Back to Journals » Clinical Epidemiology » Volume 12
Positive Predictive Value of the Giant Cell Arteritis Diagnosis in the Danish National Patient Registry: A Validation Study
Authors Hjort PE , Therkildsen P , Nielsen BD , Hansen IT, Nørgaard M , de Thurah A , Hauge EM
Received 17 April 2020
Accepted for publication 11 June 2020
Published 12 July 2020 Volume 2020:12 Pages 731—736
DOI https://doi.org/10.2147/CLEP.S258219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Henrik Sørensen
Peter Engholm Hjort,1,2,* Philip Therkildsen,1,2,* Berit Dalsgaard Nielsen,1– 3 Ib Tønder Hansen,1,2 Mette Nørgaard,2,4 Annette de Thurah,1,2 Ellen-Margrethe Hauge1,2
1Department of Rheumatology, Aarhus University Hospital, Aarhus, Denmark; 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 3Diagnostic Centre, Silkeborg Regional Hospital, Silkeborg, Denmark; 4Department of Clinical Epidemiology, Aarhus University Hospital, Aarhus, Denmark
*These authors contributed equally to this work
Correspondence: Philip Therkildsen
Department of Rheumatology, Aarhus University Hospital, Aarhus, Denmark
Email [email protected]
Purpose: To investigate the positive predictive value (PPV) of the giant cell arteritis (GCA) diagnosis in the Danish National Patient Registry (DNPR).
Patients and Methods: A total of 293 patients aged ≥ 50 years with a first-time diagnosis of GCA in the DNPR between January 2012 and December 2017 were included. Patients were sampled from two secondary and one tertiary care hospitals in the Central Region Denmark. Two independent investigators (PH & PT) reviewed all medical files, including medical records, treatment, biochemistry, histopathology and imaging, and either confirmed or dismissed the diagnosis of GCA. In case of disagreement, a consensus agreement was reached. Sub-analyses including number of redeemed prescriptions performed temporal artery biopsies (TABs), and number of GCA-related hospital contacts were performed.
Results: We confirmed the diagnosis of GCA in 183/293 patients resulting in a PPV of 62% (95% CI: 57– 68). In patients with ≥ 3 redeemed prescriptions of glucocorticoids (GCs), we confirmed the diagnosis in 166/214 resulting in a PPV of 78% (95% CI: 71– 83). In patients with ≥ 3 redeemed prescriptions of GCs and ≥ 3 GCA-related hospital contacts, we confirmed the diagnosis in 88/95 resulting in a PPV of 93% (95% CI: 85– 96); however, this only included 88/183 confirmed GCA patients.
Conclusion: This is the first study to validate the diagnostic code of GCA in the DNPR. The overall PPV of GCA in the DNPR was 62%. Requiring redeemed prescriptions of GCs and/or GCA-related hospital contacts increase the PPV, but also excludes a significant number of GCA patients.
Keywords: rheumatology, giant cell arteritis, validation, positive predictive value, Danish National Patient Registry
Introduction
Giant cell arteritis (GCA) is the most frequent primary systemic vasculitis with an annual incidence rate of 15–25 per 100,000 in Caucasians ≥50 years of age and it primarily affects medium- and large-sized vessels.1,2 GCA is a clinical diagnosis. Patients often present with nonspecific symptoms and raised inflammatory markers, and histopathology and/or imaging can help to establish the diagnosis. Although the temporal artery biopsy (TAB) is often referred to as the gold standard, its sensitivity has been reported in the range of 40–87%.3,4 Establishing an accurate diagnosis of GCA can be difficult and GCA has been recognized as a previously underdiagnosed disease.5 Large population-based registries constitute an important source of data for epidemiological research; however, the validity of diagnostic codes is of crucial importance to the quality and interpretation of registry-based research. The main administrative health registry in Denmark is the Danish National Patient Registry (DNPR), containing information regarding every hospital admission in Denmark since 1977. Since 1995 all emergency visits and outpatient visits have been included as well. The positive predictive values (PPV) of different diagnostic codes in the DNPR range from <15% up to 100% depending on the diagnosis.6 The validity of the diagnostic code of GCA in the DNPR is unknown. Therefore, the aim of this study is to investigate the PPV of GCA in the DNPR.
Materials and Methods
Setting
Denmark is divided into five comparable geographical regions,7 and this cross-sectional population-based validation study was conducted within the Central Denmark Region. The Central Denmark Region has a source population of 1.3 million inhabitants which accounts for 23% of the Danish population. Typically, every region has one major tertiary care hospital and several smaller secondary care hospitals.
Data Sources
Denmark has several large population-based registries. For the purpose of this paper, we used information from the DNPR and the Danish National Prescription Registry (NPrR). All diagnoses in the DNPR have been coded according to the International Classification of Diseases, 10th revision (ICD-10) since 1994.6 NPrR is an administrative database containing information on all prescriptions redeemed at community pharmacies in Denmark since 1995.8
Study Population
Inclusion criteria were defined as patients ≥50 years of age with a first-time primary or secondary discharge diagnosis of GCA (ICD-10: M31.5 “giant cell arteritis with or without PMR” or M31.6 “other giant cell arteritis”) registered in the DNPR at one of three hospitals in the Central Region of Denmark during the period 01.01.2012 to 31.12.2017. To ensure a first-time discharge diagnosis, patients with a diagnosis of GCA prior to the defined period were not considered for inclusion. There are a number of distinct ICD-10 codes for both small and medium-sized vessel vasculitis and for Takayasu arteritis. The diagnostic codes M31.5 and M31.6 are specifically used for GCA. By linking with the NPrR, we divided patients into four groups based on the number of redeemed GC prescriptions (ATC: H02AB06 and H02AB07) within 6 months following the diagnosis (0, 1, 2 and ≥3). Through the DNPR, we also identified the number of GCA-related hospital contacts and divided patients into three groups (1, 2, and ≥3). GCA-related hospital contacts were defined as any in- or outpatient hospital contacts registered in the DNPR with a diagnostic code of GCA. The cut-off value of ≥3 prescriptions and ≥3 GCA-related hospital contacts was determined prior to analyses and were selected because higher cut-off values were believed to increase selection- and immortal-time bias without increasing the PPV significantly.
Medical Record Review
Review of medical records was used to confirm or dismiss the diagnosis of GCA among included patients and was based on the expert opinion of investigators. The review included an assessment of symptoms, clinical findings, treatment, pathological descriptions of TABs, imaging reports (magnetic resonance imaging (MRI), computerized tomography (CT), fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET/CT), and vascular ultrasound), biochemical results (hemoglobin, c-reactive protein (CRP) and erythrocyte sedimentation reaction (ESR)), and the course of disease over time including evaluation of the treatment response, wherever possible. In case a second TAB showed positivity, patients were defined as having a positive TAB. Two investigators (PH and PT) independently reviewed all medical records and either confirmed or dismissed the diagnosis. In case of disagreement between the two investigators, a third investigator (ITH) reviewed the material and a consensus agreement was reached. The review process included the complete material for a period of minimum of 6 months after the time of the diagnosis. When collecting information on clinical symptoms/findings we used a predefined variable definition scheme, ensuring a uniform and unbiased way of collecting data (Supplementary Table 1). Data were collected and managed using REDCap (Vanderbilt, TN) at Aarhus University.9
Statistics
We calculated the PPV with a 95% CI using a binomial exact model. The PPV was computed as the proportion of confirmed GCA cases amongst all patients sampled from the DNPR with a diagnostic code of GCA. In the planned sensitivity analyses, we assessed PPVs for the following subsets: Number of prescriptions of GCs (0, 1, 2, and ≥3), number of GCA-related hospital contacts (1, 2, and ≥3), TAB performed, year of diagnosis, and a combination of the above. Statistical analysis was performed using Stata 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
Ethics
This study has been approved by the Danish Data Protection Agency (case no. 1-16-02-705-17) and the Danish Patient Safety Authority (case no. 3-3013-2340/1).
Results
Patient Characteristics
A random sample of 301 patients with a first-time discharge diagnosis of GCA were drawn; 105 from Aarhus University Hospital, 106 from Silkeborg Regional Hospital and 90 from Randers Regional Hospital. Of the sampled patients, a total of 293/301 (97%) were included and the remaining eight were excluded, two because the medical records could not be obtained and six because medical records lacked sufficient information to evaluate the diagnosis. Baseline characteristics of the included patients are shown in Table 1.
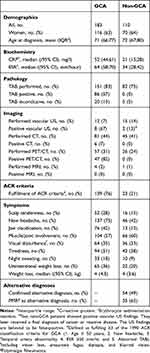 |
Table 1 Patient Characteristics |
Positive Predictive Values of First-Time GCA Diagnosis
In a total of 183/293 patients, the GCA diagnosis was confirmed, resulting in an overall PPV of 62% (95% CI: 57–68). In most cases the diagnosis was supported by TAB and/or imaging results; however, in 11/183 GCA and 17/110 non-GCA patients, the diagnosis was based solely on clinical symptoms and laboratory findings.
In patients in whom a TAB was performed (Table 1), 151/233 had a confirmed diagnosis of GCA, resulting in a PPV of 65% (95% CI: 58–71). There was no difference in the PPV when considering the year of the diagnosis.
In the sensitivity analyses, the PPV increased with an increasing number of GCA-related hospital contacts and number of redeemed GC prescriptions. In patients with ≥3 redeemed GC prescriptions, the diagnosis was confirmed in 166/214 resulting in a PPV of 78% (95% CI: 71–83). In patients with ≥3 GCA-related hospital contacts, the diagnosis was confirmed in 95/110 resulting in a PPV of 86% (95% CI: 79–92). Lastly, in patients with ≥3 redeemed GC prescriptions and ≥3 GCA-related hospital contacts the diagnosis was confirmed in 88/95 resulting in a PPV of 93% (95% CI: 85–96). However, this only included 88 (48%) out of 183 patients with a confirmed GCA diagnosis. PPV values are shown in Table 2. An alternative diagnosis was established in 54/110 (49%) of non-GCA patients, of which 65% were diagnosed with polymyalgia rheumatica (PMR). Other diagnoses included thromboembolic events, infections, reactive disease, cancer, osteoarthritis, and other autoimmune diseases.
 |
Table 2 Positive Predictive Value (PPV) of the Giant Cell Arteritis (GCA) Diagnosis |
Discussion
This is the first study to validate the diagnostic code of GCA in the Danish National Patient Registry, using medical record review as the reference standard, including symptoms, clinical findings, treatment, biochemistry, histopathology, imaging, and course of disease in the review process. It shows an overall PPV of 62% for a first-time GCA diagnosis in the DNPR in the period 2012–2017. This is similar to other medical diagnoses in the DNPR.6 We were able to increase the PPV if we required patients to have an increasing number of redeemed GCs prescriptions and/or GCA-related hospital contacts. However, this also significantly decreased the number of included GCA patients. Using the combination of the GCA diagnosis with ≥3 redeemed prescriptions of GCs is a balanced way of ensuring both a high PVV of 78% and that 91% of GCA patient were included. However, this also introduces the risk of both selection bias and immortal time bias since patients are assumed to have survived until they redeem the third GC prescription. Knowledge of the PPV is of the utmost importance when interpreting results of large, populations-based studies.
Due to the introduction of new diagnostic tools (vascular ultrasound and FDG PET/CT), we expected the PPV of the GCA diagnosis to increase over time. However, we observed no difference in the PPV when considering the time of diagnosis. Vascular ultrasound is highly user-dependent and not fully implemented in all clinics, which should be considered when evaluating these findings.
Similar findings have been shown by Linauskas et al10 validating the diagnostic code of rheumatoid arthritis (RA) in the DNPR. They found an overall PPV of 62% (95% CI: 57–67). Combining the diagnostic code of RA with relevant treatment codes increased the PVV to 88% (95% CI: 83–92), showing that linking diagnostic codes with treatment codes is a reliable method of strengthening the PPV. A recent study from France validated the diagnostic code of GCA in a French hospital electronic database with the 1990 American College of Rheumatology (ACR) classification criteria as the reference standard.11 They found an overall PPV of 94% (95% CI: 90–97). However, they only included 170/302 (56%) of the total sampled population in the analysis and exclusion criteria are sparsely described. This introduces a risk of bias, and the “true” PVV could be up to half of the reported.
Also, the 1990 ACR classifications criteria for GCA are not intended for diagnosis. The diagnostic specificity of the 1990 ACR classification criteria has been estimated to be only 64%.12 Hence, using the 1990 ACR classification criteria as diagnostic criteria would misclassify a large number of patients. A central problem when validating the diagnostic code of GCA is the definition of a “true” case. As discussed, ACR criteria are inadequate to distinguish GCA and non-GCA patients and no clear objective definition of a “true” GCA case exists. The use of more objective prediction models to identify “true” GCA cases has been suggested;13 however, these have only been validated among biopsy-positive GCA patients and require further validation. We defined a “true” GCA case as the investigators' expert opinion, which was based on the complete review of all medical records over time including symptoms, clinical findings, histopathology, imaging, biochemistry, and treatment response. To ensure a valid diagnosis, two independent investigators evaluated all the medical records, and in case of disagreement, patients were evaluated by a third investigator and a consensus agreement was reached. We consider this a strength of our study. Also, we included patients diagnosed with GCA at any department, including ophthalmological departments, from both secondary and tertiary care hospitals, thereby ensuring the generalizability of our results. Our study also has potential limitations. We excluded a few number of patients because their medical files did not include sufficient information to determine whether or not the patient had GCA. This introduces the risk of selection bias. However, due to the small number of patients excluded, it is unlikely to have significantly affected the PPV.
In conclusion, the overall PPV of GCA in the DNPR is 62%. Requiring redeemed GC prescriptions and/or GCA-related hospital contacts increases the PPV, but also excludes a significant number of GCA patients. These findings show the importance of validating diagnostic codes prior to performing large, population-based studies.
Disclosure
The abstract of this paper was presented at the European Congress of Rheumatology 2019 as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in Annals of the Rheumatic Diseases (ARD): http://dx.doi.org/10.1136/annrheumdis-2019-eular.6852.
Berit Dalsgaard Nielsen reports personal and speaker’s bureau fees from Roche and consulting fee from Sanofi, outside the submitted work. Ellen-Margrethe Hauge reports grants from Aarhus University and Danish Rheumatism Association, during the conduct of the study; grants from Danish Regions Medicine Grants, Novo Nordic Foundation, Roche, and Novartis, and travel expenses from Sobi, outside the submitted work. The authors report no other possible conflicts of interest in this work.
References
1. Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum. 2009;61:1454–1461. doi:10.1002/art.24459
2. Brekke LK, Diamantopoulos AP, Fevang BT, Abetamus J, Espero E, Gjesdal CG. Incidence of giant cell arteritis in Western Norway 1972–2012: a retrospective cohort study. Arthritis Res Ther. 2017;19:278. doi:10.1186/s13075-017-1479-6
3. Ashton-Key MR, Gallagher PJ. False-negative temporal artery biopsy. Am J Surg Pathol. 1992;16:634–635. doi:10.1097/00000478-199206000-00014
4. Niederkohr RD, Levin LA. A Bayesian analysis of the true sensitivity of a temporal artery biopsy. Invest Ophthalmol Vis Sci. 2007;48:675–680. doi:10.1167/iovs.06-1106
5. Meller J, Sahlmann CO, Gurocak O, Liersch T, Meller B. FDG-PET in patients with fever of unknown origin: the importance of diagnosing large vessel vasculitis. Q J Nucl Med Mol Imaging. 2009;53:51–63.
6. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
7. Henriksen DP, Rasmussen L, Hansen MR, Hallas J, Pottegard A. Comparison of the Five Danish Regions regarding demographic characteristics, healthcare utilization, and medication use–a descriptive cross-sectional study. PLoS One. 2015;10:e0140197. doi:10.1371/journal.pone.0140197
8. Pottegard A, Schmidt SAJ, Wallach-Kildemoes H, Sorensen HT, Hallas J, Schmidt M. Data resource profile: the Danish National prescription registry. Int J Epidemiol. 2017;46:798–f. doi:10.1093/ije/dyw213
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi:10.1016/j.jbi.2008.08.010
10. Linauskas A, Overvad K, Johansen MB, Stengaard-Pedersen K, de Thurah A. Positive predictive value of first-time rheumatoid arthritis diagnoses and their serological subtypes in the Danish National patient registry. Clin Epidemiol. 2018;10:1709–1720. doi:10.2147/CLEP.S175406
11. Caudrelier L, Moulis G, Lapeyre-Mestre M, Sailler L, Pugnet G. Validation of giant cell arteritis diagnosis code in the French hospital electronic database. Eur J Intern Med. 2019;60:e16–e7. doi:10.1016/j.ejim.2018.10.004
12. Seeliger B, Sznajd J, Robson JC, et al. Are the 1990 American College of Rheumatology vasculitis classification criteria still valid? Rheumatology. 2017;56:1154–1161. doi:10.1093/rheumatology/kex075
13. Ing EB, Miller NR, Nguyen A, et al. Neural network and logistic regression diagnostic prediction models for giant cell arteritis: development and validation. Clin Ophthalmol. 2019;13:421–430. doi:10.2147/OPTH.S193460
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
