Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
Post COVID-19 Vaccination-Associated Neurological Complications
Authors Assiri SA , Althaqafi RMM, Alswat K, Alghamdi AA, Alomairi NE, Nemenqani DM, Ibrahim ZS , Elkady A
Received 8 October 2021
Accepted for publication 12 January 2022
Published 2 February 2022 Volume 2022:18 Pages 137—154
DOI https://doi.org/10.2147/NDT.S343438
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Roger Pinder
Sara A Assiri,1 Raad MM Althaqafi,2 Khaled Alswat,2 Ahmed Abdullah Alghamdi,3 Naif E Alomairi,2,4 Dalal M Nemenqani,2 Zein S Ibrahim,5 Ahmed Elkady4
1Otolaryngology-Head and Neck Surgery Department, King Faisal Hospital, Taif, Saudi Arabia; 2College of Medicine, Taif University, Taif, Saudi Arabia; 3College of Medicine, Al-Baha University, Al Baha, Saudi Arabia; 4Neurology Department, Saudi German Hospital, Jeddah, Saudi Arabia; 5Department of Physiology, Faculty of Veterinary Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt
Correspondence: Sara A Assiri, Email [email protected]
Purpose: Neurological sequelae after COVID-19 vaccination are rare. We investigated the possible pathogenesis behind the development of neurological complications within a short period after Saudi residents received a COVID-19 vaccine.
Patients and Methods: We evaluated 18 patients who recently received a COVID-19 vaccine (Comirnaty and Vaxzevria vaccines) and presented with neurological complications to the Saudi German Hospitals in Jeddah, Saudi Arabia. Neurologists assessed the patients’ clinical presentation, radiological investigations, and laboratory findings.
Results: Three patients who received the first dose of the Vaxzevria vaccine experienced severe cerebral venous thrombosis, two of them were complicated by intracranial hemorrhage. Their laboratory investigations showed very high d-dimers and severe thrombocytopenia, which have been linked to higher mortality and poor outcome. Ischemic stroke occurred in eight cases (44.4%) with a predominance in older male patients. Three patients presented with seizures, two had optic neuritis. Guillain–Barré syndrome (GBS) and Miller Fisher syndrome (MFS) occurred in two male patients following vaccination with Comirnaty.
Conclusion: Neurological complications after COVID-19 vaccinations are very rare, and only a few cases have been reported worldwide. The shared pathophysiological basis between COVID-19 viral infection and COVID-19 vaccines stands behind the very rare neurological complications resulting from the hypercoagulable state triggered by the general inflammatory condition. We suspect some differences in the pathogenesis of ischemic stroke caused by COVID-19 infection and COVID-19 vaccines, which render COVID-19 vaccine-associated ischemic stroke more responsive to treatment. To date, no definitive association between the vaccine and GBS has been proven by any strong evidence, but it has recently been added as a very rare side effect of the Janssen COVID-19 vaccine. No possible links of Miller Fisher syndrome to COVID-19 vaccines have been reported before the one reported in this study.
Keywords: vaccine, ischemic stroke, cerebral venous thrombosis, seizures, optic neuritis
A Letter to the Editor has been published for this article.
Introduction
Health and economies have been affected globally by the devastating pandemic resulting from coronavirus disease 2019 (COVID-19). As of December 23rd, 2021, more than 279,572,610 cases had been reported with 5,398,772 documented deaths.1 Health organizations worldwide have reached a decision that widespread vaccination in all countries is inevitable to survive this deadly pandemic.
A number of vaccines have been approved based on randomized, blinded, controlled trials that started in December 2020. These approved vaccines include two mRNA-based vaccines that encode the spike protein antigen of SARS-CoV-2 encapsulated in lipid nanoparticles: Comirnaty (BNT162b2, Pfizer-BioNTech) and Spikevax mRNA-1273 (Moderna). The other two vaccines are a recombinant chimpanzee adenoviral vector encoding the spike glycoprotein of SARS CoV-2 called Vaxzevria (ChAdOx1 nCov-19, AstraZeneca) and a recombinant adenovirus type 26 vector encoding SARS-CoV-2 spike protein (Ad26.COV2.S, Johnson & Johnson/Janssen).2
Putting aside the uneven vaccine distribution between countries, certain countries have had a high percentage of vaccinated citizens. More than 90.68% of the population in the United Arab Emirates have received a second dose of a vaccine. More than 77.12% of Canadian people, 69.28% of the United Kingdom, and 73.83% of the Italian population older than 18 years old have received a first dose.3 In the European Union, as of December 23rd, 2021, more than 305,786,002 of the total population aged 18 years and above (about 83.6%) have received at least one dose of a vaccine.4
In Saudi Arabia, the Saudi Food and Drug Authority (SFDA) approved the registration of the Pfizer-BioNTech Comirnaty COVID-19 Vaccine on December 10, 2020. Later, the SFDA approved the importation and use of the Oxford-AstraZeneca Vaxzevria COVID-19 Vaccine on February 18th, 2021. Johnson & Johnson and Spikevax (Moderna) vaccines were also approved shortly after. By December 23rd, 2021, about 49,253,475 out of the total population of 35.34 million in Saudi Arabia had received two vaccine doses, which is at least 67% of the targeted population, and 73% has received at least one dose.5
Due to this rapid uptake of the vaccine and the huge number of people who have been vaccinated or will be vaccinated, safety profiles should be carefully considered. It is essential to monitor adverse reactions following any newly introduced vaccine, and monitoring does not undermine the extreme importance of vaccines in saving lives. Vaccines are considered some of the safest and most effective drugs, but adverse reactions are inevitable, especially during the process of mass immunization, which highlights the complexity and uniqueness of individual human bodies’ response to vaccines.
The Vaxzevria ChAdOx1n CoV-19 vaccine (AstraZeneca) has been claimed to be associated with a safety alert of particular concern.2,6 Because of the popularity and widespread use of the AstraZeneca Vaxzevria vaccine, the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Medicines Agency (EMA) have conducted intensive reviews of the risks reported to be associated with it, including abnormal clotting, cerebral venous thrombosis (CVT), and thrombocytopenia. Both agencies emphasize the overwhelming favorable risk–benefit ratio for the vaccines against SARS-CoV-2 since the risk of venous thromboembolism associated with the vaccines has been proven not to be higher than the background risk in the general population. Although a clear causal link has not been proven (Government of the United Kingdom), the EMA has acknowledged that vaccination against SARS-CoV-2 may be associated with the occurrence of rare but serious CVT and thrombocytopenia.7,8
As of April 28, 2021, around 9000 reports of adverse vaccine reactions have been submitted to Adverse Event Reporting System (VAERS). Expected acute transient effects were listed as neurological symptoms such as muscle spasm, myalgia, paresthesia, headaches, and dizziness. Rare reports of tremor, diplopia, seizures, reactivation of herpes zoster, and dysphonia have been mentioned. Additionally, reports to VAERS have included 17 stroke cases, 32 cases of Guillain–Barré syndrome (GBS), and 6 cases of acute disseminated encephalomyelitis.9 The reports to VAERS reached 284,043 cases of adverse reactions to the vaccines as of June 8, 2021, by which more than 51% of the total United States population had been given at least the first dose of a COVID-19 vaccination.10
A wide range of severe neurological complications following the COVID-19 vaccine has been reported during the last year including encephalopathy, transverse myelitis, and bell’s palsy. Guillain–Barré syndrome has also been reported, post-vaccination peripheral nerve damage due to autoanti-body molecular mimicry is the currently proposed pathogenesis.11 Cerebral venous thrombosis and thrombocytopenia have been reported among patients who had autoantibodies against platelet factor 4 and high d-dimer.12,13 A picture similar to heparin-induced thrombocytopenia.14 It is important to be extremely careful when evaluating the association between vaccines and neurological complications. If side effects are wrongfully connected to the vaccine, the harm of depriving nations from vaccinations might outweigh the risk of the very rare complications. To gain a complete picture of the possibility of a causal link between vaccination and neurological complications among Saudi residents, we investigated 18 cases presenting with neurological sequelae following COVID-19 vaccination.
Patients and Methods
This retrospective study included a total of 18 patients, of which 10 received the Oxford-AstraZeneca Vaxzevria COVID-19 vaccine, and the remaining 8 patients received the Pfizer-BioNTech Comirnaty COVID-19 vaccine. The patients included comprised all patients who were referred to or presented to the Saudi German Hospitals in Jeddah, Saudi Arabia, from March to September 2021 with neurological complications after receiving a COVID-19 vaccine.
All patients had a confirmed negative polymerase chain reaction (PCR) result for COVID-19 before admission. Blood samples were taken from all patients, and all available laboratory tests were recorded and included the following: hemoglobin (13–15 g/dl), platelets (140–440,109/L), fibrinogen <150 mg/dL, blood glucose, HbA1c% (4.8–6), creatinine (0.5–1.1 mg/dL), troponin-I (10–26 pg/mL), and prothrombin time (PT) (10.5–14 Sec), from which INR ratios were calculated. In addition, thyroid-stimulating hormone (TSH) (0.4–4.0 mU/L), alanine transaminase (ALT) (<55 U/L), high-density lipoprotein (>60 mg/dL), low-density lipoprotein (<100 mg/dL), total cholesterol (<200 mg/dL), and triglycerides (<150 mg/dL) were included. All patients were subjected to echocardiography with ejection fraction taken as a marker for the efficiency of cardiac functions. Measurements of blood pressure and heart rate were used to evaluate hypertension.
All patients were seen by a consultant neurologist who evaluated them according to Modified Rankin Scale (MRS) and Glasgow Coma Scale/Score (GCS). Patients were subjected to brain computerized tomography (CT) or magnetic resonance imaging (MRI) and vessel imaging, such as magnetic resonance angiography (MRA), magnetic resonance venography (MRV), CT venography (CTV), and cerebral angiography when clinically indicated. Data were analyzed using IBM SPSS Statistics for Windows, Version 23.0. Means, standard deviations (SDs), medians, interquartile ranges (IQRs), and percentages were calculated. The Mann–Whitney U-test was used to compare the two independent groups for nonparametric parameters. We used an independent t-test for parametric variables.
We applied the FAPIC predictive scoring model for mortality15 to our first three patients. If any of the following was applicable, a point was scored: age less than or 60 years, platelet count of less than 25109/mL, fibrinogen less than 150 mg/dL, intracranial hemorrhage (ICH), and CVT. The predicted mortality increased with each point: 2.08% with FAPIC score 0, 6.66% with FAPIC score 1, 19.31% with FAPIC score 2, 44.54% with FAPIC score 3, 72.94% with FAPIC score 4, and 90.05% with FAPIC score 5.
Results
The total cohort’s mean age was 48.8 years (IQR 36–59 years), and there was no gender predominance. The mean age of female patients was 46.2 years (IQR 26–69 years), and the mean age of male patients was 51.4 years (IQR 39.5–60.5 years). The number of days between receiving the vaccine and presenting with various neurological complications ranged between 4 and 25 days with an average of 10 days (Figure 1), while the average days of hospital admission was 8 days. Of the 18 patients studied, ischemic stroke was encountered in eight patients (constituting 44.4% of all presented cases), and severe CVT occurred in 16.6% of cases.
Patients Who Received the Oxford-AstraZeneca COVID-19 Vaccine (Vaxzevria)
The 10 patients who received the first dose of the Oxford-AstraZeneca Vaxzevria COVID-19 vaccine had a mean age of 43.6 years (IQR 28–58 years). Table 1 shows all clinical, laboratory and radiological data of patients who presented with neurological sequelae after receiving this vaccine.
 |  |  |
Table 1 Patients Who Received the Vaxzevria Oxford – AstraZeneca Vaccine |
Cerebral Venous Thrombosis (CVT)
Three patients had severe CVT (Table 1, cases 1, 2, and 3) after the first dose of the Vaxzevria vaccine by 10, 12, and 4 days, respectively, with a median of 10 days. All patients were females under 40 years of age. Two of the three CVT patients who died had an intracerebral hemorrhage with herniation after rebleeding (Table 1, cases 1 and 2). Their initial complaint was headache, followed by clinical deterioration of GCS to scores of 8 and 6 points for the first and second cases, respectively. Laboratory results revealed thrombocytopenia as low as 11,109/L in the first case, 17,109/L in the second case, and 21,109/L in the third one. Overall, they had the highest D-dimers readings among our total cohort of 18 patients.
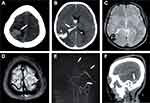
Prothrombin time was also elevated in all three cases. The first two patients had a low hemoglobin level, and the second case had a low fibrinogen level of 128 mg/dl. Lipid profile results revealed high triglyceride levels, low HDL, and normal total cholesterol levels in all three patients, and the first two cases had elevated LDL. We calculated the FAPIC score for all three patients, and the first case scored 4 points, so her expected probability of death was 72.94% (fibrinogen was not available for the first case, so it was not included). The second case scored 5 points, and her expected probability of death was 90.05%, while the third case scored 3 points and had an expected probability of death of 44.54%. Figure 2 shows the radiological brain imaging of cases 1, 2, and 3 presenting with CVT.
Optic Neuritis
Bilateral optic neuritis occurred in a 24-year-old female, and right unilateral optic neuritis occurred in a 42-year-old male patient after the first dose of Vaxzevria Oxford-AstraZeneca vaccine by 14 and 19 days, respectively (Table 1, cases 9 and 10). Both were medically free and presented with decreased visual acuity and eye pain. The 42-year-old male patient was a smoker and had elevated triglyceride and LDL levels, as well as mildly elevated blood pressure. Both recovered completely after less than 10 days of hospital admission.
Ischemic Stroke
Four male patients had an ischemic cerebrovascular accident (Table 1, cases 4, 5, 6, and 7). All of them had comorbidities and were older than 40 years old. The oldest patient was 66 years old (Table 1, Case 4) and presented with a seizure, low blood pressure, aphasia, and dysphagia, in addition to motor, sensory, and visual affection at 10 days after receiving the first dose of the AstraZeneca vaccine. He had multiple comorbidities, including diabetes that was poorly controlled with HbA1C of 10.6, as well as a previous stroke. He spent the longest duration in the intensive care unit (ICU), and his MRI showed a right middle cerebral infarction.
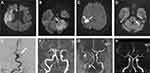
A 46-year-old Filipino male (Table 1, case 5) presented with a seizure, ataxia, dysarthria, and motor and sensory affection at 7 days after receiving the first dose of the AstraZeneca vaccine. He had a known case of poorly controlled diabetes with HbA1C of 13.7 and highly elevated triglyceride, total cholesterol, and LDL readings. Figure 3C and G show the MRI and MRA done for this case.
The third ischemic stroke patient was 56 years old (Table 1, case 6). His MRI showed a right lacunar thalamic stroke, and he presented at 2 days after receiving the vaccine with ataxia and vertigo. He was diagnosed as a case of vestibular neuritis. The fourth case was an Indian hypertensive patient and a 62-year-old male (Table 1, case 7). His MRI showed pontine infarction, as shown in Figure 3D. Figure 3H shows his MRA. He presented 4 days after receiving the vaccine with dysarthria and motor, sensory, and visual affection.
Seizures
A 58-year-old female patient with a known case of controlled hypothyroidism and hypertension (Table 1, case 8) presented with an isolated first-time seizure after receiving the first dose of Vaxzevria the vaccine within 25 days. Her GCS on presentation was 10, and she was admitted to the ICU. Her MRI and CT brain were unremarkable, and her TSH and blood pressure were within normal limits. She spent a total of 4 days of admission. Additionally, two of the patients who had an ischemic stroke also presented with first-time seizure.
Patients Who Received the Pfizer-BioNTech Comirnaty COVID-19 Vaccine
The eight patients who received the Comirnaty vaccine had a mean age of 55.3 years (IQR 36.5–69.5) years. Table 2 demonstrates all clinical, laboratory, and radiological data of these patients.
 |  |  |
Table 2 Patients Who Received the Comirnaty Pfizer Vaccine |
Guillain–Barre Syndrome (GBS) and Miller Fisher Syndrome
A 36-year-old male patient was diagnosed with GBS (Table 2, case 17). He presented with muscular weakness four days after receiving the second dose of the Pfizer-BioNTech vaccine. Similarly, a 37- year-old male patient presented initially with dysphagia and dysarthria nine days after the first dose of the Pfizer-BioNTech vaccine, and then he was diagnosed with a GBS variant: Miller Fisher syndrome (MFS). Both did not have any previous comorbidities and were admitted for a total of 7 days, during which they received intravenous immune globulin (IVIG).
Seizures and Encephalopathy
An 80-year-old female patient presented with motor, sensory, and visual affection in addition to seizures, vertigo, dysphagia, and dysarthria after receiving the second dose of the Pfizer-BioNTech vaccine by 16 days. She was admitted to the ICU for 8 days. She had a known case of hypertension, diabetes, and epilepsy, and she had been seizure free for years on Depakine.
Ischemic Stroke
Four patients had an ischemic cerebrovascular incident (Table 2, cases 11, 12, 13, and 14), and one female patient had a transient ischemic attack (Table 2, case 15). All of them had comorbidities and were older than 30 years old. A 59-year-old Saudi male patient presented with sensory affection in addition to ataxia, vertigo, aphasia, dysphagia, dysarthria, and a GCS score of 9 after receiving the first dose of Pfizer-BioNTech vaccine by 12 days. He had a known case of uncontrolled diabetes, and upon presentation, his blood sugar was 458, and his HbA1c was 10.3. He was also hypertensive, presenting with a blood pressure of 458/210 mmHg. Additionally, his lipid profile showed high triglyceride, cholesterol, and LDL levels. He was admitted to the ICU for 15 days, his MRI brain showed a large left cerebral infarction, and MRA showed left posterior inferior cerebellar artery occlusion. Figure 3B and F show the MRI and MRA done for this case.
A 59-year-old Palestinian male presented after receiving the second dose of the Pfizer-BioNTech vaccine by 23 days with motor and sensory affection in addition to dysphagia and dysarthria. He had dyslipidemia, and on admission, his triglyceride level was 187 mg/dl, and his total cholesterol was 285 mg/dl, while his LDL was 169 mg/dl. Figure 3A and F show the MRI and cerebral angiography done for this case, revealing right middle cerebral artery infarction.
The oldest among this group of ischemic stroke patients was an 80-year-old Ethiopian woman who received the second dose of Pfizer-BioNTech vaccine, and then seven days later, she presented with motor, sensory, and visual affection in addition to aphasia, dysphagia, and dysarthria. She was admitted to the ICU for 18 days, where her MRI and MRA showed left middle cerebellar artery occlusion. The youngest patient was a 36-year-old asthmatic female who received the first dose of the Pfizer-BioNTech vaccine and presented 6 days later with motor and sensory affection, in addition to aphasia and dysphagia. Her brain imaging revealed a left middle cerebellar artery occlusion. One 56-year-old hypertensive female patient had a transient ischemic attack after the first dose of Pfizer-BioNTech vaccine by 8 days. She had an episode of aphasia and dysarthria. Table 3 shows a comparison of the coagulation profiles between the two groups receiving the AstraZeneca and Pfizer vaccines.
 |
Table 3 Comparison of the Laboratory Data of INR, PT, Platelets and D-Dimer Between the Two Groups of Vaccinated Patients |
Discussion
This study investigated neurological complications for their possible association with COVID-19 vaccination among Saudi residents. In accordance with a recent report,6 the timing of the neurological symptoms’ presentation among our patients ranged from 4 to 25 days with an average of 10 days after vaccination.
CVT
CVT was severe in three female patients under 40 years old, and all of them received the first dose of the Oxford-AstraZeneca Vaxzevria vaccine. This is in accordance with EMA and WHO reports.8,16 Thrombosis with thrombocytopenia syndrome (TTS) has been reported as a very rare complication following vaccination with Vaxzevria with 70% predilection to middle-aged females; it is caused by IgG antibodies that recognize platelet factor 4 (PF4) leading to platelet activation.17
The predominance of CVT among middle-aged females who were vaccinated with the Vaxzevria vaccine was also observed in a case series by Greinacher et al.2 CVT occurrence in young females, as presented in the current study, and its resemblance to the available literature should raise attention and prompt further investigation of the Vaxzevria vaccine’s risk in this particular population.
Similarly, CVT following vaccination with the Johnson & Johnson vaccine, another adenoviral vector-based vaccine, has been reported and compared with CVT following the Vaxzevria vaccine.
They have shown no difference in mortality between the two groups, but patients who received the Vaxzevria vaccine presented earlier after a median of 10 days,18 which matches the results in the present study. Two out of the three CVT patients in the current study died, and their radiological investigations showed intracranial hemorrhage, while labs showed severe thrombocytopenia. Like previously reported cases,19 mortality was associated with intracranial hemorrhage and very low platelet counts. The FAPIC score is a recent novel scoring system suggested by Hwang et al to predict the probability of death among patients presenting with thrombotic thrombocytopenia after receiving an adenoviral vaccine. The scores depend on the age, platelet count, fibrinogen level, and intracranial hemorrhage, in addition to CVT.15
It is important to note that the two deceased patients had the highest D-dimers readings overall among our 18 studied cases. Thus, in the current study, D-dimer elevation indicates the severity of the coagulopathy and its related complications, including CVT and its association with poor outcomes in COVID-19-vaccinated individuals, especially those receiving the Vaxzevria vaccine, in a similar way to COVID-19 patients.20 Severe CVT occurred in 16.6% of our 18 cases, which approaches the reported incidence of 21% of CVT among COVID-19 patients.21 Interestingly, COVID-19 infection-associated CVT has been reported in patients who have various co-morbidities.22 However, our three cases who had CVT were medically free and did not receive any previous medications. This may indicate an insignificant role of the suggested risk factors in those individuals and could refocus clinicians’ attention to depend mainly on the clinical symptoms at the time of the patient’s admission.
Ischemic Stroke
In the current study, the equal distribution of the ischemic stroke occurrence between those receiving the Oxford-AstraZeneca and Pfizer-BioNTech COVID-19 vaccines may indicate a general reaction of the body that is prompted by the vaccines. Although the mechanism of the presented neurological complications after COVID-19 vaccination has not been precisely explained, it could be attributed to the COVID-19 vaccine-triggered inflammatory condition like in the course of COVID-19 viral infection, which induces disseminated intravascular coagulation (DIC)23 concurrent with vascular endothelial dysfunction,24 leading to large-vessel stroke.25 This hypothesis may be the main cause, especially in mRNA-based vaccines, which depend on giving the mRNA code of spike protein to human cells. In turn, this directs the cell machinery to synthesize the spike protein, and after being released from the cells, it stimulates the immune system to recognize and memorize it and attack it in the future.26
Based on this hypothesis, the vaccine-triggered inflammatory condition may be considered the main pathway for the COVID-19 vaccines’ neurological complications, in addition to cytokine-mediated neuroinflammation.27 Of course, this is separate from the local effect of viral-induced neurodegeneration in cases of COVID-19 infection, including the viral-induced effect on the blood brain barrier (BBB) and its associated neurons and neuroglia cell inflammation.28 The likelihood of ischemic stroke is increased with COVID-19 infection, increasing mortality among patients.29 Out of the 18 cases studied, ischemic stroke was encountered in eight cases, who were mostly elderly males, with four in each vaccine group.
The occurrence of stroke in COVID-19 vaccinated individuals in the current study seems to have the same tendency as those occurring in COVID-19 viral infections, with a predominance in older age.30 This is inconsistent with a previous retrospective study from Wuhan, China.31 Moreover, in this study, MRI and vessel imaging studies revealed that the majority of patients who experienced stroke showed large vessel infarction, similar to younger COVID-19 infected patients who have been reported with similar presentation.32 Further investigation is still needed to determine whether this observation distinguishes stroke presenting in young patients who have a natural COVID-19 infection from older vaccinated individuals.
Notably, in the current study, most of patients who experienced stroke had risk factors and common comorbidities such as dyslipidemia, hypertension, and diabetes, which is in agreement with previously reported stroke-predisposing factors in COVID-19 patients.33 In contrast to previous studies showing high mortality associated with stroke in COVID-19 patients,34 all patients who experienced stroke in the current study survived and were discharged home after, with only 3 of them requiring extended care. This may raise the question of whether COVID-19 vaccine-associated stroke is more responsive to treatment than natural COVID-19 infection-associated stroke. The complicated pathogenesis of COVID-19 infection-associated thrombus might render it more resistant and difficult to treat when compared to thrombus resulting from COVID-19 vaccines. This could be due to the viral induced-vascular endothelial changes,35 in addition to COVID-19-associated inflammation in the neurons and neuroglia cells.28
Seizures
Out of the 10 cases injected with the first dose of the Oxford-AstraZeneca COVID-19 vaccine, three cases presented with seizures as a neurological complication. COVID-19 infection has been reported to cause seizures as one of the neurological complications of this viral infection.36 However, it was hypothesized to occur due to viral invasion of the central nervous system (CNS) inducing the microglia to initiate an inflammatory cascade,37 with the release of the astrocytes and microglia inflammatory cytokines including TNF-α, IL-6, IL-1B, NO, PGE2, and free radicals.36,38 It was also hypothesized to be caused by severe hypoxia from respiratory failure,36 which causes a cytokine storm release leading to organ failure.39
Seizures were also reported after vaccination in a 68-year-old man in India.40 In another study, an 83-year-old woman and a 76-year-old man with no previous neurological diseases presented with seizures and encephalopathy after receiving Spikevax Moderna COVID-19 vaccination.41 These COVID-19 vaccine-associated seizures could be attributed to the vaccine-induced cell machinery synthesizing and releasing spike protein, which stimulates an exaggerated inflammatory condition and causes hyperthermia. It is worth mentioning that hyperthermia has been recognized to induce glial cell activation and increase BBB permeability.42 Increased BBB permeability permits peripheral blood cells and albumin passage to the CNS, causing disruption of its osmotic balance43,44 and allowing the passage of peripheral cytokines into it, causing seizures.37 The occurrence of seizures in the current study was mostly among the group vaccinated with Oxford-AstraZeneca Vaxzevria, which might indicate that it causes a strong inflammatory reaction.
Optic Neuritis
In the current study, bilateral optic neuritis occurred in a 24-year-old female, and right unilateral optic neuritis occurred in a 42-year-old male after the first dose of the Oxford-AstraZeneca vaccine by 14 and 19 days, respectively. COVID-19 vaccine-associated optic neuritis could be attributed to myelin sheath destruction and axonal degeneration,45 which are caused by inflammatory mediators produced by peripheral blood cells that have migrated through the hyperthermia-disrupted BBB.42 The occurrence of optic neuritis among the Oxford-AstraZeneca Vaxzevria group may add additional evidence to the severity of the body’s reaction to this vaccine in individuals with currently undetermined risk factors. Intriguingly, COVID-19 infection resulting in optic neuritis has been reported in older age in a 63-year-old man,45 and a 64-year-old woman.46 However, the results of the current study indicate that the optic neuritis associated with Oxford-AstraZeneca Vaxzevria occurred in young and middle-aged patients. It is still unknown whether the body’s reaction or the pathogenesis of the optic neuritis differs between COVID-19-infected and COVID-19-vaccinated individuals, resulting in some variation in the age distribution.
GBS
Two male patients among our cohort who received the Pfizer Comirnaty vaccine were diagnosed with GBS and MFS. In February 2021, the first reported case of GBS following the first dose of the Pfizer Comirnaty vaccine was an 82-year-old female patient.47 A 65-year-old male patient was diagnosed with GBS after receiving the first dose of the Pfizer Comirnaty vaccine,48 and a 67-year-old man was diagnosed with GBS 15 days after receiving the first dose of the AstraZeneca Vaxzevria vaccine.49
GBS following COVID-19 infection was also highlighted and reported in more than 40 cases.50 However, to date, there are no specific GBS features distinguishing those cases presumably caused by COVID-19, and additionally, molecular mimicry linking COVID-19 to GBS has not been proven by any strong evidence.51 There is no definitive association linking SARS-COV-2 to the sporadic incidence of GBS, and since COVID-19 vaccines are synthesized based on the same spike protein of SARS-COV-2, there is lack of causative link between the vaccine and GBS.52 More recently, the EMA has reported that GBS will be added as a very rare side effect of the Janssen COVID-19 vaccine.53
MFS is a variant of GBS characterized by ataxia, ophthalmoplegia, and areflexia. MFS has been reported in 7 cases after COVID-19 infection, and most patients were men.54 However, the occurrence of MFS after vaccination is extremely rare and has been published as case reports following seasonal influenza vaccination.55 To our knowledge, our patient who was diagnosed with MFS nine days following the Pfizer vaccination is the first reported case to be possibly linked to COVID-19 vaccine (Table 2, case 18).
Limitations
The current study does not establish a causal link between the reported 18 patients’ complications and COVID-19 vaccines. Descriptive case series such as our study help to monitor the safety of vaccines, but they do not clarify whether the link between such complications and vaccines is causal or coincidental. One of the many limitations that we faced was the difficulty in reaching the total number of all vaccinated individuals within the area served by the hospital where we performed our study, and hence, we were unable to express the incidence of neurological complication among the total number of vaccinated individuals. Moreover, anti-PF4 antibody testing was unavailable during the study period. Additionally, we could not obtain the previous history of COVID-19 infection in our 18 patients, although all patients had confirmed negative PCR results for COVID-19 infection before admission. During the study period, there were no patients vaccinated with Johnson & Johnson or Moderna vaccines presenting with neurological complications to our hospital, which is why this study did not include patients vaccinated with vaccines other than the Oxford-AstraZeneca Vaxzevria or Pfizer-BioNTech Comirnaty vaccines.
Conclusion
Neurological complications associated with mRNA vaccination seem to be induced by a shared pathophysiological basis with COVID-19 viral infection, including spike protein attachment to human cells triggering a general inflammatory state. This inflammatory state can lead to hypercoagulopathy manifesting as an ischemic stroke or CVT complicated by intracranial hemorrhage. Strokes encountered in this study showed better response to treatment than those reported in association with COVID-19 viral infection. The body’s reaction to COVID-19 vaccines is stronger with the Oxford-AstraZeneca Vaxzevria vaccine than with the Pfizer-BioNTech vaccine Comirnaty, which results in severe neurological complications and mortality in individuals with currently undetermined risk factors. Careful and continuous documentation and monitoring of the adverse reactions encountered following vaccinations may help to identify risk factors that render some individuals susceptible to specific complications.
Ethics Approval and Informed Consent
Approval from the Saudi German Hospital ethical committee was obtained on May 21, 2021. Patients’ consent to review their medical records was not required and it was waived by the Saudi German Hospital Ethical Committee due to the retrospective nature of the study. Privacy and anonymization of the participants are maintained with confidentiality and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
We greatly acknowledge the Taif University Researchers Supporting Program (TURSP-2020/233) of Taif University, Taif, Saudi Arabia, for facilitating this work.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors confirm that there are no competing interests to disclose.
References
1. Johns Hopkins University & Medicine, Coronavirus Resource Center. COVID-19 Map. Available from: https://coronavirus.jhu.edu/map.html. Accessed December 23, 2021.
2. Greinacher A, Thiele T, Warkentin TE, Weisser K, Kyrle PA, Eichinger S. Thrombotic thrombocytopenia after ChAdOx1 nCov-19 vaccination. N Engl J Med. 2021;384(22):2092–2101. doi:10.1056/NEJMoa2104840
3. Our world in data June, 8, 2021. Available from: https://ourworldindata.org/Covid-vaccinations.
4. European Centre for Disease Prevention and Control (ECDC). COVID-19 vaccine tracker. 2021.
5. The New York Times - Tracking Coronavirus Vaccinations Around the World By Josh Holder. Covid world vaccination tracker. Available from: https://www.nytimes.com/interactive/2021/world/covid-vaccinations-tracker.html. Accessed December 23, 2021.
6. Schultz NH, Sørvoll IH, Michelsen AE, et al. Thrombosis and thrombocytopenia after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384:2124–2130. doi:10.1056/NEJMoa2104882
7. European Medicines Agency. AstraZeneca’s COVID-19 vaccine: EMA finds possible link to very rare cases of unusual blood clots with low blood platelets. 2021.
8. European Medicines Agency. COVID-19 Vaccine AstraZeneca: benefits still outweigh the risks despite possible link to rare blood clots with low blood platelets. 2021.
9. Goss AL, Samudralwar RD, Das RR, Nath A. ANA investigates: neurological complications of COVID-19 vaccines. Am Neurol Assoc. 2021;89(5):856–857.
10. VAERS vaccine adverse event reporting system. Available from: https://vaers.hhs.gov/index.html.
11. Garg RK, Paliwal VK. Spectrum of neurological complications following COVID-19 vaccination. Neurol Sci. 2021;1–38. doi:10.1007/s10072-021-05662-9
12. Scully M, Singh D, Lown R, et al. Pathologic antibodies to platelet factor 4 after ChAdOx1 nCoV-19 vaccination. N Engl J Med. 2021;384(23):2202–2211. doi:10.1056/NEJMoa2105385
13. Tiede A, Sachs UJ, Czwalinna A, et al. Prothrombotic immune thrombocytopenia after COVID-19 vaccine. Blood. 2021;138:350–353. doi:10.1182/blood.2021011958
14. Iba T, Levy JH, Warkentin TE. Recognizing vaccine-induced immune thrombotic thrombocytopenia. Crit Care Med. 2021. doi:10.1097/CCM.0000000000005211
15. Hwang J, Park SH, Lee SW, et al. Predictors of mortality in thrombotic thrombocytopenia after adenoviral COVID-19 vaccination: the FAPIC score. Eur Heart J. 2021;42(39):4053–4063. doi:10.1093/eurheartj/ehab592
16. WHO. Statement of the COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) on safety signals related to the Johnson & Johnson/Janssen COVID-19 vaccine; 2021 Available from: https://www.who.int/news/item/19-05-2021-statement-gacvs-safety-johnson-johnson-janssen-Covid-19-vaccine.
17. Douxfils J, Favresse J, Dogné J-M, et al. Hypotheses behind the very rare cases of thrombosis with thrombocytopenia syndrome after SARS-CoV-2 vaccination. Thromb Res. 2021;203:163–171. doi:10.1016/j.thromres.2021.05.010
18. Hwang J, Lee SB, Lee SW, et al. Comparison of vaccine-induced thrombotic events between ChAdOx1 nCoV-19 and Ad26.COV.2.S vaccines. J Autoimmun. 2021;122:102681. doi:10.1016/j.jaut.2021.102681
19. Pavord S, Scully M, Hunt BJ, et al. Clinical features of vaccine-induced immune thrombocytopenia and thrombosis. N Engl J Med. 2021;385:1680–1689. doi:10.1056/nejmoa2109908
20. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1–14.
21. Lodigiani C, Iapichino G, Carenzo L, et al.; Humanitas COVID-19 Task Force. Venous and arterial thromboembolic complications in COVID19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. 2020;191:9–14. doi:10.1016/j.thromres.2020.04.024
22. Abdalkader M, Shaikh SP, Siegler JE, et al. Cerebral venous sinus thrombosis in COVID-19 patients: a multicenter study and review of literature. J Stroke Cerebrovasc Dis. 2021;30(6):105733. doi:10.1016/j.jstrokecerebrovasdis.2021.105733
23. Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. 2020;12(1):69. doi:10.1186/s13195-020-00640-3
24. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi:10.1016/S0140-6736(20)30566-3
25. Gonçalves de Andrade E, Šimončičová E, Carrier M, Vecchiarelli HA, Robert MÈ, Tremblay MÈ. Microglia fighting for neurological and mental health: on the central nervous system frontline of COVID-19 pandemic. Front Cell Neurosci. 2021;15:647378. doi:10.3389/fncel.2021.647378
26. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;4:195–197.
27. Muccioli L, Pensato U, Cani I, et al. COVID-19-associated encephalopathy and cytokine-mediated neuroinflammation. Ann Neurol. 2020;88:860–861. doi:10.1002/ana.25855
28. Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95:e1060–e1070. doi:10.1212/WNL.0000000000009937
29. Escalard S, Maïer B, Redjem H, et al. Treatment of acute ischemic stroke due to large vessel occlusion with COVID-19: experience from Paris. Stroke. 2021;51:2540–2543. doi:10.1161/STROKEAHA.120.030574
30. Ahmadi Karvigh S, Vahabizad F, Banihashemi G, et al. Ischemic stroke in patients with COVID-19 disease: a report of 10 cases from Iran. Cerebrovasc Dis. 2021;50(2):239–244. doi:10.1159/000513279
31. Mao L, Wang M, Chen S, et al. Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. SSRN Electronic Journal. 2020. doi:10.2139/ssrn.3544840
32. Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of covid-19 in the young. N Engl J Med. 2020;382(20):e60. doi:10.1056/NEJMc2009787
33. Qureshi AI, Abd-Allah F, Alsenani F, et al. Management of acute ischemic stroke in patients with COVID-19 infection: report of an international panel. Int J Stroke. 2020;15:1747493020935396.
34. Ntaios G, Michel P, Georgiopoulos G, et al. Characteristics and outcomes in patients with COVID-19 and acute ischemic stroke. Stroke. 2020;51:
35. Eschera R, Breakeya N, Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi:10.1016/j.thromres.2020.04.014
36. Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
37. Farnaz N, Mohammadkhanizadeh A, Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Mult Scler Relat Disord. 2020;46:102535. doi:10.1016/j.msard.2020.102535
38. Tufan A, Güler AA, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50(SI–1):620–632. doi:10.3906/sag-2004-168
39. Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The Cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi:10.1016/j.cytogfr.2020.05.003
40. Ritwik G, Dubey S, Roy D, Mandal A, Naga D, Benito-León J. Focal onset non-motor seizure following COVID-19 vaccination: a mere coincidence. Diabetes Metab Syndr. 2021;15(3):1023–1024. doi:10.1016/j.dsx.2021.05.003
41. Liu BD, Ugolini C, Jha P. Two cases of post-moderna COVID-19 vaccine encephalopathy associated with nonconvulsive status epilepticus. Cureus. 2021;13(7):e16172. doi:10.7759/cureus.16172
42. Kiyatkin EA, Sharma HS. Permeability of the blood–brain barrier depends on brain temperature. Neuroscience. 2009;161(3):926–939. doi:10.1016/j.neuroscience.2009.04.004
43. Van Vliet E, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130(2):521–534. doi:10.1093/brain/awl318
44. Rana A, Musto AE. The role of inflammation in the development of epilepsy. J Neuroinflammation. 2018;15(1):144. doi:10.1186/s12974-018-1192-7
45. Žorić L, Rajović-Mrkić I, Čolak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. 2021;14:349–355. doi:10.2147/IMCRJ.S315103
46. Novi G, Rossi T, Pedemonte E. Acute disseminated encephalomyelitis after SARS-CoV-2 infection,” (in eng). Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. doi:10.1212/NXI.0000000000000797
47. Waheed S, Bayas A, Hindi F, et al. Neurological complications of COVID-19: Guillain-Barre syndrome following Pfizer COVID-19 vaccine. Cureus. 2021;13(2):e13426. doi:10.7759/cureus.13426
48. Hughes DL, Brunn JA, Jacobs J, Todd PK, Askari FK, Fontana RJ. Guillain-Barré syndrome after COVID-19 mRNA vaccination in a liver transplant recipient with favorable treatment response. Liver Transplant. 2021. doi:10.1002/lt.26279
49. Azam S, Khalil A, Taha A. Guillain-Barré Syndrome in a 67-year-old Male Post COVID-19 Vaccination (Astra Zeneca). Am J Med Case Rep. 2021;9(8):424–427. doi:10.12691/ajmcr-9-8-10
50. Abu-Rumeileh S, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain–Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2020;268(4):1133–1170. doi:10.1007/s00415-020-10124-x
51. Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain. 2021;144(2):682–693. doi:10.1093/brain/awaa433
52. Lunn MP, Cornblath DR, Jacobs BC, et al. COVID-19 vaccine and Guillain-Barré syndrome: let’s not leap to associations. Brain. 2021;144(2):357–360. doi:10.1093/brain/awaa444
53. EMA. COVID-19 Vaccine Janssen: Guillain-Barré syndrome listed as a very rare side effect. 2021.
54. Li Z, Li X, Shen J, Chan MTV, Wu WKK. Miller Fisher syndrome associated with COVID-19: an up-to-date systematic review. Environ Sci Pollut Res. 2021;28(17):20939–20944. doi:10.1007/s11356-021-13233-w
55. Shoamanesh A. Postvaccination Miller Fisher syndrome. Arch Neurol. 2011;68(10):1327. doi:10.1001/archneurol.2011.236
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.