Back to Journals » Cancer Management and Research » Volume 11
Postmastectomy radiotherapy using three different techniques: a retrospective evaluation of the incidental dose distribution in the internal mammary nodes
Authors Wang W , Zhang Y, Xu M, Shao Q, Sun T, Yu T, Liu X, Li J
Received 16 October 2018
Accepted for publication 27 December 2018
Published 30 January 2019 Volume 2019:11 Pages 1097—1106
DOI https://doi.org/10.2147/CMAR.S191047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Wei Wang,1 Yingjie Zhang,1 Min Xu,1 Qian Shao,1 Tao Sun,2 Ting Yu,1,3 Xijun Liu,1 Jianbin Li1
1Department of Radiation Oncology, Shandong Cancer Hospital affiliated to Shandong University, Jinan, Shandong 250117, China; 2Department of Medical Physics, Shandong Cancer Hospital affiliated with Shandong University, Jinan, Shandong 250117, China; 3School of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, Jinan, Shandong 250117, China
Objective: To evaluate the incidental coverage dose to the internal mammary nodes (IMN) in patients treated with postmastectomy radiotherapy (PMRT) and its relationship with the treatment plan.
Patients and methods: We retrospectively analyzed 138 patients undergoing PMRT and divided them into three groups: three-dimensional conformal radiotherapy (3D-CRT), field-in-field forward intensity-modulated radiotherapy (F-IMRT), and inverse intensity-modulated radiotherapy (I-IMRT). The IMN were contoured according to the Radiation Therapy Oncology Group consensus and not included in the planning target volume. We analyzed incidental IMN dose coverage and its relationship with the lung and heart.
Results: The mean dose (Dmean) to the IMN was 32.85 Gy for all patients, and the dose delivered to the IMN showed no differences in 3D-CRT, F-IMRT, and I-IMRT (33.80, 29.65, and 32.95 Gy, respectively). In addition, 10.42%, 2.04%, and 9.76% of patients achieved ≥45 Gy with 3D-CRT, F-IMRT, and I-IMRT, respectively. No differences were evident among the three treatment plans regarding IMN dose in the first three intercostal spaces (ICS1–3). The Dmean, V20, V30, V40, and V50 of ICS2 and ICS3 were superior to those of ICS1 for all three plans. For 3D-CRT, a moderate positive correlation was evident between the Dmean to the IMN and the Dmean to the heart. For F-IMRT and I-IMRT, positive correlations were evident between the Dmean of the IMN and the Dmean and V20 of the lung.
Conclusion: The mean incidental dose to the IMN for IMRT (F-IMRT and I-IMRT) and 3D-CRT after modified radical mastectomy was insufficient to treat subclinical disease. A substantial dose was delivered to the IMN in some patients. Higher incidental doses to the IMN were associated with a higher heart mean dose for 3D-CRT and a higher dose to the lung for IMRT. Future prospective studies should further explore subgroups that do not require IMN irradiation.
Keywords: postmastectomy radiotherapy, internal mammary chain incidental irradiation dose, three-dimensional conformal radiotherapy, field-in-field forward intensity-modulated radiotherapy, inverse IMRT
Background
Radiotherapy (RT) after breast conserving surgery (BCS) or mastectomy can provide significant locoregional control, decrease the risk of distant metastases, and translate into significant survival benefits,1–3 especially for patients with given molecular subtypes.4–6 The American College of Surgeons Oncology Group (ACOSOG) Z0011 Phase III trial has confirmed that, for patients with clinical T1–T2 invasive breast cancer who are treated with lumpectomy and who have one or two identified sentinel lymph nodes (SLNs) containing metastases, the use of SLN dissection (SLND) alone compared with axillary lymph node dissection (ALND) does not result in inferior overall survival (OS) or disease-free survival (DFS).7,8 Furthermore, the same observations apply to patients with negative SLNs, and the National Surgical Adjuvant Breast and Bowel Project Phase III protocol B-32 (NSABP B-32) has corroborated the superiority of SLND to the ALND treatment approach in terms of OS, DFS, and regional control.9,10 In both studies, adjuvant systemic therapy was delivered to >85% of the patients, and a portion of the level I–II axilla was irradiated in patients who underwent standard opposing tangential irradiation. Therefore, although a portion of the lymph nodes (LNs) was not irradiated, a portion of the postsurgical breast patients who received adjuvant systemic therapy (chemotherapy, hormonal therapy, or targeted therapy) combined with LN incidental irradiation during RT still exhibited diminished locoregional recurrence.
The ACOSOG Z0011 and NSABP B-32 trials have provoked speculations regarding whether the incidental radiation dose to ALNs with tangential radiation therapy fields could have prevented axillary recurrences in the SNB-only arm.7,9 In addition, recent studies have evaluated the axillary incidental irradiation dose coverage.11–14 To date, whether the incidental radiation dose to the axilla is responsible for eliminating additional hidden metastases in ALNs when the breast alone is irradiated remains unclear. Along with ALNs, internal mammary nodes (IMN) are the first filter stations for the lymphatic drainage of the breast, and the unexpected control of IMN that accompanies incidental IMN irradiation has been an ongoing controversy, especially for one to three LN-positive breast cancer and high-risk, node-negative stage II breast cancer.15–18
Despite the high incidence of IMN metastases,19–21 the overall rate of clinically detectable recurrences in IMN after primary systemic breast cancer treatment is <1%, even when regional IMN are not irradiated.22,23 As with ALN, the low recurrence rates in IMN are attributable not only to patients receiving adjuvant systemic therapy but also to IMN incidental irradiation.23,24 The literature is sparse on the incidental doses received with no internal mammary LN surgical dissection, especially with newer techniques. Recently, reports on the incidental irradiation of IMN in breast cancer have examined treatment with three-dimensional conformal RT (3D-CRT).24–27 Therefore, in this study, we compared the dosimetric parameters for incidental irradiation to the IMN during chest wall RT with 3D-CRT, field-in-field forward intensity-modulated radiotherapy (F-IMRT), and inverse intensity-modulated radiotherapy (I-IMRT). In addition, we aimed to examine these dose distribution patterns and their possible clinical relevance.
Patients and methods
Patient selection and instructions
Eligibility criteria for our study were as follows. Patients with diagnoses of invasive carcinoma or ductal carcinoma in situ of the breast who received adjuvant RT at our institution between April 2013 and May 2017 were enrolled. Patients were treated with modified radical mastectomy (MRM) with SLND or ALND, and without immediate reconstruction. Also, these patients with breast cancer who had been irradiated postoperatively were included in this retrospective study. All patients were confirmed as having no clinical or pathological evidence of IMN involvement at the time of diagnosis. The institutional research ethics board of Shandong Cancer Hospital approved this study (SDTHEC201703014), and the requirement to obtain written informed consent from patients was waived due to the retrospective nature of the investigation. Also, this study was carried out in accordance with the Declaration of Helsinki principles.
Delineation of target volume and organs at risk
The clinical target volumes (CTVs) of the chest wall and supraclavicular fossa (SCF), heart, ipsilateral lung (IPSL), and spinal cord were delineated, and the IMN were not included in the CTV. The planning target volume (PTV) margin was 5 mm from the CTV. In addition, a 5 mm bolus was used over the chest wall. The IMN were contoured according to the Radiation Therapy Oncology Group breast cancer consensus: from the first to third intercostal spaces (ICS1–3) through the topography of the internal thoracic vessels (available online at: http://www.rtog.org/LinkClick.aspx?fileticket=vzJFPaBipE%3d&tabid=236). PTV IMN were designed to include an expansion of 5 mm around the IMN.
Treatment plan and dosimetric evaluation
The prescription dose was 50 Gy in 25 fractions (2 Gy per fraction) to the PTV, 5 days/week.
Three-dimensional conformal radiotherapy
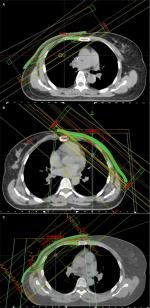
The chest wall was treated with two opposite tangential fields (two oblique beams with an interval of 180°) using 6 MV photon beams and an ipsilateral SCF with a single anterior field. The criterion of the 3D-CRT plan was to ensure that at least 90% of the PTV received the prescription dose (Figure 1A).
Forward intensity-modulated radiotherapy
The chest wall treatment plan involved using the tangential field technique with static multileaf collimator segments, with two parallel-opposed tangential fields using 6 MV photon beams. Two to five segmented fields were manipulated to maintain dose delivery to organs at risk (OARs), such as the IPSL, and heart within normally accepted tolerances and to reduce the volumes of hot spots in the treatment field. Four to five fields were designed toward the SCF to guarantee dose uniformity. The criterion of the F-IMRT plan was to ensure that at least 95% of the PTV received the prescription dose (Figure 1B).
Inverse intensity-modulated radiotherapy
The common isocenter was located in the center of the PTV. The tangential field technique was set to the entire PTV, and additional 0° and 40° multi-leaf collimator (MLC) segments were constructed toward the SCF. Additional subfields were set to reduce hot regions generated by the primary tangential fields and improve PTV dose uniformity to achieve dose homogeneity (Figure 1C).
Based on the patient’s dose-volume histogram (DVH), the mean doses (Dmean) and the volumes receiving equal to or more than 20, 30, 40, and 50 Gy (V20, V30, V40, and V50, respectively) to the IMN were analyzed.
Statistical analysis
Statistical analysis was performed with the SPSS statistical analysis software package. Based on the normality of the distributions, the Kruskal–Wallis H test was used for each dosimetric parameter in the three treatment plans. Wilcoxon signed-rank tests were used for comparisons of the dosimetric parameters of the first and third ICS. Spearman rank correlation test was used to examine the relationship between the dosimetric parameters of the IMN and the dosimetric parameters of the OARs. All tests were two sided. Data were regarded as statistically significant when P<0.05.
Results
Patients and treatment
One hundred and thirty-eight patients with breast cancer who received adjuvant postmastectomy RT (PMRT) at our institution were enrolled. Table 1 outlines the patient characteristics. One hundred and thirty-four patients were diagnosed with invasive ductal carcinoma, three patients with invasive lobular carcinoma, and one patient was diagnosed with invasive papillary carcinoma. A total of 48, 49, and 41 of the patients underwent 3D-CRT, F-IMRT, and I-IMRT followed by MRM, respectively.
  | Table 1 Patient and treatment characteristics |
Incidental IMN dose coverage
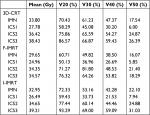
The Dmean to the IMN was 32.85 Gy for all patients. According to the treatment plan technique type, the Dmean values to the IMN in the patients undergoing 3D-CRT, F-IMTR, and I-IMRT were 33.80, 29.65, and 32.95 Gy, respectively (H=2.412, P=0.299). The detailed values of each parameter for the total IMN and the first ICS (ICS1), second ICS (ICS2), and third ICS (ICS3) are shown in Table 2. The Dmean to each ICS of the IMN in this study showed no differences among the 3D-CRT, F-IMRT, and I-IMRT groups. Representative axial images of all three plans, including target volume and field distributions, are shown in Figure 1.
Thirty, 24, and 26 patients who underwent 3D-CRT, F-IMRT, and I-IMRT, respectively achieved 30 Gy for the incidental dose distribution to the IMN. Also, the incidental doses to the IMN of 10.42%, 2.04%, and 9.76% of the patients who underwent 3D-CRT, F-IMRT, and I-IMRT, respectively, reached 45 Gy. The IMN dosimetric parameters for each level of the 3D-CRT, F-IMRT, and I-IMRT plans are shown in Table 3, and the dosimetric parameters for each level of both the IMN and ICS1–3 were equal.
Regardless of the technique, the parameters, including Dmean, V20, V30, V40, and V50, indicated that the dose delivered to the IMN was lower in ICS1 than in ICS2 and ICS3. For 3D-CRT, F-IMRT, and I-IMRT, the Dmean values to the IMN in ICS2 and ICS3 were higher than 8.16/10.65, 9.39/9.61, and 8.16/12.72 Gy, respectively, compared to that in ICS1. For 3D-CRT, no significant differences were evident between ICS2 and ICS3. However, for patients who underwent F-IMRT, the V20, V30, and V40 in ICS3 were higher than those in ICS2 (Z=–2.053,–2.032, –2.021; P=0.040, 0.042, 0.043), and no differences were evident for Dmean and V50. For I-IMRT, ICS3 exceeded ICS2 in terms of Dmean, V20, V30, and V40 (Z=–2.287,–2.749, −4.840,–2.876; P=0.022, 0.006, 0.004, 0.004), and no difference was evident in V50 (Z=–1.626, P=0.104).
Analyses of OARs: the IPSL and heart
The OAR doses and volume results of the three groups are presented in Table 4. The IPSL volume for all breast cancer patients (P=0.290) and the heart volume for the left-sided breast cancer patients (P=0.985) were well balanced. Compared to the 3D-CRT plan, the F-IMRT and I-IMRT plans had decreased dose distributions to the IPSL (Dmean and V20). However, the dose delivered to the heart showed no differences in these plans with tangential techniques (Table 4). The correlation between the dose delivered to the IMN and OARs showed mixed features for 3D-CRT and IMRT (Table 5). A moderately positive correlation was found between the Dmean of the IMN and the Dmean of the heart for patients who underwent 3D-CRT (r=0.338, P=0.01). In addition, no dosimetric correction was found between the IMN and IPSL, whereas for the F-IMRT and I-IMRT groups, positive correlations were found only between the Dmean of IMN and the Dmean and V20 of the IPSL.
Discussion
The incidental irradiated dosimetric reviews of the IMN from the published articles described above are summarized in Table 6. Our study showed a relatively similar Dmean in the IMN region. Notably, these trials only used the 3D-CRT technique on the targeted volume. To the best of our knowledge, our study on IMN incidental irradiation coverage is the first to address this topic using three different techniques and to examine these dose distribution patterns and OAR dose correlations. Compared to 3D-CRT, the improved conformity of IMRT indicates that less irradiation was delivered outside of the PTV. Conventional parallel-opposed tangential fields were still mainly used in F-IMRT and I-IMRT, and the IMN were in the rear of the inner tangential semi-opposed beams. Therefore, the IMN were incidentally irradiated with an equal dose of radiation to that of 3D-CRT. This finding indicates that in every analysis we performed concerning these three RT techniques, no group ultimately received an adequate or effective prophylactic treatment. Adequate coverage of the IMN, defined as ≥45 Gy, was achieved in 10.42%, 2.04%, and 9.76% of the patients with 3D-3CRT, F-IMRT, and I-IMRT techniques, respectively. Thus, tailored RT for individual patients might be needed.
Previous reports have shown that relatively low doses (10–30 Gy) can sterilize subclinical metastases and microscopic tumors of ovarian, bladder, and breast carcinomas and of head and neck neoplasms.28,29 Recently, Lee et al found that for breast cancer, the estimated 50% tumor control dose (TCD50) values were 19.3 Gy for MCF7 (luminal) cells and 44.9 Gy for SUM159 (basal) cells.30 In addition, of the enrolled patients in our study, 62.5%, 48.98%, and 63.41% achieved 30 Gy for the incidental dose distribution to the IMN and 10.42%, 2.04%, and 9.76% of the patients who underwent 3D-CRT, F-IMRT, and I-IMRT, respectively, reached 45 Gy. Therefore, for invasive breast carcinoma, the incidental dose to the IMN does not reach standard clinically therapeutic levels in MRM breast cancer patients treated with any of these three RT techniques, but a worthwhile benefit can be achieved by the incidental doses delivered when only chest wall ± SCF are irradiated without the IMN region. This finding is also confirmed by the overall low recurrence rates in the IMN after PMRT, even when these nodes are not excised or irradiated.22,23 The European Organization for Research and Treatment of Cancer (EORTC) Radiation Oncology and Breast Cancer groups found that for patients with stage I, II, and III cancers with centrally or medially located primary tumors, in the whole breast irradiation (WBI) or chest wall irradiation alone group, the internal mammary recurrence rate was 0.8%, and in the nodal irradiation group, the rate was 0.2%.23 Kanyilmaz et al showed that the incidental Dmean to the IMN in patients treated with 3D-CRT after MRM was 32.8 Gy, and that advanced T and N stages were the prognostic factors that affected OS and progression-free survival, which were poorly affected by unplanned irradiation doses to the IMN.24 No IMN relapses occurred during the median 38-month (range 3–80) follow-up time.
In our hospital, internal mammary SLN (IM-SLN) biopsy was performed with the modified injection technique, and the IM-SLNs were detected by preoperative lymphoscintigraphy and/or intraoperative gamma probe detection. The results showed that the IM-SLNs were concentrated in the second and third ICS (80.8%). ICS3 had the highest incidence of positive IMN, followed by the second and first spaces (50.2%, 30.6%, and 1.6% for ICS3, ICS2, and ICS1, respectively).31 Krishan et al analyzed the anatomical distribution of IMN metastases and found that 78% of nodal metastases were in the first three ICSs, whereas 14% and 8% were located in ICS3 and ICS1, respectively.32 The regularity of the distributions of the IMN metastases was consistent with that of IM-SLN metastases. Our results showed that regardless of the RT technique, the Dmean values to the IMN in ICS2 and ICS3 were higher than 8.16–12.72 Gy compared to that of ICS1. In addition, the dose may be conducive for detecting the ICS2 and ICS3 recurrence rate. The anatomical position and topography of the internal thoracic vessels in ICS1 are deeper than those in ICS2 and ICS3, which may lead to the IMN dose differences of the different ICSs. Sapienza et al evaluated the relationship between the unintentional coverage of the IMN and the type of surgery employed, and the authors found that the Dmean values to the IMN after MRM and MRM with immediate reconstruction (MRM + R) were greater than those for BCS (30.34 Gy for MRM, 30.26 Gy for MRM + R, and 18.67 Gy for BCS).25 MRM changed the chest wall thickness, which resulted in the variation in IMN depth, possibly leading to the IMN dose differences of each operative approach.
Darby et al reconstructed the RT regimens of 2,168 women on computed tomography scans with typical anatomy and found a dose-dependent increase in the risk of late ischemic heart disease associated with RT for cancer of the left breast.33 We also found that for patients who underwent 3D-CRT, the incidental dose to the IMN was positively correlated with the Dmean of the heart. In addition, this moderate positive correlation also existed in ICS3. However, in our stratified analyses, little of the heterogeneity was explained by the IPSL volume for all breast cancer patients or the heart volume for left-sided early breast cancer patients. With equal dose distribution patterns for all three techniques, the Dmean of the heart was further decreased by ~2 Gy in the IMRT cohort (F-IMRT and I-IMRT), and no correlation was evident between the incidental dose to the IMN and the dose to the heart. Contemporary IMRT likely reduced the risk to the heart, and RT-induced cardiotoxicity may have been mitigated by the medical progress made in the treatment of ischemic heart disease over the past decade. Therefore, the incubation time of late cardiac complications may be prolonged,34 so the long-term hazards in the general population will still need to be monitored with additional follow-up and summaries in most cases.
EORTC trial 22922/10925 found that lung toxicity was significantly increased with irradiation of the IMN and SCF treatment (fibrosis, 0.9% vs 2.8%; dyspnea, 0.1% vs 0.7%; pneumonitis, 0.1% vs 0.7%; any lung toxicity, 4.3% vs 1.3%).17 In our study, although the IMN were not included in the treatment volume, a significant association was evident between the incidental dose to the IMN and the Dmean and V20 to the IPSL for IMRT. Therefore, the next research direction will involve identifying the influencing factors of the incidental coverage to the IMN and screening out potential breast cancer patients whose incidental IMN doses can achieve clinically therapeutic levels that do not require IMN RT.
Conclusion
F-IMRT and I-IMRT can deliver lower and clinically less relevant doses than 3D-CRT to OARs. The incidental dose to the IMN of patients treated with 3D-CRT, F-IMRT, and I-IMRT were equal when the IMN were routinely uninvolved in the target volume. However, a higher incidental dose to the IMN was associated with a higher Dmean to the heart for 3D-CRT, and a higher incidental dose to the IMN was associated with a higher dose to the IPSL for patients who underwent F-IMRT and I-IMRT. Although none of these three RT techniques ultimately achieved an adequate and effective prophylactic treatment for the IMN, a substantial dose was still delivered to the IMN for partial patients. A more careful data analysis or long-term clinical trial is warranted for detailed explorations of the subgroups that do not require IMN irradiation, and additional follow-up is needed to determine whether the different incidental dose to the IMN would result in a different recurrence rate in the IMN.
Acknowledgments
This manuscript was edited by American Journal Experts. The National Key Research Program of China (No. 2016YFC0904700), National Natural Science Foundation of China (No. 81703038 and 81502314), Natural Science Foundation of Shandong Province (No. ZR2017PH006), and the Key Research Development Program of Shandong Province (No. 2017GSF18102) supported the study.
Disclosure
The authors report no conflicts of interest in this work.
References
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG), Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. | ||
EBCTCG (Early Breast Cancer Trialists’ Collaborative Group), McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. | ||
Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: an American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. J Clin Oncol. 2016;34(36):4431–4442. | ||
Sjöström M, Lundstedt D, Hartman L, et al. Response to radiotherapy after breast-conserving surgery in different breast cancer subtypes in the Swedish Breast Cancer Group 91 radiotherapy randomized clinical trial. J Clin Oncol. 2017;35(28):3222–3229. | ||
Liu FF, Shi W, Done SJ, et al. Identification of a low-risk luminal a breast cancer cohort that may not benefit from breast radiotherapy. J Clin Oncol. 2015;33(18):2035–2040. | ||
Langlands FE, Horgan K, Dodwell DD, Smith L. Breast cancer subtypes: response to radiotherapy and potential radiosensitisation. Br J Radiol. 2013;86(1023):20120601. | ||
Giuliano AE, Mccall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. | ||
Giuliano AE, Ballman K, Mccall L, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: long-term follow-up from the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 randomized trial. Ann Surg. 2016;264(3):413–420. | ||
Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised Phase 3 trial. Lancet Oncol. 2010;11(10):927–933. | ||
Land SR, Kopec JA, Julian TB, et al. Patient-reported outcomes in sentinel node-negative adjuvant breast cancer patients receiving sentinel-node biopsy or axillary dissection: national surgical adjuvant breast and bowel project Phase III protocol B-32. J Clin Oncol. 2010;28(25):3929–3936. | ||
Kataria T, Bisht SS, Gupta D, et al. Incidental radiation to axilla in early breast cancer treated with intensity modulated tangents and comparison with conventional and 3D conformal tangents. Breast. 2013;22(6):1125–1129. | ||
Aguiar A, Gomes Pereira H, Azevedo I, Gomes L. Evaluation of axillary dose coverage following whole breast radiotherapy: variation with the breast volume and shape. Radiother Oncol. 2015;114(1):22–27. | ||
Lee J, Kim SW, Son SH. Dosimetric evaluation of incidental irradiation to the axilla during whole breast radiotherapy for patients with left-sided early breast cancer in the IMRT era. Medicine. 2016;95(26):e4036. | ||
Belkacemi Y, Allab-Pan Q, Bigorie V, et al. The standard tangential fields used for breast irradiation do not allow optimal coverage and dose distribution in axillary levels I–II and the sentinel node area. Ann Oncol. 2013;24(8):2023–2028. | ||
Moreno AC, Shaitelman SF, Buchholz TA. A clinical perspective on regional nodal irradiation for breast cancer. Breast. 2017;34(1):S85–S90. | ||
Whelan TJ, Olivotto IA, Parulekar WR, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373(4):307–316. | ||
Matzinger O, Heimsoth I, Poortmans P, et al. Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol. 2010;49(1):24–34. | ||
Thorsen LB, Offersen BV, Danø H, et al. DBCG-IMN: a population-based cohort study on the effect of internal mammary node irradiation in early node-positive breast cancer. J Clin Oncol. 2016;34(4):314–320. | ||
Nikolaevich NS, Vasilevich KS. Why do we need irradiation of internal mammary lymph nodes in patients with breast cancer: analysis of lymph flow and radiotherapy studies. Rep Pract Oncol Radiother. 2017;22(1):37–41. | ||
Li Z, Gu X, Tong J, et al. A meta-analysis of internal mammary lymph node metastasis in breast cancer patients. Onkologie. 2013;36(12):747–752. | ||
Huang O, Wang L, Shen K, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008;107(3):379–387. | ||
Xie L, Higginson DS, Marks LB. Elective regional nodal irradiation in patients with early-stage breast cancer. Semin Radiat Oncol. 2011;21(1):66–78. | ||
Poortmans PM, Collette S, Kirkove C, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373(4):317–327. | ||
Kanyilmaz G, Aktan M, Koc M, Demir H, Demir LS. Unplanned irradiation of internal mammary lymph nodes in breast cancer. Radiol Med. 2017;122(6):405–411. | ||
Sapienza LG, Chen MJ, Gomes MJ, Mansur DB. Unintended irradiation of internal mammary chain – is that enough? Rep Pract Oncol Radiother. 2016;21(1):25–30. | ||
Arora D, Frakes J, Scott J, et al. Incidental radiation to uninvolved internal mammary lymph nodes in breast cancer. Breast Cancer Res Treat. 2015;151(2):365–372. | ||
Chung Y, Kim JW, Shin KH, et al. Dummy run of quality assurance program in a Phase 3 randomized trial investigating the role of internal mammary lymph node irradiation in breast cancer patients: Korean Radiation Oncology Group 08-06 study. Int J Radiat Oncol Biol Phys. 2015;91(2):419–426. | ||
Marks LB. A standard dose of radiation for “microscopic disease” is not appropriate. Cancer. 1990;66(12):2498–2502. | ||
Withers HR, Suwinski R. Radiation dose response for subclinical metastases. Semin Radiat Oncol. 1998;8(3):224–228. | ||
Lee CT, Zhou Y, Roy-Choudhury K, et al. Subtype-specific radiation response and therapeutic effect of FAS death receptor modulation in human breast cancer. Radiat Res. 2017;188(2):169–180. | ||
Qiu PF, Cong BB, Zhao RR, et al. Internal mammary sentinel lymph node biopsy with modified injection technique: high visualization rate and accurate staging. Medicine. 2015;94(41):e1790. | ||
Jethwa KR, Kahila MM, Hunt KN, et al. Delineation of internal mammary nodal target volumes in breast cancer radiation therapy. Int J Radiat Oncol Biol Phys. 2017;97(4):762–769. | ||
Darby SC, Ewertz M, Mcgale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–998. | ||
Yun-Jiu C, Xiao-Ying N, Cheng-Cheng J, et al. Long-term cardiovascular risk after radiotherapy in women with breast cancer. J Am Heart Assoc. 2017;6(5):e005633. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.