Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Post-Stroke Depression and Estimated Glomerular Filtration Rate: A Prospective Stroke Cohort
Authors Lin S, Luan X, He W, Ruan Y, Yuan C, Fan A , Chen X, He J
Received 3 August 2019
Accepted for publication 12 November 2019
Published 21 January 2020 Volume 2020:16 Pages 201—208
DOI https://doi.org/10.2147/NDT.S225905
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jun Chen
Shasha Lin, 1,* Xiaoqian Luan, 1,* Weilei He, 1,* Yiting Ruan, 1 Chengxiang Yuan, 1 Aiyue Fan, 1 Xiachan Chen, 2 Jincai He 1
1Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, People’s Republic of China; 2Department of Neurology, Wenzhou 325000, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jincai He
Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou 325000, Zhejiang Province, People’s Republic of China
Tel/Fax +86 577 5557 9363
Email [email protected]
Purpose: Post-stroke depression (PSD) is a frequent comorbidity in patients presenting with acute ischemic stroke. Impaired kidney function has been associated with depression in non-stroke subjects. We would like to evaluate whether the estimated glomerular filtration rate (eGFR) on admission is associated with the development of PSD.
Patients and methods: Total of 268 patients with acute ischemic stroke were recruited and completed 1-month follow-up visit. eGFR was calculated from the serum creatinine value, race, age, and sex by using the chronic kidney disease epidemiology collaboration equation (CKD-EPI creatinine equation). The 17-item Hamilton Depression Scale was used to evaluate depression symptoms. Patients with a depression score of ≥ 7 were evaluated using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th edition, for diagnosing post-stroke depression at 1 month. Meanwhile, 114 normal control subjects were also recruited.
Results: Ninety-three (34.7%) patients were diagnosed as having PSD at 1 month. There was a significant intergroup difference in eGFR levels within 24 hrs after admission (F=13.608, p< 0.001). The levels of eGFR within 24 hrs after admission were significantly lower in both non-PSD patients and PSD patients than in normal controls. In logistic regression, the level of eGFR (< 82mL/min/1.73m 2) was independently associated with increased risk of PSD even after adjusting for confounders (OR=2.328, 95% CI:1.092– 4.965, p=0.029).
Conclusion: Reduced eGFR was found to be correlated with the development of PSD and it suggests the need for greater attentions and potential interventions for depression in patients with stroke and with reduced eGFR.
Keywords: estimated glomerular filtration rate, eGFR, post-stroke depression, stroke, Depression
Introduction
Post-stroke depression (PSD), a serious and undertreated mood disorder, is regarded as important complication following stroke, with prevalence ranging from 29% to 39%.1–3 Study had shown that factors including history of depression before stroke, history of a previous stroke, stroke severity, and disability after stroke would contribute to the development of PSD.3–5 It is urgent for neurologists to early recognize and diagnose PSD because the presence of PSD not only affects the quality of life but also reduces functional ability, worsens rehabilitation outcomes, and increases mortality.3,6–8 However, being unable to understand the pathophysiological mechanisms completely makes it difficult to prevent and manage PSD.
Estimated glomerular filtration rate is an index reflecting the function of kidney. The lower the eGFR is, the worse the kidney function will be. Kidney disease, common among stroke patients,9 increases the risk of stroke10,11 and carotid atherosclerosis.12 In a large, community-based population, an independent, graded association was observed between renal dysfunction as was estimated with glomerular filtration rate (GFR) and the risk of death, cardiovascular events, and hospitalization.13 Study shows that when the glomerular filtration rate was <81.0 mL/min/1.73 m 2, with each 10-unit drop, death and nonfatal cardiovascular events would increase by 1.1-fold.14 Furthermore, reduced eGFR was found to negatively correlate with stroke outcome,15 and the risk of recurrent stroke was significantly increased with the decline of eGFR.16 In addition, studies had observed that reduced kidney function is independently associated with cognitive decline.17 The elderly with impaired kidney function have a more rapid rate of cognitive decline.18 Also, anxiety symptoms are common in patients with CKD.19 As for depression, studies have indicated the association between reduced kidney function and depression,20,21 while others have different attitudes.22 The prevalence of depression in patients suffering from chronic kidney disease (CKD) is approximately 20–30%, which is higher than that of other chronic diseases,23 such as 11% for diabetes mellitus,24 14% for congestive heart failure,25 and 16% for coronary artery disease after acute myocardial infarction.26 Interestingly, depression could also in turn accelerate the progression of CKD and lead to poor outcomes or earlier death in patients with CKD.21
However, to date, no previous study has explored the association between the estimated glomerular filtration rate (eGFR) on admission and the development of PSD. Considering the involvement of eGFR level in stroke as well as depression, we conducted this study to examine the extent to which the estimated glomerular filtration rate (eGFR) might be associated with PSD.
Materials and Methods
Subjects
The study was a prospective cohort study, which was approved by the Medical Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Participants were consecutively recruited from all patients with recent ischemic stroke hospitalized in the Stroke Unit of our hospital from October 2013 to June 2015, with written informed consents signed. And this study was conducted in accordance with the Declaration of Helsinki.
The inclusion criteria were: (1) Chinese ethnicity; (2) between 18 and 80 years old; (3) onset of acute stroke events was less than 7 days at admission; (4) diagnosed by cranial-computed tomography (CT) or magnetic resonance imaging (MRI) at admission. The exclusion criteria included: (1) transient ischemic attack (TIA) or primary haemorrhagic stroke; (2) pre-stroke diagnosis of significant cognitive impairment or dementia; (3) patients with a history of depression (clinical diagnosis or previous treatment) or other psychiatric disorders; (4) history of severe central nervous system diseases such as craniocerebral trauma, Parkinson’s disease or hydrocephalus; (5) a history of nootropic or antipsychotic drug use; (6) any situation that make them failed to the neuropsychological assessments such as visual or auditory impairment, chaotic conscious state, severe aphasia or dysarthria; (7) history of chronic kidney disease (clinical diagnosis or previous treatment); (8) comorbid malignancy or clinically thyroid disease, or undergoing glucocorticoid therapy at admission. Meanwhile, 114 healthy control subjects were recruited from a health survey. The subjects with any personal or familial history of psychiatric illness were excluded. All subjects were free of severe physical diseases including acute ischemic stroke.
Clinical Measurements
A standard questionnaire was administered by trained staff to obtain information on demographic characteristics. Patient’s age, sex, income, years of education and marital status, personal history (smoking or drinking) and medical history (hypertension, diabetes, coronary artery disease or previous stroke) were recorded. Cranial computerized and magnetic resonance imaging were performed within 24 to 72 hrs after admission in order to assess the site of the brain infarct.
Assessment
The 17-item Hamilton depression Rating Scale (HAMD) was adopted to assess the depressive symptoms in this population at 1 month after stroke.27 And there are already many studies using 1 month as evaluation point for PSD.28–30 A score of 0 to 7 is considered to be normal, while a score of ≥7 is indicative of depression. Patients with a HAMD score of ≥7 were evaluated using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) for the diagnosis of post-stroke depression by a neurologist/psychiatrist who was blind to the laboratory results of stroke patients.31
Stroke severity was assessed by trained neurologists using the National Institutes of Health Stroke Scale (NIHSS) within 24 hrs of admission. Functional outcome was evaluated by the modified Rankin Scale (mRS) and the Barthel Index (BI) at discharge. Cognition function was assessed by the Mini-Mental State Examination (MMSE) at 1 month.
Laboratory Tests
Peripheral blood was drawn from fasting patients on the second day after admission. Blood urea nitrogen (BUN), serum creatinine (Scr), hemoglobin, uric acid and high-sensitivity C-reactive protein (hs-CRP) were determined with a Beckman Coulter AU5800 automatic analyzer at our hospital’s laboratory. The eGFR was calculated on the basis of Scr concentration, race, age and sex using the chronic kidney disease epidemiology collaboration equation (CKD-EPI creatinine equation). According to the number of patients and the distribution of the eGFR level with the highest differences in this study, eGFR levels were further divided into tertiles (<82mL/min/1.73m2, 82–97mL/min/1.73m2, and >97mL/min/1.73m2).
Statistical Analyses
The results were represented as percentages for categorical variables, while continuous variables depending on their normal distribution were expressed as mean standard deviation (SD) or median (interquartile range, IQR). The Chi-squared test was employed for proportions and the normally distributed variables were compared using Student’s t-test and analysis of variance (ANOVA), while the Mann–Whitney U-test was employed for the asymmetrically distributed variables. Pearson correlation coefficient was performed for bivariate correlation. Binary logistic regression analysis was used to analyze association of PSD and reduced eGFR. The odds ratio (OR) value of PSD were recorded after adjusting for potential confounding variables. Data were managed and analyzed using SPSS version21.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 (two-tailed) was considered to be statistically significant.
standard deviation (SD) or median (interquartile range, IQR). The Chi-squared test was employed for proportions and the normally distributed variables were compared using Student’s t-test and analysis of variance (ANOVA), while the Mann–Whitney U-test was employed for the asymmetrically distributed variables. Pearson correlation coefficient was performed for bivariate correlation. Binary logistic regression analysis was used to analyze association of PSD and reduced eGFR. The odds ratio (OR) value of PSD were recorded after adjusting for potential confounding variables. Data were managed and analyzed using SPSS version21.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 (two-tailed) was considered to be statistically significant.
Results
Characteristics of Patients in the PSD Group, Non-PSD Group, and Normal Group
Among 512 subjects with acute stroke screened, 347 met the study entry criteria and were included in the study and 268 completed 1-month follow-up (Figure 1). Overall, 93 (34.7%, 49 men, 44 women) were into the group with post-stroke depression and 175 (65.3%, 122 men, 53 women) had no depressive symptoms after stroke. The clinical characteristics of patients in the PSD group, non-PSD group, and normal group are presented in Table 1.
 |
Table 1 Clinical and Demographic Characteristics of the Samples Under Study |
 |
Figure 1 Study recruitment profile. PSD indicates post-stroke depression. |
The mean (±SD) eGFR level for all stroke patients was 89.58±19.42 mL/min/1.73m2; the mean (±SD) eGFR levels in normal subjects, non-PSD patients and PSD patients were 98.31±16.44 mL/min/1.73m2, 92.69 ± 19.02 mL/min/1.73m2 and 83.71 ±18.91 mL/min/1.73m2, respectively. A significant intergroup difference in eGFRs within 24 hrs after admission was revealed (F=13.608, p<0.001). Indeed, the eGFR was significantly lower in non-PSD patients and PSD patients than in normal controls; the eGFR was significantly decreased in PSD patients than in non-PSD patients.
Patients with post-stroke depression also had more severe strokes (z= - 5.10, p<0.001) and poorer functional outcomes (z= - 5.92, p<0.001) and cognitive function (z= - 2.60, p<0.05). In addition, post-stroke depression group was more likely to be female, older and more current drinking and have higher blood urea nitrogen levels (all p<0.05). There were no significant differences in the distribution of stroke lesion between the two groups. Also, no associations were found in serum creatinine and hemoglobin and uric acid between PSD and non-PSD groups, as well as hs-CRP (p>0.05) (Table 1).
eGFR Level Tertiles of Patients
Significant differences were found between the PSD and non-PSD groups in eGFR level tertiles of patients (P = 0.011). Indeed, the proportion of patients in the lowest tertile (<82mL/min/1.73m2) was significantly higher in the PSD group (P=0.032), while the proportion of patients in the highest quartile (>97mL/min/1.73m2) was significantly lower in the PSD group (P =0.003) (Table 2).
 |
Table 2 eGFR Level Tertiles of Patients |
Predictors of PSD After Stroke
The Logistic regression analysis showed that the risk of post-stroke depression significantly increased with the decline of eGFR (<82mL/min/1.73m2), which was still significant after adjusting for age, sex, current drinking, NIHSS score, mRS score, BI score, MMSE score, Hs-CRP and Diabetes mellitus and(OR=2.328, 95% CI: 1.029–4.965, p=0.029). In addition, the NIHSS scores at admission (OR=1.189, 95% CI: 1.028–1.376, p=0.020) were independently associated with depression, after adjusting for the above variables (Table 3).
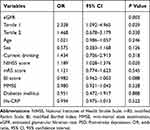 |
Table 3 Multivariate Logistic Model of the Clinical Determinants of PSD |
Discussion
To the best of our knowledge, this is the first study evaluating the relationship between the estimated glomerular filtration rate (eGFR) and the development of depression in stroke survivors. Our results point out that the eGFR is an important indicator of risk of the development of PSD.
We estimated the prevalence of post-stroke depression at 1 month. About 34.7% of stroke patients met the diagnosis of PSD, which was in line with the result of previous studies.1,32 Importantly, our results also demonstrated that patients with more severe stroke and more serious physical disability were susceptible to develop depression, which broadly agreed with the findings of earlier studies.3,33 Some studies had suggested that the location of stroke lesion might play a role in the etiology of post-stroke depression.34 However, in the present study, there is no association between the location of lesion and the presence of depression, consistent with other studies.35 Additionally, we did not find any significant association between the development of PSD and other controversial variables, such as MMSE scores,3,33,36 although previous studies had provided inconclusive results in these aspects. These relationships need to be further explored with more advanced imaging techniques and accurate analysis in future studies.
As mentioned earlier, a large number of studies have already reported the relationship between the reduced eGFR and the poor outcome, and greater mortality of ischemic stroke. However, limited studies have explored the relationship between the reduced eGFR and depression, especially in patients with acute ischemic stroke. We found that the levels of eGFR were significantly lower in stroke patients groups than in normal controls, which was consistent with previous studies.10,37,38 In this stroke patients-based prospective cohort study, our results indicated that the eGFR was decreased enormously in patients with PSD than non-PSD, which was in agreement with previous studies.39 Likewise, individuals with reduced eGFR were increasingly likely to experience lower Health-related quality of life.40 Depression and suicidal ideation were related closely with reduced kidney function. The risk of having depression and suicidal ideation increased even in patients with mild-reduced kidney function.41 Campbell et al found, whether proteinuria exists or not, lower eGFR was associated with a markedly increased risk of depressive symptoms after taking cardiovascular comorbidities into account among older adults with diabetes.39 Similarly, Hedayati et al showed that a major depressive episode was common in patients with earlier stages of CKD, and participants with CKD who had diabetic were twice as likely to have a major depressive episode compared with those without diabetes.23
The mechanisms of reduced eGFR and depressive symptoms remained not completely understood. Acute ischemic stroke triggered a series of inflammatory responses including central and peripheral, characterized by rapid infiltration of microglia and upregulation of proinflammatory cytokines.42 Inflammatory response plays an important role in the pathogenesis of depression,43 which accorded well with one study conducted by Raison et al that pro-inflammatory cytokines participated in many of the pathophysiological mechanisms of depression, involving neuroendocrine function, neurotransmitter metabolism, synaptic plasticity, and behaviour.44 Evidence shows that higher concentrations of pro-inflammatory cytokines have been identified among clinically depressed individuals and those with symptoms of depression.45,46 Meanwhile, it has been suggested that systemic inflammation and oxidative stress are known to be aggravated with reductions of kidney function.47 In addition, the orbitofrontal cortex (OFC) is recognised as a vital part of networks involving emotional processing.48 It is proposed to make decisions in experiencing pleasure and reward which are likely to be impaired in depression.49 At the same time, a study found that higher scores on the Hamilton Depression Rating Scale (HDRS) were associated with decreased cerebral glucose metabolism of the OFC in pre-dialysis CKD patients.50 It is in line with studies showing that in CKD patients global gray matter clusters reduced in OFC.51,52 Furthermore, a study conducted by Schaefer et al revealed that the amino acid neurotransmitter milieu altered in uremic rats, suggesting that uremic toxins may influence mental health and behavior.53 Besides, multiple comorbidities that are related to precede kidney function decline, such as hypertension, diabetes, stroke and chronic heart disease could also play roles in increasing the risk of depression.54 Therefore, these factors may affect mental health even in asymptomatic subjects whose kidney function is only mildly decreased. Thus, considering the role of reduced kidney function in ischemic stroke as well as depression, it may involve in the development of PSD.
There are still some limitations in our study. Firstly, eGFR levels were measured only 1 time at admission, suggesting that further studies are needed to evaluate how eGFR levels changed across time following stroke and whether its levels increased at later points improving stroke outcomes. Secondly, patients with aphasia or a serious condition were excluded, which might underestimate the actual incidence of PSD, and in the future, these patients should be evaluated using other scales such as Stroke Aphasic Depression Questionnaire (SADQ).55 Thirdly, further follow-up is necessary so that we could further explore the link between eGFR and PSD. Finally, the application of our conclusion may be limited in those minor stroke subjects, for most of the patients with higher NIHSS scores have been excluded, and patients with higher NIHSS scores should be included in future researches.
Conclusion
In summary, our study demonstrates an important relationship between declining eGFR levels and the development of PSD. Furthermore, the prevalence of these mental health problems after stroke increased obviously even when eGFR level is reduced mildly. Future studies on larger populations should be encouraged to confirm these findings.
Acknowledgments
This study was supported by Wenzhou Municipal Sci-Tech Bureau Program (Y20160002), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number: 2015BAI13B01) as well as the Projects of National Science Foundation of China (No. 81873799). We are greatly indebted to the staff and to the patients for their contributions to this study. Shasha Lin, Xiaoqian Luan, and Weilei He should be considered co-first authors for this study.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(6):1330–1340. doi:10.1161/01.STR.0000165928.19135.35
2. Ayerbe L, Ayis S, Crichton S, Wolfe CD, Rudd AG. The natural history of depression up to 15 years after stroke: the South London Stroke Register. Stroke. 2013;44(4):1105–1110. doi:10.1161/STROKEAHA.111.679340
3. Ayerbe L, Ayis S, Wolfe CD, Rudd AG. Natural history, predictors and outcomes of depression after stroke: systematic review and meta-analysis. Br J Psychiatry. 2013;202(1):14–21. doi:10.1192/bjp.bp.111.107664
4. Kutlubaev MA, Hackett ML. Part II: predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. 2014;9(8):1026–1036. doi:10.1111/ijs.12356
5. Shi Y, Yang D, Zeng Y, Wu W. Risk factors for post-stroke depression: a meta-analysis. Front Aging Neurosci. 2017;9:218. doi:10.3389/fnagi.2017.00218
6. Lindén T, Blomstrand C, Skoog I. Depressive disorders after 20 months in elderly stroke patients: a case-control study. Stroke. 2007;38(6):1860–1863. doi:10.1161/STROKEAHA.106.471805
7. Masskulpan P, Riewthong K, Dajpratham P, Kuptniratsaikul V. Anxiety and depressive symptoms after stroke in 9 rehabilitation centers. J Med Assoc Thai. 2008;91(10):1595–1602.
8. Whyte EM, Mulsant BH. Post stroke depression: epidemiology, pathophysiology, and biological treatment. Biol Psychiatry. 2002;52(3):253–264. doi:10.1016/S0006-3223(02)01424-5
9. Ovbiagele B, Schwamm LH, Smith EE, et al. Patterns of care quality and prognosis among hospitalized ischemic stroke patients with chronic kidney disease. J Am Heart Assoc. 2014;3(3):e000905. doi:10.1161/JAHA.114.000905
10. Lee M, Saver JL, Chang KH, et al. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. doi:10.1136/bmj.c4249
11. Ninomiya T, Perkovic V, Verdon C, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am J Kidney Dis. 2009;53(3):417–425. doi:10.1053/j.ajkd.2008.08.032
12. Bui AL, Katz R, Kestenbaum B, et al. Cystatin C and carotid intima-media thickness in asymptomatic adults: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2009;53(3):389–398. doi:10.1053/j.ajkd.2008.06.025
13. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi:10.1056/NEJMoa041031
14. Anavekar NS, McMurray JJ, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351(13):1285–1295. doi:10.1056/NEJMoa041365
15. El Husseini N, Fonarow GC, Smith EE, et al. Renal dysfunction is associated with poststroke discharge disposition and in-hospital mortality: findings from get with the guidelines-stroke. Stroke. 2017;48(2):327–334. doi:10.1161/STROKEAHA.116.014601
16. Wang IK, Lien LM, Lee JT, et al. Renal dysfunction increases the risk of recurrent stroke in patients with acute ischemic stroke. Atherosclerosis. 2018;277:15–20.
17. Berger I, Wu S, Masson P, et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016. 14(1):206.
18. Buchman AS, Tanne D, Boyle PA, et al. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology. 2009;73(12):920–927. doi:10.1212/WNL.0b013e3181b72629
19. Loosman WL, Rottier MA, Honig A, Siegert CE. Association of depressive and anxiety symptoms with adverse events in Dutch chronic kidney disease patients: a prospective cohort study. BMC Nephrol. 2015;16:155. doi:10.1186/s12882-015-0149-7
20. Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007;2(5):919–925. doi:10.2215/CJN.00820207
21. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946–1953. doi:10.1001/jama.2010.619
22. Remy JH, Martens JP, Kooman CDA, et al. Albuminuria is associated with a higher prevalence of depression in a population-based cohort study: the Maastricht Study. Nephrol Dial Transplant. 2018;33(1):128–138.
23. Hedayati SS, Minhajuddin AT, Toto RD, Morris DW, Rush AJ. Prevalence of major depressive episode in CKD. Am J Kidney Dis. 2009;54(3):424–432. doi:10.1053/j.ajkd.2009.03.017
24. Mitchell AJ, Chan M, Bhatti H, et al. Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol. 2011;12(2):160–174. doi:10.1016/S1470-2045(11)70002-X
25. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069–1078. doi:10.2337/diacare.24.6.1069
26. Frasure-Smith N, Lespérance F, Talajic M. Depression following myocardial infarction. Impact on 6-month survival. JAMA. 1993;270(15):1819–1825. doi:10.1001/jama.1993.03510150053029
27. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi:10.1136/jnnp.23.1.56
28. Chen H, Luan X, Zhao K, et al. The association between neutrophil-to-lymphocyte ratio and post-stroke depression. Clin Chim Acta. 2018;486:298–302. doi:10.1016/j.cca.2018.08.026
29. Qiu H, Liu Y, He H, et al. The association between mean platelet volume levels and poststroke depression. Brain Behav. 2018;8(10):e01114. doi:10.1002/brb3.2018.8.issue-10
30. Huang G, Chen H, Wang Q, et al. High platelet-to-lymphocyte ratio are associated with post-stroke depression. J Affect Disord. 2019;246:105–111. doi:10.1016/j.jad.2018.12.012
31. Na N. Diagnostic and statistical manual of mental disorders, 4th edition. Alzheimer Dis Assoc Disord. 1996;10(2):20–22.
32. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–1025. doi:10.1111/ijs.12357
33. Zhang W-N, Pan Y-H, Wang X-Y, Zhao Y. A prospective study of the incidence and correlated factors of post-stroke depression in China. PLoS One. 2013;8(11):e78981.
34. Choi-Kwon S, Han K, Choi S, et al. Poststroke depression and emotional incontinence: factors related to acute and subacute stages. Neurology. 2012;78(15):1130–1137. doi:10.1212/WNL.0b013e31824f8090
35. Tang WK, Liang H, Chu WC, et al. Association between high serum total bilirubin and post-stroke depression. Psychiatry Clin Neurosci. 2013;67(4):259–264. doi:10.1111/pcn.12051
36. Hackett ML, Anderson CS. Predictors of depression after stroke: a systematic review of observational studies. Stroke. 2005;36(10):2296–2301. doi:10.1161/01.STR.0000183622.75135.a4
37. Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67(2):224–228. doi:10.1212/01.wnl.0000229099.62706.a3
38. Nakayama M, Metoki H, Terawaki H, et al. Kidney dysfunction as a risk factor for first symptomatic stroke events in a general Japanese population–the Ohasama study. Nephrol Dial Transplant. 2007;22(7):1910–1915. doi:10.1093/ndt/gfm051
39. Campbell KH, Huang ES, Dale W, et al. Association between estimated GFR, health-related quality of life, and depression among older adults with diabetes: the Diabetes and Aging Study. Am J Kidney Dis. 2013;62(3):541–548. doi:10.1053/j.ajkd.2013.03.039
40. Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–1301. doi:10.2215/CJN.05541008
41. Jhee JH, Lee E, Cha MU, et al. Prevalence of depression and suicidal ideation increases proportionally with renal function decline, beginning from early stages of chronic kidney disease. Medicine. 2017;96(44):e8476. doi:10.1097/MD.0000000000008476
42. Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87(5):779–789. doi:10.1189/jlb.1109766
43. Song C, Wang H. Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):760–768. doi:10.1016/j.pnpbp.2010.06.020
44. Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi:10.1016/j.it.2005.11.006
45. Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi:10.1016/j.biopsych.2009.09.033
46. Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi:10.1097/PSY.0b013e3181907c1b
47. Himmelfarb J. Uremic toxicity, oxidative stress, and hemodialysis as renal replacement therapy. Semin Dial. 2009;22(6):636–643. doi:10.1111/sdi.2009.22.issue-6
48. Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi:10.1016/S0006-3223(03)00171-9
49. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi:10.1038/nrn1747
50. Song SH, Kim IJ, Kim SJ, Kwak IS, Kim YK. Cerebral glucose metabolism abnormalities in patients with major depressive symptoms in pre-dialytic chronic kidney disease: statistical parametric mapping analysis of F-18-FDG PET, a preliminary study. Psychiatry Clin Neurosci. 2008;62(5):554–561. doi:10.1111/j.1440-1819.2008.01849.x
51. Zhang LJ, Wen J, Ni L, et al. Predominant gray matter volume loss in patients with end-stage renal disease: a voxel-based morphometry study. Metab Brain Dis. 2013;28(4):647–654. doi:10.1007/s11011-013-9438-7
52. Qiu Y, Lv X, Su H, et al. Structural and functional brain alterations in end stage renal disease patients on routine hemodialysis: a voxel-based morphometry and resting state functional connectivity study. PLoS One. 2014;9(5):e98346. doi:10.1371/journal.pone.0098346
53. Schaefer F, Vogel M, Kerkhoff G, et al. Experimental uremia affects hypothalamic amino acid neurotransmitter milieu. JASN. 2001;12(6):1218–1227.
54. Katon WJ. Epidemiology and treatment of depression in patients with chronic medical illness. Dialogues Clin Neurosci. 2011;13(1):7–23.
55. Sutcliffe LM, Lincoln NB. The assessment of depression in aphasic stroke patients: the development of the stroke aphasic depression questionnaire. Clin Rehabil. 1998;12(6):506–513. doi:10.1191/026921598672167702
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
