Back to Journals » Drug Design, Development and Therapy » Volume 10
Position and enforcement practice of the People's Republic of China's pharmaceutical data exclusivity protection
Received 20 January 2016
Accepted for publication 1 April 2016
Published 22 June 2016 Volume 2016:10 Pages 2015—2020
DOI https://doi.org/10.2147/DDDT.S104642
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Wei Duan
Na Li,1 Xiang Yu,2 Michael Pecht3
1School of Law, Ningbo University, Ningbo, People’s Republic of China; 2School of Law and Politics, Ningbo Institute of Technology, Zhejiang University, Ningbo, People’s Republic of China; 3Center for Advanced Life Cycle Engineering, University of Maryland, College Park, MD, USA
Abstract: The concept of pharmaceutical data exclusivity protection comes from the West. The Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) establishes the basic rules for pharmaceutical data exclusivity protection. People’s Republic of China’s domestic law is consistent with the TRIPS agreement. In the drug registration approval process of the People’s Republic of China’s Drug Supervision Department, pharmaceutical data exclusivity protection has encountered some problems, including data authentication, exclusive rights to data, number of drugs requiring data to be submitted, and drug costs. In view of the long-term interests of the People’s Republic of China’s pharmaceutical industry and intellectual property protection trends, there are a lot of difficulties in the enforcement of pharmaceutical data exclusivity protection law that need to be overcome. Some measures can be taken, such as establishing a shorter data exclusivity protection period, only protecting the data submitted and relied on in the People’s Republic of China, only protecting the drugs that use new chemical components, allowing application and necessary research before the expiry of pharmaceutical data exclusivity protection period of generic drugs.
Keywords: drug, developing countries, approval, competition
Introduction
In ancient times, people believed that curative drugs came from gods such as Imhotep (ancient Egypt), Asclepius (ancient Greek), and Mercurius (ancient Rome). Today’s drugs are created in science labs, and generally the development of a drug, whether from animal or plant extracts or synthetics, requires complicated trials that consume time and money.1 Pharmaceutical companies are aware of the value of pharmaceutical data on drug effectiveness and safety, and competitors are very eager to get such data to help them save trial time and money. Governments also attach great importance to such data, especially for determining the risk to public health and safety.2
In the 1980s, more than 50 countries disapproved the patenting of drugs. Nevertheless, pharmaceutical companies built a protective layer for pharmaceutical data, so that others would not get the data, while vigorously lobbying for drug patent protection.3 In particular, they advocated that pharmaceutical data exclusivity protection reduces their development risks so that people afford effective and safe drugs.4 The Hatch-Waxman Act (United States, 1984) created a period of pharmaceutical data exclusivity protection. Those pharmaceutical companies who owned drugs patents, when applying for the marketing approval, could also request the government to protect their pharmaceutical experimental data.5 During this period, unauthorized pharmaceutical companies were prohibited from applying for new drug marketing with these pharmaceutical data. The EU Council adopted the European Directive 87/21/EEC in 1986 and took measures similar to those in the Hatch-Waxman Act to encourage new drug research and development, that is, the pharmaceutical manufacturers were prohibited from seeking market permission using pharmaceutical data from others within 6 years after approval of the new drug.6 The Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS) in 1995 upgraded the pharmaceutical data exclusivity protection legislation to the standard of international law. Paragraph 3 in Article 39 of the Agreement stipulates that:
the submission of undisclosed test or other data, the origination of which involves a considerable effort, shall protect such data against unfair commercial use.
In addition, Members shall protect such data against disclosure, except where necessary to protect the public,7 or unless steps are taken to ensure that the data are protected against unfair commercial use.8
In the People’s Republic of China and some other countries, the prospect of pharmaceutical data exclusivity protection has been confounded by the accession to TRIPS. In fact, the relationship between pharmaceutical data and drug patent has become more complicated and confusing.
Materials and methods
In the People’s Republic of China, there was no law about pharmaceutical data exclusivity protection until 2001. The government and the people had insufficient understanding of the value of pharmaceutical data. Some pharmaceutical manufacturers made unlimited use of the pharmaceutical data that had been obtained by others when applying for drug registration.
When the People’s Republic of China wanted accession to the WTO, the deadlock was broken. In paragraph 284 of the Report on China’s Accession to WTO Work Group, Chinese representatives stated that, in order to comply with paragraph 3 of Article 39 of TRIPS, the People’s Republic of China would provide effective protection for undisclosed data or other data submitted to Chinese authorities as required to apply for marketing approval of drugs or agricultural chemicals using new chemical components. Such exclusivity protection includes the adoption and development of laws and regulations to ensure that no one other than the data provider shall apply for product marketing approval based on the data without the permission of the data provider at least within 6 years after the Chinese government grants marketing approval for the data provider. In this period, any second applicant can only be granted marketing approval when submitting its own data. All drugs or agricultural chemicals using new chemical components can be subject to such data exclusivity protection, regardless of whether it is subject to patent protection.
The legal outcome of the implementation of this commitment was the “Regulations for the Implementation of Drug Administration Law of the People’s Republic of China” in 2002. Article 35 of the Regulations stipulates that:
The State protects undisclosed data of pharmaceutical study which are independently acquired and submitted by pharmaceutical manufacturers or sellers to obtain production or marketing approval of the drugs which contain new chemical entities.
No one may make unfair commercial use of this data. Within 6 years from the date that exclusivity protection began, other applicants could not use such pharmaceutical data to apply for production or marketing approval of the drug. In two types of situations, the government may disclose the pharmaceutical data exclusivity protection: 1) for the need of public interests or 2) the pharmaceutical data exclusivity caused unfair commercial use.
Another law developed by China Food and Drug Administration (CFDA) also involves pharmaceutical data exclusivity protection issues. Article 20 of The Drug Registration Regulations (Third Version) in 2007 stipulates that:
During period of 6 years from the pharmaceutical date exclusivity protection took effect, CFDA refused the subsequent application, unless the original applicant permitted the subsequent application using these undisclosed data, or the subsequent applicant carried out the drug trials by itself.
The pharmaceutical data exclusivity protection mainly focuses on drugs that “contain new chemical components”, which are generally named as “new drugs”.9 The CPL (Patent Law of the People’s Republic of China) identified the “new drugs”, that is, the drugs must contain new chemical components that are different from the patented drugs. In addition, traditional Chinese medicines and natural drugs are considered different from the chemical drugs and do not obey pharmaceutical data exclusivity protection rules. Second, CFDA is only responsible for protecting the pharmaceutical data obtained by drug manufacturers and sellers.10 Third, drug manufacturers are not required to provide pharmaceutical data for each drug, but are obliged to provide the pharmaceutical data of drugs not sold at home and abroad or the drugs with new components and new processes.11 Fourth, the government is obliged to respect the confidentiality of pharmaceutical data within 6 years after drug registration and does not allow other drug manufacturers to rely on such data. After the expiration of 6 years, the drug imitators can refer to the data archived by the government authorities.12
Results and discussion
There is a “Berlin Wall” in the territory of pharmaceutical industry in today’s world. On one side are the EU, USA, and Japan; these countries own lot of drug patents and have huge pharmaceutical industry output; on the other side are developing countries that rely on imports and generic drugs (Figure 1). Pharmaceutical data exclusivity protection raised this wall, because developing countries cannot obtain and use the pharmaceutical data within the exclusivity protection period to promote the pharmaceutical level even if they can publicly obtain only some preliminary information from drug patent index. In the past decade, the pharmaceutical data were mainly provided by the branches of some giant multinational pharmaceutical companies in the People’s Republic of China, but now this pattern is slowly changing. At present, the People’s Republic of China has thousands of drug laboratories and pharmaceutical companies, and the CFDA has preserved a lot of pharmaceutical data in the process of accepting applications for drug registration.13 However, there is still some confusion about the People’s Republic of China’s pharmaceutical data exclusivity protection policies. In the following subsections, we describe these policies in detail.
  | Figure 1 Share of the global pharmaceutical market (2014). |
Submission of pharmaceutical data to government
The first step is data submission. Drug registration is an administrative licensing act. First, pharmaceutical companies need to submit data to the authorities of different countries. However, according to TRIPS, if the pharmaceutical data submitted by a pharmaceutical company has been approved by one country (say, the applicant country), another country that accepts the drug listing application is not obliged to protect the pharmaceutical data. The People’s Republic of China has dozens of pharmaceutical companies coestablished with transnational pharmaceutical giants, such as Xi’an-Janssen and TSKF.14 The drugs produced by them are mostly mature pharmaceutical dosage forms that are subjected to pharmaceutical data exclusivity protection in their home country. Some pharmaceutical data exclusivity protection periods have expired. But in the People’s Republic of China, they also hope to get exclusivity protection to prevent domestic pharmaceutical companies from using the data, because CFDA received thousands of drug registration applications, and only approved a small portion of the application, imported drugs and generic drugs were easy to get approved. This means that although the pharmaceutical company submitted the pharmaceutical data of more than two kinds of drugs to the CFDA, similiar data had been submitted to other countries’ authorities as well. Chinese researchers suggested that the CFDA would not protect published or publicly available drug data.15 Currently, the CFDA does not specifically study such problems because the Chinese government has carried out many policies favorable to foreign enterprises over the past 30 years16 (Figure 2).
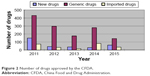  | Figure 2 Number of drugs approved by the CFDA. |
Whether the CFDA can use the pharmaceutical data
A “Proviso” in Article 35 of Regulations for the Implementation of Drug Administration Law stipulates that the CFDA can use these data for the need of public interest or after taking measures to ensure that such data will not be improperly used for commercial exploitation.17 In other words, the CFDA has the right to use the pharmaceutical data under special circumstances, such as the drugs responding to major public health events and approval of vaccines. Except these two important exceptions, due to rapid growth of the drug registration application number (Figure 3), the CFDA tends to evaluate the efficacy and safety of the drugs applied for listing by means of the pharmaceutical data submitted by R&D enterprises and finally determines whether to approve listing. Such a review constitutes indirect use of pharmaceutical data and results in the earning of money. This issue is of concern not only in the People’s Republic of China but has also existed in other countries. On the one hand, the US, EU, and other countries insist that pharmaceutical data should enjoy exclusivity as a commercial secret. As such, no government has the right to unseal and use these data. On the other hand, many developing countries believe that governments have no incentive to “steal” the trade secrets of pharmaceutical companies and commit “unjustifiable commercial utilization”, as mentioned in TRIPS, which refers to unfair competition between drug manufacturers.
  | Figure 3 Number of drug registration applications to the CFDA. |
The government’s use of pharmaceutical data for drug marketing approval should not be confused with competition between drug manufacturers. In this regard, the TRIPS does not give a clear guidance.18 The Chinese legislation, which is highly consistent with the TRIPS in terms of representation, does not explicitly recognize that pharmaceutical data is a trade secret and does not judge the acquisition and use of pharmaceutical data by pharmaceutical companies to obtain profits, competitive advantages, customer loyalty, and other benefits as constituting unfair competition. In view of the fact that there are mostly generic drugs in the the People’s Republic of China’s current drug registration approval process and that patent technology specifications and literature have disclosed full drug information for similar drugs, the CFDA can make judgments without relying on the exclusivity protection data before an approval and can consider more how to establish fair competition rules for the protection of domestic and foreign drug manufacturers through drug data protection laws.19
Width of protection scope of pharmaceutical data
Pharmaceutical data are diversified. Some pharmaceutical data describe new chemical components, which may be the world’s first, or it may not have been applied for listing in one country but may have existed in other countries. Another type of pharmaceutical data is expected to treat new therapeutic indications for existing compound entities. In the People’s Republic of China, the CPL and the Drug Administration Law fail to adequately address this issue. In relation to the protection of the People’s Republic of pharmaceutical industry and objections for the People’s Republic of China to assume the obligations of TRIPS PLUS, one view is that the People’s Republic of China should only protect the pharmaceutical data for drugs using new chemical components rather than protect the pharmaceutical data for drugs with new ingredients or new uses and should define the concept of drugs using new chemical components.
The People’s Republic of China has been facing a difficult problem in how to deal with pharmaceutical data exclusivity protection of both registered drugs without patent protection and registered drugs with expired patents. According to TRIPS, the pharmaceutical data exclusivity protection and patent protection of chemical components of related drugs are stipulated, respectively, and are two separate, parallel, and different intellectual property rights. A considerable number of drugs are not patented, and for a small number of drugs, patent protection is about to expire in the People’s Republic of China.20 These patents are facing a risk of being imitated in a short time, because the competitors are already very familiar with these drugs. It is still difficult to determine whether pharmaceutical data exclusivity protection can play a role similar to the “firewall” of patent protection, because pharmaceutical data are also a type of intellectual property right and generic drug manufacturers are unwilling to comply with it.
Pharmaceutical data exclusivity protection and high drug prices
Many consumers in the People’s Republic of China complain about the expensive drug prices. As a result, the government has come up with various solutions to the “difficult and expensive medical treatment” problem. But a key question is whether the costs are associated with pharmaceutical data exclusivity protection. The general logic is that if the pharmaceutical data are excessively protected, then drug developers will require competitors to repeat various safety and efficacy trials that have been conducted by drug developers, resulting in a high total cost of drug development, thereby affecting the market pricing.
The monopoly that foreign pharmaceutical companies have, as well as their inflexible drug pricing system, promotes the higher drug prices. One example is Guangzhou Basi Ltd (Guangzhou, Guangdong, People’s Republic of China), which since 2003 has been selling its famotidine tablets, as approved by the Price Bureau, at one-sixth the price of the same product sold by Japan Yamanouchi Ltd (Shimotakai, Nagano, Japan). In this case, Yamanouchi did not enjoy pharmaceutical data exclusivity protection in the People’s Republic of China. Furthermore, Guangzhou Basi prosecuted an administrative lawsuit and required lowering the price of drugs of foreign manufacturers to an acceptable degree. But, in the end, the Chinese domestic pharmaceutical manufacturer lost the lawsuit. This case tells us that drug pricing in the Chinese market does not consider the costs brought by pharmaceutical data exclusivity protection under some circumstances.
Data R&D effort level
A drug trial is a long-term and difficult activity requiring a huge investment. The trial data are critical to determine whether drugs are defective. Drug regulatory authorities often refuse to disclose trial data to research institutions for a long time, and they hire experts to analyze drug safety and efficacy. As a result, for the diabetes drug named “Avandia” accidentally developed by GSK (Brentford, UK) and painkiller named “Vioxx” accidentally developed by Merck & Co (Kenilworth, NJ, USA), the European Medicines Agency and US Food and Drug Administration failed to find drug defects from the trial data. In contrast, the German IQWIG Institute paid close attention to the benefits and harmful effects of the antidepressant named “Reboxetine” developed by Pfizer (New York, NY, USA) for the treatment of major depressive disorders in adults. The scientists analyzed the results of 13 clinical tests, five of which had been published. However, the published data had obvious publication bias, overestimated its benefits, and underestimated the harm. So far, nearly three-fourths of the clinical test data of this drug has not been published, but more and more scientists believe that the truth is that Reboxetine is ineffective on the whole and potentially harmful.21 Under sustained pressure, the European Medicines Agency decided to open its database in July 2012 and allowed independent researchers to have access to clinical trial data. The Chinese pharmaceutical data face similar problems. The first problem has to do with the acquisition process. The CFDA checked 134 clinical trial agencies for drugs in 2012 and found that 38 drug evaluation centers had low staffing level, a limited amount of reliable data, and a lack of supervision of third-party auditing companies. The second problem is the poor state of technical specification. The data of some trials carried out in the People’s Republic of China currently are not widely recognized internationally.
Conclusion
For pharmaceutical data exclusivity protection to benefit Chinese local companies, some measures can be taken, such as establishing a shorter pharmaceutical data exclusivity protection period, only protecting the pharmaceutical data submitted and relied on in the People’s Republic of China, only protecting the drugs that use new chemical components, allowing application and necessary research before the expiry of pharmaceutical data exclusivity protection period of generic drugs. But in the long run, the Chinese pharmaceutical R&D capability will rise to a new level. It has a longer-term value to activate vitality of Chinese pharmaceutical industry through pharmaceutical data exclusivity protection and establish the common action rules for pharmaceutical R&D under the intellectual property framework to meet the increasingly sharp public health demand contradiction. Therefore, the People’s Republic of China’s drug legislation needs to clearly tell the world that pharmaceutical data exclusivity protection is a strict exclusive right protection and a mean to deterring unfair competition.22 Meanwhile, for the increasing pharmaceutical data, it is also necessary to take some preventive measures, such as requiring manufacturers to provide all pharmaceutical data to the regulatory authorities, allowing researchers to freely access data on registered drugs, establishing data sharing among the regulatory authorities of all countries, and reevaluating drugs refused by other countries.
Acknowledgments
This research was sponsored by KC Wong Magna Fund in Ningbo University. The authors also thank the following funders: Zhejiang Soft Science Research Projects, Ningbo Municipal Government – Chinese Academy of Social Sciences Collaborative Research Base, and the Law and Economic Research Center of Zhejiang University.
Disclosure
The authors report no conflicts of interest in this work.
References
Anand B, Galetovic A. How market smarts can protect property rights. Harv Bus Rev. 2004;82(12):72–79. | ||
John JF. Data exclusivity – the generics market’s third hurdle. IMS Health. 2001;17(10):2021. Available from: http://omaha.sanofi.com/Images/22810_20F_2010.pdf. Accessed June 30, 2015. | ||
Crowne EA, MihalceanuC. Innovators and generics: proposals for balancing pharmaceutical patent protection and public access to cheaper medicines in Canada. IDEA. 2011;51:693. Available from: http://ssrn.com/abstract=1665342. Accessed June 30, 2015. | ||
Costa-Font J, Burakoff A. Overview of progress in the international regulation of the pharmaceutical industry. Pierce L Rev. 2002;1:103. | ||
Kara W. Food and drug law as intellectual property law: historical reflections. Wis L Rev. 2011;12:331–352. | ||
Bai T, Chen J, Shi LW. Analysis of the protection to marketing approval data under the TRIPS Agreement. Chin J New Drugs. 2009;18(19):1823–1826. | ||
Liu JJ, Yang Y. Study on improving the data protection system in China. Chin J New Drugs. 2012;21(1):6–9. | ||
Zhang XM, Qiu JX. Discussion on development of drug data protection in China. China Pharm. 2005;16(21):1607. | ||
Chen Y, Li J, Li JA. Knowledge representation method for pharmaceutical products in China. Mod Lib Infor. 2013;234(6):9–15. | ||
Ohly DC, Patel SK. The Hatch-Waxman act: prescriptions for innovative and inexpensive medicines. U Balt Intell Prop LJ. 2011;19:107–179. Available from: http://www.schiffhardin.com/Templates/Media/files/publications/PDF/ohly-Hatch_Waxman_Prescriptions-42010.pdf. Accessed June 30, 2015. | ||
Park S. Drug approval-patent linkage systems in the US and Canada. J Kor Pharm Sci. 2008;38:207. | ||
Shreya M. Do developing countries need a pharmaceutical data-exclusivity regime? Eur Intellect Prop Rev. 2010;32(6):268–273. | ||
Miller JS. Joint defense or research joint venture? Reassessing the patent-challenge-bloc’s antitrust status. Stan Tech L Rev. 2011;3:5–12. | ||
Galbraith CD. Dying to know: a demand for genuine public access to clinical trial results data. Miss LJ. 2008;78:705. Available from: http://www.law.tulane.edu/uploadedfiles/WIPIP/2008/Galbraith_Abstract.doc. Accessed June 30, 2015. | ||
Andanda P. Managing intellectual property rights over clinical trial data to promote access and benefit sharing in public health. IIC. 2013;44(2):140–177. | ||
Rowe EA. Striking a balance: when should trade-secret law shield disclosures to the government. Iowa L Rev. 2010;96:791. | ||
Weissman R. Public health-friendly options for protecting pharmaceutical registration data. IJIPM. 2006;1(1–2):113–130. | ||
Weissman R. Long strange trips: the pharmaceutical industry drive to harmonize global intellectual property rules, and the remaining WTO legal alternatives available to third world countries. U Pa J Int’l Econ L. 1996;17:1069. | ||
Galantucci R. Data protection in a US-Malaysia free trade agreement: new barriers to market access for generic drug manufacturers. Fordham Intell Prop Media Ent L J. 2006;17:1083. Available from: http://ir.lawnet.fordham.edu/cgi/viewcontent.cgi?article=1388&context=iplj. Accessed June 30, 2015. | ||
Abughanm S. The protection of pharmaceutical patents and data under TRIPS and US-Jordan FTA: exploring the limits of obligations and flexibilities: a study of the impacts on the pharmaceutical sector in Jordan. Toronto, ON: University of Toronto; 2012:16–18. Available from: http://hdl.handle.net/1807/32296. Accessed June 30, 2015. | ||
Ho C. Access to medicine in the global economy: international agreements on patents and related rights. Oxford, UK: Oxford University Press; 2011:45–47. | ||
Basheer S. Protection of regulatory data under Article 39.3 of TRIPs: the Indian context. Intellectual Property Institute (IPI). 2006. Available from SSRN: http://ssrn.com/abstract=934269. Accessed June 30, 2015. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
