infection.
A few studies have analyzed the potential outcome of arthroscopic I&D of a seroma. Most of the literature regarding arthroscopic I&D includes single patient case studies or technique papers.65–67 It is unclear in the literature whether arthroscopic I&D is viable for large or more complicated seromas.
I&D of the fluid collection is often difficult with potential complications including infection, continued drainage, and enlargement of the seroma/hematoma. Meticulous hemostasis and elimination of nonviable tissue is paramount to prevent the formation of another fluid collection. Closing the wound in a multi-layered (quilted) fashion has been shown to decrease the recurrence of a seroma/hematoma. Placing a subcutaneous drain and primarily closing the incision along with NPWT is highly recommended to help manage the resultant edema and inflammation after an I&D procedure.
In rare instances where the wound does not respond to the above management strategies and the wound is isolated to the subcutaneous tissues, healing via secondary intention is an option although not preferred. This can be a tedious process, sometimes taking 3–12 months to heal depending on the size of the seroma.64 At this juncture, coordinating with a wound care specialist and a plastic surgeon will be necessary, especially if soft tissue coverage will be necessary in the future. During this time, the surgeon may elect to keep the patient on chronic antibiotics to help protect the prosthesis during the healing.
Discussion
We have highlighted similarities and differences between seroma and hematoma. It is important to distinguish between the two because treatment can differ. POFCs are among one of the top causes for patient readmission within one month of receiving a TJA.68,69 In 2015, the total average cost of a TJA was just over 8200 US dollars. In addition, Medicare claims and economic burden for revision THA and TJA were about 19% and 8%, respectively, in 2003, totaling almost 30% in total for Medicare claims for a total burden of about 1.5 billion dollars.70 Most importantly, POFCs are important to prevent, distinguish, manage, and treat correctly to improve patient outcomes.
Timely identification of a POFC is beneficial to ensure a proper treatment strategy is initiated. Table 1 summarizes the different aspects of seromas and hematomas that may aid in diagnosis. For a suspected fluid collection, the surgeon must remain cognizant of the associated inflammatory mediators. Further soft tissue insult can lead to further liquefaction of subcutaneous fat, which contributes to increased fluid accumulation, which may further perpetuate the inflammatory cycle and enlarge the POFC.24
 |
Table 1 Overview of the Similarities and Differences Between a Seroma and Hematoma in the Setting of TJA |
A peri-incisional US can help localize a POFC as well as assess characteristics, such as echogenicity. A POFC located in the superficial subcutaneous that are relatively anechoic on US tend to favor a diagnosis of seroma, while the converse holds true for a more complex collection such as a hematoma. Therefore, if the incision is healed, aspiration of a symptomatic the collection is usually first-line management. A large seroma can easily contain over 100 mL of fluid; therefore, the location of the POFC should be identified with one of the aforementioned imaging modalities to ensure proper localization before aspiration. Because a seroma exists mostly in a liquefied state, manual pressure around the periphery of the seroma can help to divert the fluid to the aspiration needle. Most hematomas will consolidate on their own, but if the POFC becomes symptomatic or creates a mass effect on the surrounding tissues and incision it may have to be evacuated. Although an aspiration with a large bore needle can be attempted, it is highly unlikely it will be successful unless the hematoma is early and not coagulated.
If the decision is to aspirate, sterile technique is employed with the skin prepared with an iodine-based solution. Aspiration is usually at the apex or distal aspect of the POFC. Afterwards, a compression bandage or peri-incisional wound vac is applied in attempts to help eliminate the remaining potential space and slow the POFC from returning. Pausing anticoagulation and rehabilitation are also suggested to prevent further soft tissue irritation from shearing of the subcutaneous tissues. It is sometimes necessary to perform serial aspirations as the fluid collection may slowly recur. Each subsequent aspiration should yield a less fluid indicating convalesce. If the collection appears to be increased in size, advanced imaging to further define the parameters of the POFC is necessary. A CT or MRI angiogram would be considered if there was suspicion of continued blood loss, such as with diminishing hemoglobin/hematocrit levels, to aid in locating adjacent vascular structures or lymphatic channels that may be contributing to the POFC.
A chronic POFC, usually persisting for more than 6 weeks, may become encapsulated in a cystic membrane, which may lead to increased pain in adjacent soft tissues; thus, operative intervention would be necessary. The same meticulous operative technique would be employed with strong consideration in a post-operative subcutaneous drain, sclerotherapy, or ADM can be employed. Close post-operative follow-up is highly recommended in these cases. A pre-operative consultation with the plastic surgery team for planning purposes should be considered. In rare circumstances, a seroma can form after the debridement of a hematoma, thus meticulous surgical technique with the use of an intralesional drain is a common management strategy.
Future technology will likely become more common to help diagnose a POFC. Fagotti et al71 studied the reliability of two hematoma classification systems in THA. Their objective classification systems, which employed a dedicated computer algorithm, demonstrated improved accuracy in classifying the severity of hematomas in arthroplasty. Finally, because a seroma is an exudate, proper protein nutrition during healing is a very important consideration. It is recommended that patients consume at least 0.8 grams of protein/kg of body weight/day for proper healing to occur.72–74
Conclusion
In summary, POFCs are common after TJA and fortunately most resolve on their own. In instances where they become large enough to become symptomatic, prompt identification and management are paramount, especially when they occur adjacent to a joint replacement. Future well-designed studies are necessary to explore the interplay of inflammatory mediators in seroma/hematoma formation and to define the role of lymphatics for fluid collection resolution. A strong clinical suspicion with appropriate clinical exam is required along with select imaging modalities that are necessary to assist diagnosis. Management of POFC begins with steps towards prevention, which include meticulous surgical dissection and adequate hemostasis. When symptomatic cases occur, aspiration is usually attempted before further surgical intervention. Further investigation into sclerotherapy agents, ADM, and the timing of I&D would provide more clarity on the overall management of a POFC.
Acknowledgments
The authors acknowledge the support from Kassandra Flores (Illustrations) at 1Marshall University, Joan C. Edwards School of Medicine, Huntington, WV and Dr Krista Denning, Department of Pathology, Marshall University, Joan C. Edwards School of Medicine, Huntington, WV.
Disclosure
Dr Matthew Bullock reports as a consultant for Smith & Nephew, outside the submitted work; and a member of American Association of Hip and Knee Surgeons Patient Education Committee. The authors have no relevant financial or non-financial interests to disclose.
References
1. Wagenaar F-CB, Löwik CA, Zahar A, Jutte PC, Gehrke T, Parvizi J. Persistent wound drainage after total joint arthroplasty: a narrative review. J Arthroplasty. 2019;34(1):175–182. doi:10.1016/j.arth.2018.08.034
2. Aggarwal VK, Rasouli MR, Parvizi J. Periprosthetic joint infection: current concept. Indian J Orthop. 2013;47(1):10–17. doi:10.4103/0019-5413.106884
3. Pasternak H, Bullock A, Bartus S, Schweizer R. Pelvic lymphocele complicating total hip replacement after renal transplantation. JBJS. 1977;59(2):272–273. doi:10.2106/00004623-197759020-00030
4. Sloan M, Sheth NP. Length of stay and inpatient mortality trends in primary and revision total joint arthroplasty in the United States, 2000–2014. J Orthopaedics. 2018;15(2):645–649. doi:10.1016/j.jor.2018.05.021
5. Motififard M, Nazem K, Meshkati M. Determining the relation between total knee arthroplasty surgery site drainage in two weeks after surgery with periprosthetic joint infection (PJI) in two years. Int J Burns Trauma. 2021;11(1):62.
6. Nichols CI, Vose JG. Clinical outcomes and costs within 90 days of primary or revision total joint arthroplasty. J Arthroplasty. 2016;31(7):1400–1406. e1403. doi:10.1016/j.arth.2016.01.022
7. Pachowsky M, Gusinde J, Klein A, et al. Negative pressure wound therapy to prevent seromas and treat surgical incisions after total hip arthroplasty. Int Orthop. 2012;36(4):719–722. doi:10.1007/s00264-011-1321-8
8. Granzier RW, van Bastelaar J, van Kuijk SM, et al. Reducing seroma formation and its sequelae after mastectomy by closure of the dead space: the interim analysis of a multi-center, double-blind randomized controlled trial (SAM trial). Breast. 2019;46:81–86. doi:10.1016/j.breast.2019.05.002
9. Memtsoudis SG, Della Valle AG, Besculides MC, Gaber L, Sculco TP. In-hospital complications and mortality of unilateral, bilateral, and revision TKA: based on an estimate of 4,159,661 discharges. Clin Orthop Relat Res. 2008;466(11):2617–2627. doi:10.1007/s11999-008-0402-5
10. Biber R, Brem M, Singler K, Moellers M, Sieber C, Bail HJ. Dorsal versus transgluteal approach for hip hemiarthroplasty: an analysis of early complications in seven hundred and four consecutive cases. Int Orthop. 2012;36(11):2219–2223. doi:10.1007/s00264-012-1624-4
11. Bettiol P, Cox C, Gerzina C, Simpson J, MacKay B. Novel Use of a Porcine Bladder Extracellular Matrix Scaffold to Treat Postoperative Seroma in a Total Knee Arthroplasty Patient. Arthroplast Today. 2021;7:143–147. doi:10.1016/j.artd.2020.12.007
12. Woodworth PA, McBoyle MF, Helmer SD, Beamer RL. Seroma formation after breast cancer surgery: incidence and predicting factors/discussions. Am Surg. 2000;66(5):444.
13. López-Cano M, Armengol-Carrasco M. Use of vacuum-assisted closure in open incisional hernia repair: a novel approach to prevent seroma formation. Hernia. 2013;17(1):129–131. doi:10.1007/s10029-011-0837-6
14. Scolaro JA, Chao T, Zamorano DP. The Morel-Lavallee Lesion: diagnosis and Management. J Am Acad Orthop Surg. 2016;24(10):667–672. doi:10.5435/JAAOS-D-15-00181
15. Chetlen AL, Kasales C, Mack J, Schetter S, Zhu J. Hematoma formation during breast core needle biopsy in women taking antithrombotic therapy. Am J Roentgenol. 2013;201(1):215–222. doi:10.2214/AJR.12.9930
16. Eicken JJ, Morrow D. Limb threatening thigh hematoma diagnosis accelerated by emergency physician bedside ultrasound. SAGE Open Medical Case Reports. 2019;7:2050313X19848589. doi:10.1177/2050313X19848589
17. Ma Y, Zhou Y, Wu F, Ji W, Zhang J, Wang X. The bidirectional interactions between inflammation and coagulation in fracture hematoma. Tissue Eng Part B Rev. 2019;25(1):46–54. doi:10.1089/ten.teb.2018.0157
18. Hughes S, Cammarata A, Steinmann S, Pellegrini JV. Effect of standard total knee arthroplasty surgical dissection on human patellar blood flow in vivo: an investigation using laser Doppler flowmetry. J South Orthop Assoc. 1998;7(3):198–204.
19. Hartog C, Metzler C, Meier C, Kalberer F, Wahl P. Anatomy of the lateral circumflex femoral artery: does the direct anterior approach to the hip jeopardize vascularization of the proximal femur? Orthopaedics Traumatology. 2019;105(7):1257–1264.
20. Loo WT, Chow LW. Factors predicting seroma formation after mastectomy for Chinese breast cancer patients. Indian J Cancer. 2007;44(3):99. doi:10.4103/0019-509X.38940
21. Pogson C, Adwani A, Ebbs S. Seroma following breast cancer surgery. Eur J Surgical Oncol. 2003;29(9):711–717. doi:10.1016/S0748-7983(03)00096-9
22. Hoff P, Maschmeyer P, Gaber T, et al. Human immune cells’ behavior and survival under bioenergetically restricted conditions in an in vitro fracture hematoma model. Cell Mol Immunol. 2013;10(2):151–158. doi:10.1038/cmi.2012.56
23. Chow LW, Loo WT, Yuen KY, Cheng C. The study of cytokine dynamics at the operation site after mastectomy. Wound Repair Regeneration. 2003;11(5):326–330. doi:10.1046/j.1524-475X.2003.11503.x
24. Oertli D. Lymphadenektomie der Axilla. Der Chirurg. 2007;78(3):194–202. doi:10.1007/s00104-006-1297-x
25. Pang C, Fan KS, Wei L, Kolar MK. Gene therapy in wound healing using nanotechnology. Wound Repair Regeneration. 2021;29(2):225–239. doi:10.1111/wrr.12881
26. Parvizi J, Zmistowski B, Berbari EF, et al. New definition for periprosthetic joint infection: from the Workgroup of the Musculoskeletal Infection Society. Clin Orthopaedics Related Research®. 2011;469(11):2992–2994. doi:10.1007/s11999-011-2102-9
27. Singh A, Anand A, Mittal S, Sonkar AA. Morel-Lavallee seroma (post-traumatic pseudocyst) of back: a rarity with management conundrum. Case Rep. 2016;2016:bcr2016216122.
28. Mortazavi SJ, Hansen P, Zmistowski B, Kane PW, Restrepo C, Parvizi J. Hematoma following primary total hip arthroplasty: a grave complication. J Arthroplasty. 2013;28(3):498–503. doi:10.1016/j.arth.2012.07.033
29. Gioacchini M, Bottoni M, Grassetti L, Scalise A, Di Benedetto G, Simple A. Reliable, and Inexpensive Method for Seroma Drainage. Arch Plast Surg. 2015;42(3):361–362. doi:10.5999/aps.2015.42.3.361
30. Hoefnagels EM, Obradov M, Reijnierse M, Anderson PG, Swierstra BA. Sonography after total hip replacement: reproducibility and normal values in 47 clinically uncomplicated cases. Acta orthopaedica. 2007;78(1):81–85. doi:10.1080/17453670610013457
31. Kong K, Jeyagopal N, Davies S. Should we still stitch the subcutaneous fat layer? A clinical and ultrasound assessment in 50 hip operations. Ann R Coll Surg Engl. 1993;75(1):23.
32. Bialecki J, Bartosz P, Marczynski W, Zajac J. Usefulness of ultrasonography in the diagnosis of hematoma after primary hip arthroplasty. J Ultrason. 2017;17(70):149–153. doi:10.15557/JoU.2017.0022
33. Cho K-H, Park B-H, Yeon KM. Ultrasound of the adult hip. Paper presented at: seminars in Ultrasound, CT and MRI. 2000.
34. Lee M-J, Kim S, Lee S-A, et al. Overcoming artifacts from metallic orthopedic implants at high-field-strength MR imaging and multi-detector CT. Radiographics. 2007;27(3):791–803. doi:10.1148/rg.273065087
35. Mushtaq N, To K, Gooding C, Khan W. Radiological Imaging Evaluation of the Failing Total Hip Replacement. Front Surgery. 2019;6:35. doi:10.3389/fsurg.2019.00035
36. Roth TD, Maertz NA, Parr JA, Buckwalter KA, Choplin RH. CT of the hip prosthesis: appearance of components, fixation, and complications. Radiographics. 2012;32(4):1089–1107. doi:10.1148/rg.324115183
37. Park SY, Kim EY, Park HK, et al. Computed Tomographic Findings of Postoperative Seroma in Breast Cancer Patients. J Breast Dis. 2014;2(2):64–68. doi:10.14449/jbd.2014.2.64
38. Fritz J, Lurie B, Miller TT. Imaging of hip arthroplasty. Paper presented at: seminars in musculoskeletal radiology. Int J Med. 2013;2:654.
39. Buckwalter KA. Optimizing imaging techniques in the postoperative patient. Semin Musculoskelet Radiol. 2007;11(3):261–272. doi:10.1055/s-2008-1038315
40. Aliprandi A, Sconfienza LM, Randelli F, Bandirali M, Di Leo G, Sardanelli F. Magnetic resonance imaging of painful total hip replacement: detection and characterisation of periprosthetic fluid collection and interobserver reproducibility. Radiol Med. 2012;117(1):85–95. doi:10.1007/s11547-011-0706-5
41. Khattar NK, Parry PV, Agarwal N, et al. Total hip arthroplasty complicated by a gluteal hematoma resulting in acute foot drop. Orthopedics. 2016;39(2):e374–e376. doi:10.3928/01477447-20160307-04
42. Butt A, McCarthy T, Kelly I, Glynn T, McCoy G. Sciatic nerve palsy secondary to postoperative haematoma in primary total hip replacement. J Bone Joint Surg Br. 2005;87(11):1465–1467. doi:10.1302/0301-620X.87B11.16736
43. Davies A, Hall A, Strouhal P, Evans N, Grimer R. The MR imaging appearances and natural history of seromas following excision of soft tissue tumours. Eur Radiol. 2004;14(7):1196–1202. doi:10.1007/s00330-004-2255-y
44. Gupta S, Augustine A, Horey L, Meek R, Hullin M, Mohammed A. Electrocautery of the patellar rim in primary total knee replacement: beneficial or unnecessary? J Bone Joint Surg Br. 2010;92(9):1259–1261. doi:10.1302/0301-620X.92B9.24467
45. Peng Z, Lin X, Kuang X, Teng Z, Lu S. The application of topical vancomycin powder for the prevention of surgical site infections in primary total hip and knee arthroplasty: a meta-analysis. Orthopaedics Traumatology. 2020.
46. Dial BL, Lampley AJ, Green CL, Hallows R. Intrawound vancomycin powder in primary total hip arthroplasty increases rate of sterile wound complications. Hip Pelvis. 2018;30(1):37. doi:10.5371/hp.2018.30.1.37
47. Borgquist O, Ingemansson R, Malmsjö M. Wound edge microvascular blood flow during negative-pressure wound therapy: examining the effects of pressures from–10 to–175 mmHg. Plast Reconstr Surg. 2010;125(2):502–509. doi:10.1097/PRS.0b013e3181c82e1f
48. Kosins AM, Scholz T, Cetinkaya M, Evans GR. Evidence-based value of subcutaneous surgical wound drainage: the largest systematic review and meta-analysis. Plast Reconstr Surg. 2013;132(2):443–450. doi:10.1097/PRS.0b013e3182958945
49. Keeney JA, Cook JL, Clawson SW, Aggarwal A, Stannard JP. Incisional negative pressure wound therapy devices improve short-term wound complications, but not long-term infection rate following hip and knee arthroplasty. J Arthroplasty. 2019;34(4):723–728. doi:10.1016/j.arth.2018.12.008
50. Matar HE, Emms N, Raut V. Effectiveness of Negative-Pressure Wound Therapy following Total Hip and Knee Replacements. J Long Term Eff Med Implants. 2019;29(1):51–57. doi:10.1615/JLongTermEffMedImplants.2019030593
51. Suh H, Lee A-Y, Park EJ, Hong JP. Negative pressure wound therapy on closed surgical wounds with dead space: animal study using a swine model. Ann Plast Surg. 2016;76(6):717. doi:10.1097/SAP.0000000000000231
52. Kilpadi DV, Cunningham MR. Evaluation of closed incision management with negative pressure wound therapy (CIM): hematoma/seroma and involvement of the lymphatic system. Wound Repair Regeneration. 2011;19(5):588–596. doi:10.1111/j.1524-475X.2011.00714.x
53. Meloni M, Izzo V, Vainieri E, Giurato L, Ruotolo V, Uccioli L. Management of negative pressure wound therapy in the treatment of diabetic foot ulcers. World J Orthop. 2015;6(4):387. doi:10.5312/wjo.v6.i4.387
54. Panayi AC, Leavitt T, Orgill DP. Evidence based review of negative pressure wound therapy. World J Dermatology. 2017;6(1):1. doi:10.5314/wjd.v6.i1.1
55. Ma Z, Shou K, Li Z, Jian C, Qi B, Yu A. Negative pressure wound therapy promotes vessel destabilization and maturation at various stages of wound healing and thus influences wound prognosis. Exp Ther Med. 2016;11(4):1307–1317. doi:10.3892/etm.2016.3083
56. Pagani NR, Moverman MA, Puzzitiello RN, Menendez ME, Kavolus JJ. The Cost-Effectiveness of Closed Incisional Negative Pressure Wound Therapy for Infection Prevention after Revision Total Knee Arthroplasty. J Knee Surg. 2021. doi:10.1055/s-0041-1724137
57. Nherera LM, Trueman P, Karlakki SL. Cost‐effectiveness analysis of single‐use negative pressure wound therapy dressings (sNPWT) to reduce surgical site complications (SSC) in routine primary hip and knee replacements. Wound Repair Regeneration. 2017;25(3):474–482. doi:10.1111/wrr.12530
58. Premkumar A, Kolin DA, Farley KX, et al. Projected Economic Burden of Periprosthetic Joint Infection of the Hip and Knee in the United States. J Arthroplasty. 2021;36(5):1484–1489. e1483. doi:10.1016/j.arth.2020.12.005
59. Doman DM, Young A, Buller LT, Deckard ER, Meneghini RM. Comparison of Surgical Site Complications with Negative Pressure Wound Therapy Versus Silver Impregnated Dressing in High-Risk Total Knee Arthroplasty Patients: a Matched Cohort Study. J Arthroplasty. 2021;36:3437–3442. doi:10.1016/j.arth.2021.05.030
60. Reich MS, Ezzet KA. A nonsurgical protocol for management of postarthroplasty wound drainage. Arthroplasty Today. 2018;4(1):71–73. doi:10.1016/j.artd.2017.03.009
61. Boháč M, Danišovič Ľ, Koller J, Dragúňová J, Varga I. What happens to an acellular dermal matrix after implantation in the human body? A histological and electron microscopic study. Eur J Histochem. 2018;62(1):2873. doi:10.4081/ejh.2018.2873
62. Dagenais S, Haldeman S, Wooley JR. Intraligamentous injection of sclerosing solutions (prolotherapy) for spinal pain: a critical review of the literature. The Spine Journal. 2005;5(3):310–328. doi:10.1016/j.spinee.2004.09.011
63. Throckmorton AD, Askegard-Giesmann J, Hoskin TL, et al. Sclerotherapy for the treatment of postmastectomy seroma. Am j Surg. 2008;196(4):541–544. doi:10.1016/j.amjsurg.2008.06.020
64. Sood A, Kotamarti VS, Therattil PJ, Lee ES. Sclerotherapy for the Management of Seromas: a Systematic Review. Eplasty. 2017;17:e25.
65. Liu M, Liu L, Zhou X, et al. A novel surgical technique for treatment of Morel-Lavallée lesion: endoscopic debridement combined with percutaneous cutaneo-fascial suture. Injury. 2018;49(8):1630–1633. doi:10.1016/j.injury.2018.06.003
66. Kim S. Endoscopic treatment of Morel-Lavallee lesion. Injury. 2016;47(5):1064–1066. doi:10.1016/j.injury.2016.01.029
67. Pan C-H, Tu C-P, Ou S-Y, et al. Percutaneous Debridement of and Fibrin Glue Injection Into a Pretibial Morel-Lavallée Lesion: a Case Report and Literature Review. Ann Plast Surg. 2021;86(2S):S123–S126. doi:10.1097/SAP.0000000000002718
68. Cullen C, Johnson D, Cook G. Re-admission rates within 28 days of total hip replacement. Ann Royal Coll Surgeons Eng. 2006;88(5):475–478. doi:10.1308/003588406X116909
69. Courtney PM, Boniello AJ, Berger RA. Complications following outpatient total joint arthroplasty: an analysis of a national database. J Arthroplasty. 2017;32(5):1426–1430. doi:10.1016/j.arth.2016.11.055
70. Ong K, Mowat F, Chan N, Lau E, Halpern M, Kurtz S. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22–28. doi:10.1097/01.blo.0000214439.95268.59
71. Fagotti L, Ejnisman L, Gurgel HDMC, Miyahara H, Croci AT, Vicente JRN. Two classifications for surgical wound hematoma after total hip replacement. Acta ortopedica brasileira. 2018;26:11–15. doi:10.1590/1413-785220182601175203
72. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Sports Medicine. 2019;49(1):59–68. doi:10.1007/s40279-018-1009-y
73. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise. Am J Clin Nutr. 2014;99(1):86–95. doi:10.3945/ajcn.112.055517
74. Macnaughton LS, Wardle SL, Witard OC, et al. The response of muscle protein synthesis following whole‐body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Phys Rep. 2016;4(15):e12893. doi:10.14814/phy2.12893
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.