Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 14
Poor Glycemic Control and the Contributing Factors Among Type 2 Diabetes Mellitus Patients Attending Outpatient Diabetes Clinic at Mbarara Regional Referral Hospital, Uganda
Authors Patrick NB, Yadesa TM , Muhindo R, Lutoti S
Received 25 May 2021
Accepted for publication 24 June 2021
Published 8 July 2021 Volume 2021:14 Pages 3123—3130
DOI https://doi.org/10.2147/DMSO.S321310
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Nsheka Bonny Patrick,1 Tadele Mekuriya Yadesa,1,2 Rose Muhindo,3 Stephen Lutoti4
1Department of Pharmacy, Mbarara University of Science and Technology, Mbarara, Uganda; 2World Bank, ACE II, Pharmacy Biotechnology and Traditional Medicine Center (PHARMBIOTRAC), Mbarara University of Science and Technology, Mbarara, Uganda; 3Department of Internal Medicine, Mbarara University of Science and Technology, Mbarara, Uganda; 4Department of Pharmacy, Makerere University, Kampala, Uganda
Correspondence: Tadele Mekuriya Yadesa
Department of Pharmacy, Mbarara University of Science and Technology, PO Box 1410, Mbarara, Uganda
Email [email protected]
Background: Glycemic control is associated with long-term complications in type 2 diabetes management. However, updated reports on glycemic control that are crucial to reducing diabetes mellitus complications remain scarce.
Objective: The objective of this study is to evaluate glycemic control and contributing factors among type 2 diabetes mellhitus patients attending the outpatient diabetic clinic at Mbarara Regional Referral Hospital.
Methods: A cross-sectional study was conducted at Mbarara Regional Referral Hospital outpatient diabetes clinic between July and October 2020. Participants were subjected to a questionnaire-based interview and glycosylated hemoglobin (HbA1C) was determined as a marker of glycemic control among participants. The collected data was entered into and analyzed by Stata version 13. The odds ratio was used to determine the strength of association between variables. The cut-off value for all statistical significance tests was set at p< 0.05 with CI of 95%.
Results: A total of 223 participants were interviewed, and the majority (188, 84.3%) had poor glycemic control (HbA1C ≥ 7%). Importantly, 81.7% (49/60) and 90.0% (99/110) of those who did not adhere to diet and physical exercise recommendations respectively, had poor glycemic control. Multivariate logistic regression revealed that poor glycemic control was more prevalent among participants aged 25– 60 years (AOR=4.48, 95%CI: 1.56– 14.50, p-value=0.009) and those aged above 60 years (AOR=4.28, 95%CI: 1.18– 15.58, p-value= 0.03) compared to the youth, 18– 24 years of age.
Conclusion: The prevalence of poor glycemic control among type 2 diabetes patients in this study is high and patient’s age was identified to be an independent risk factor. We recommend any intervention by the hospital that promotes diabetes education and optimizes lifestyle and medication adherence; ultimately to achieve good glycemic control especially for adult patients.
Keywords: type 2 diabetes mellitus, poor glycemic control, glycosylated hemoglobin A1C
Introduction
According to the 2016 World Health Organization (WHO) Global Report on Diabetes, the prevalence of diabetes and risk factors has been increasing steadily with the numbers now at 2.7% and 3.0% for males and females, respectively. About 18.6% of adults are overweight and 3.9% are obese.1 The first WHO Global report on diabetes demonstrates that the number of adults living with diabetes has almost quadrupled since 1980 to 422 million adults. Diabetes is increasing at an alarming rate throughout the world and about 80% of diabetic cases live in low and middle-income countries.2 Glycemic control is the most important predictor for diabetic-related complications and deaths. Identifying factors associated with glycemic control helps health-care providers and patients to work on the areas that reduce risks of diabetic-related complications and deaths.
In Africa, glycemic control remains the major therapeutic objective for prevention of target organ damage and other complications arising from diabetes.3 In addition, elucidation of various determinants of poor glycemic control may contribute to a clearer understanding of modifiable antecedents of diabetes-related complications and help to achieve improved diabetic control and patient outcomes.
Uganda has recently experienced a significant rise in the burden of diabetes, and it is estimated that more than 400,000 people are living with diabetes.4 A major concern in the management of diabetes is the occurrence of diabetic complications related to poor glycemic control. Identification of the factors associated with poor glycemic control helps in planning for effective prevention strategies. There is no published study from western Uganda on poor glycemic control and the contributing factors. Thus, our study is aimed at determining the prevalence of poor glycemic control and the contributing factors among T2DM patients on follow-up at Mbarara Regional Referral Hospital.
Methods and Materials
Study Setting
The study was conducted at Mbarara Regional Referral Hospital in Mbarara District, 260 km from Kampala, Uganda. This is a referral hospital in the southwestern region for the districts of Mbarara, Bushenyi, Ntungamo, Ibanda, Kiruhura, Buhweju, Kazo, Mitooma, Rubirizi, Rwampara, Sheema, and Isingiro. It is also a teaching hospital for Mbarara University of Science and Technology. The hospital offers a wide range of health services in the departments of pediatrics, obstetrics and gynecology, internal medicine, surgery, cancer unit, emergency and critical care, imaging, pathology, laboratories and outpatient department. The diabetes clinic operated on Thursday of every week and this was when participants were enrolled into the study. The doctors in the diabetes outpatients clinic reviewed, diagnose, treat, and monitor ambulatory diabetic patients and referred those with serious complications to a medical ward for further management.
Study Population
The study participants were patients that were diagnosed with type 2 diabetes mellitus at least three months prior to the study who were ≥18 years old and attending the diabetes clinic at Mbarara Regional Referral Hospital. T1DM patients and those who were mentally unstable were excluded. We conducted the study between July 2020 and October 2020.
Study Design
A cross-sectional study was conducted.
Sample Size Estimation and Sampling Procedure
The sample size was calculated using formula for finite population assuming 79% prevalence of poor glycemic control, 95% confidence interval (95%CI) and 5% margin of error. A finite population correction factor was then applied. Based on this, the sample size for this study is 223.
All diabetic patients who visited Mbarara Regional Referral Hospital Diabetes Clinic in August, September and October 2020 were assigned numbers; 0 for those not to be enrolled and 1 for those to be enrolled. Participants were selected using a simple random sampling method until 223 patients were recruited.
We assigned consecutive numbers starting from 1 to each patient throughout the study period. Every week on the clinic day (Thursday) 20 out of about 50 patients, visited the diabetes clinic of MRRH every week. We used a simple random sampling technique that employed computer generated random numbers. This continued until the sample size was achieved on the twelfth week.
Data Collection Procedures
Patients with type 2 diabetes mellitus were identified from the diagnosis written in patient files. The trained research assistant was a nurse. A laboratory technologist collected venous blood from patients and used it to determined HbA1C of each participant. Data was collected during direct encounters between researcher and participating type 2 diabetic patients via a questionnaire specially designed for the research, to ensure a high level of information accuracy. The first part of the questionnaire collected sociodemographic data on the participating patients: age, marital status, gender, education level, employment status, and income. The second part sought disease factors, drug factors and health facility factors from patient medication chart while patient factors were obtained from the patients. The third part gathered information regarding contributing factors among type 2 DM patients attending outpatient diabetes clinic at Mbarara Referral hospital. Participants were weighed in kilograms in light clothing and bare feet. Height was measured using a stadiometer; participants stood in an erect position without shoes, and the measurement was recorded in meters. Fresh venous whole blood from the arm was collected using a 2 mL syringe in the morning of overnight fasting with minimum of eight hours. The same sample was used for On Call Plus glucometer to determine fasting blood glucose and automated Finecare HbA1C analyzer to measure HbA1C that was reported in percentage. We recorded the diabetes complications that were diagnosed and documented by the doctors during the routine patient care.
Data Processing, Data Analysis and Statistical Measures
The data were checked for completeness, coded and entered into EpiData 3.1, and exported to Stata program, version 13 for analysis. Descriptive statistics was used to analyze the rate of poor glycemic control and it was presented as frequencies and percentages. Chi-squared test was used to identify the occurrence of poor glycemic control among patients with different levels of adherence.
A univariate logistic regression was performed to identify the factors associated with poor glycemic control in the study sample. Then, the variables with p-values ≤0.25 by the univariate logistic regression were included in a multivariate logistic regression, to identify associations between possible risk factors and poor diabetes control. Statistical significance was set at a p-value of <0.05.
Results
General Characteristics of the Study Participants
We recruited 223 participants in this study after approaching 228 patients; with a response rate of 98%. Over two thirds (153, 68.9%) of the participants were females, almost a third (70, 31.8%) of them were over 60 years of age (61.05±4). Over two thirds (151, 69.9%) of the participants were married. Almost a half of the participants were Anglicans (99, 44.6%). Almost half of the participants had primary school education as the highest formal education (101, 45.7%). The majority (162, 73.0%) of the participants were self-employed and low income earners (Table 1 below).
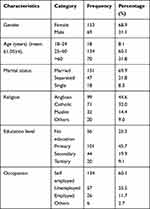 |
Table 1 Sociodemographic Characteristics of Type 2 Diabetes Patients at Diabetes Clinic of MRRH, Uganda |
Clinical Characteristics of the Participants
Almost two thirds (141, 63.5%) of the participants had had diabetes for less than five years. Half (112, 50.2%) of the participants had at least one comorbidity. The majority (188, 84.3%) of the participants had diabetic complications; most of whom (162, 85.7%) had diabetic neuropathy and almost all of the male participants (67, 97.1%) had erectile dysfunction and more than a third (89, 39.9%) of the participants were overweight (Table 2).
 |
Table 2 Clinical Characteristics Among Type 2 Diabetes Patients at Diabetes Clinic of MRRH, Uganda |
Poor Glycemic Control Among the Participants
The majority (188, 84.3%) of the participants had poor glycemic control. More than two thirds (147, 71.7%) of the participants had poor fasting blood glucose.
Poor Glycemic Control Due to Nonadherence to Lifestyle Recommendations
Only 18 (8.1%) of the participants were currently using alcohol and five (2.2%) smoked tobacco. Almost a third of the participants (60, 26.9%) were not following the recommended diet, of which most (49, 81.7%) had poor glycemic control. About a half (110, 49.3%) of the participants were not doing physical exercise and majority (90.0%) of them had poor glycemic control. The majority (16/18) of the participants that had used alcohol had poor glycemic control. Four out of five participants that were smokers had poor glycemic control (Table 3).
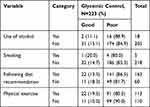 |
Table 3 Poor Glycemic Control Among Type 2 Diabetes Patients Not Adhering to Self-management Behavior at Diabetes Clinic of MRRH, Uganda |
Factors Contributing for Poor Glycemic Control
Univariate logistic regression revealed that age over 25 years (COR: 4.59, 95%CI: 1.55–13.59, p-value 0.006), duration of diabetes above 10 years (COR: 2.19, 95%CI: 0.62–7.74, p-value 0.22), medication regimens including; metformin + glibenclamide (COR: 1.96, 95%CI: 0.79–4.83, p-value 0.14), insulin (COR: 2.10, 95%CI: 0.71–6.23, p-value 0.18) and physical exercise (COR: 0.54, 95%CI: 0.25–1.18, p-value 0.13) had a p-value less than 0.25. (Table 4).
 |
Table 4 Univariate Logistic Regression of Factors Associated with Poor Glycemic Control Among Type 2 Diabetes Mellitus Patients at the Diabetes Clinic of MRRH, Uganda |
Independent factors that had a p-value of less than 0.25 at bivariate logistic regression were considered for multivariate logistic regression to obtain adjusted odds ratios and these included age, duration of type 2 diabetes mellitus, physical exercise, and antidiabetic drug(s) used.
Multivariate logistic regression revealed that poor glycemic control was more prevalent among those aged 25–60 years (OR=4.48, 95%CI: 1.56–14.50, p-value=0.006) and those aged over 60 years (OR=4.28, 95%CI: 1.18–15.58, p-value=0.03) compared to the youth (Table 5).
 |
Table 5 Multivariate Logistic Regression of Factors Associated with Poor Glycemic Control Among Type 2 Diabetes Mellitus Patients at the Diabetes Clinic of MRRH, Uganda |
Discussion
A total of 223 participants were recruited and the majority (188, 84.3%) had poor glycemic control (defined as HbA1C ≥7%). About a half (110, 49.3%) of the participants were not doing physical exercise, of which 99/110 (90.0%) had poor glycemic control. Alcohol use and tobacco smoking were recorded among 18 (8.1%) and five (2.2%) participants, respectively. More than a quarter (60, 26.9%) of the participants did not adhere to the recommended diet, of which 49/60 (81.7%) had poor glycemic control. Multivariate logistic regression revealed that poor glycemic control was more prevalent among participants aged 25–60 years (AOR=4.48, 95%CI: 1.56–14.50, p-value=0.009) and those aged over 60 years (AOR=4.28, 95%CI: 1.18–15.58, p-value=0.03) compared to the youth, 18–24 years of age.
The current prevalence (84.3%) of poor glycemic control is comparable to similar studies in Iraq, Trinidad, Tanzania, Kenya, and Ethiopia reporting up to 86.2%, 85.0%, 80.5%, 83.0%, and 72.7%, respectively. Studies conducted in Ethiopia and Bangladesh found that poor glycemic control was associated with the female gender,5 lower level of education, living in rural areas, and unemployed people or housewives.6 The finding that the proportion of males and females with poor glycemic control in our study was 85.7% (60) and 85.0% (130) respectively means that both male and female T2DM patients in our study were at an equal risk of poor glycemic control. This explains the difference in statistical significance of the above studies and our study as regards gender. Being educated, having a longer duration of diabetes and good knowledge of diabetes are associated with good diabetes management practices.7 In our study, the majority (157, 71.0%) of the participants had low education levels which may have negatively affected knowledge of the disease and self-management practices of type 2 diabetes patients leading to poor glycemic control. This may explain the high prevalence of poor glycemic control in our settings. In the current study, multivariate logistic regression revealed that increase in duration of diabetes increased likelihood of poor glycemic control for 5–10 years (AOR=1.39, 95%CI: 0.51–3.80) and over 10 years (AOR=1.68, 95%CI: 0.45–6.34) compared to <5 years of diabetes, although not statistically significant. Both insulin resistance and β-cell dysfunction are usually present at the diagnosis of T2DM and progressively worsen with disease duration.8
Whereas lifestyle modification adherence has been known to contribute to good glycemic control,9 27% (60/223) of our study participants did not adhere to dietary recommendations, and 49.3% (110/223) of the participants did not adhere to physical exercise recommendation. Importantly 81.7% (49/60) and 90.0% (99/110) of those who did not adhere to diet and physical exercise recommendations respectively, had poor glycemic control. The proportion of poor glycemic control among those nonadherent to diet and physical exercise recommendations in our study, were comparable to previous findings reporting 85.9% and 83.5%, respectively.10 In the study by Zeleke Negera and Charles Epiphianio, 2020, females and patients of age >60 years were more likely to be nonadherent to physical activity recommendation than their male and younger counterparts respectively.11 Given that majority (69.5%) of our participants were female and almost a third (31.8%) of our study participants were >60 years, this could partly explain why a large number of our study participants did not adhere to physical exercise recommendation and indeed a large proportion had poor glycemic control.
Findings in this study also indicated that being an adult, 25–60 years (AOR=4.48, 95%CI: 1.56–14.50) or being elderly, over 60 years (AOR=4.28, 95%CI: 1.18–15.58, p-value=0.03) were independently associated with poor glycemic control. This is in agreement with another study done in Ethiopia12 where patients who were 51 or over years of age had statistically significant poor glycemic control. Perhaps this is also true in our setting given that most of the young and middle-aged adult patients reported that they were most of the daytime busy at their workplace. It is likely that a busy life among asymptomatic adult diabetic patients leads to the increase in missed clinic appointments and reduced frequency of physical exercise especially when the treatment plan is for prevention of diabetic complications. Reduced access to health care through missed appointments is common among asymptomatic adults. This directly leads to irregular blood glucose monitoring at the clinic, reduces timely interventions and contributes to poor glycemic control among T2DM patients.
Multivariate logistic regression revealed that poor glycemic control was more prevalent among the elderly (AOR=4.28, 95%CI: 1.18–15.58, p-value=0.03) compared to the youth (18–24 years). Poor glycemic control among the elderly could be due to lack of strict adherence to lifestyle modification recommendation and age-related comorbidities which make patients susceptible either to hyperglycemia or hypoglycemia and other complications. Medical professionals are often not able to provide holistic health care in the absence of adequate resources13 and this is true in our setting. This results in some comorbidities being untreated for some time which could physiologically stress patient bodies and lead to poor glycemic control.
Conclusion
About eight in every ten type 2 diabetes patients that attended the diabetes outpatient clinic at Mbarara Regional Referral Hospital had poor glycemic control. This prevalence was comparable with results from other low and middle-income countries. Most of the participants did not adhere either to recommended diet or physical exercise and the majority of them had poor glycemic control. Multivariate regression revealed that being an adult or elderly was a statistically significant determinant for having poor glycemic control in this study.
Recommendation
The Mbarara Regional Referral Hospital health workers and the medical fraternity in general should educate and sensitize T2DM patients on the dangers of poor glycemic control and how to prevent associated complications. Designing an intervention that promotes diabetes education, lifestyle modification recommendation adherence and benefits of good glycemic control for adult patients is recommended.
Abbreviations
T2DM, type 2 diabetes mellitus; HbA1C, glycosylated hemoglobin; FBS, fasting blood glucose; MRRH, Mbarara Regional Referral Hospital; AOR; adjusted odds ratio, BMI, body mass index, CI; confidence interval, OR; odds ratio, WHO; World Health Organization.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethical Approval and Consent
This study was conducted in accordance with the Declaration of Helsinki.14 Approval to carry out the study was acquired from Mbarara University Research Ethics Committee (MUST-REC). Voluntary recruitment was done after the participant’s informed consent had been obtained and signed. Informed consent from participants was obtained after fully explaining the details of the study to the participants in English or in local language (Runyankole) for those who preferred the local language.
Acknowledgments
We thank Mbarara Regional Referral Hospital staff for their cooperation during the study. We are also appreciative of the study participants for their time and cooperation.
Funding
No funding was received to conduct this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health Organisation. Global report on diabetes [Online]; 2016. Available from: https://www.who.int/publications/i/item/9789241565257. Accessed 21 October, 2020.
2. Malik VS, Popkin BM, Bray GA, Després J-P, Hu FB. Sugar-sweetened beverages, obesity, type 2 diabetes mellitus, and cardiovascular disease risk. Circulation. 2010;121(11):1356–1364. doi:10.1161/CIRCULATIONAHA.109.876185
3. Kassahun T, Gesesew H, Mwanri L, Eshetie T. Diabetes related knowledge, self-care behaviours and adherence to medications among diabetic patients in Southwest Ethiopia: a cross-sectional survey. BMC Endocr Disord. 2016;16(1):28. doi:10.1186/s12902-016-0114-x
4. Mayega RW, Rutebemberwa E. Clinical presentation of newly diagnosed diabetes patients in a rural district hospital in Eastern Uganda. Afr Health Sci. 2018;18(3):707–719. doi:10.4314/ahs.v18i3.29
5. Demoz GT, Gebremariam A, Yifter H, et al. Predictors of poor glycemic control among patients with type 2 diabetes on follow-up care at a tertiary healthcare setting in Ethiopia. BMC Res Notes. 2019;12(1):207. doi:10.1186/s13104-019-4248-6
6. Afroz A, Ali L, Karim MN, et al. Glycaemic control for people with type 2 diabetes mellitus in Bangladesh-an urgent need for optimization of management plan. Sci Rep. 2019;9(1):1–10. doi:10.1038/s41598-019-46766-9
7. Alaofè H, Hounkpatin WA, Djrolo F, Ehiri J, Rosales C. Knowledge, attitude, practice and associated factors among patients with type 2 diabetes in Cotonou, Southern Benin. BMC Public Health. 2021;21(1):1–11. doi:10.1186/s12889-021-10289-8
8. Saisho Y. β-cell dysfunction: its critical role in prevention and management of type 2 diabetes. World J Diabetes. 2015;6(1):109. doi:10.4239/wjd.v6.i1.109
9. Tan S, Juliana S, Sakinah H. Dietary compliance and its association with glycemic control among poorly controlled type 2 diabetic outpatients in Hospital Universiti Sains Malaysia. Malays J Nutr. 2011;17(3):287–299.
10. Kayar Y, Ilhan A, Kayar NB, Unver N, Coban G, Ekinci I, et al. Relationship between the poor glycemic control and risk factors, life style and complications. Biomedical research (0970-938x). 2017;28(4).
11. Zeleke Negera G, Charles Epiphanio D. Prevalence and predictors of nonadherence to diet and physical activity recommendations among type 2 diabetes patients in Southwest Ethiopia: a cross-sectional study. Int J Endocrinol. 2020;2020:1–8. doi:10.1155/2020/1512376
12. Woldu M, Wami C, Lenjisa J, Tegegne G, Tesafye G, Dinsa H. Factors associated with poor glycemic control among patients with type 2 diabetes mellitus in Ambo Hospital, Ambo; Ethiopia. Endocrinol Metab Synd. 2014;3(143):2161–1017.1000143.
13. Ezenwaka C, Offiah N. Differences in glycemic control and cardiovascular risk in primary care patients with type 2 diabetes in West Indies. Clin Exp Med. 2010;1(2):91–98. doi:10.1007/s10238-001-8018-z
14. Shrestha B, Dunn L.Declaration of Helsinki – ethical principles for medical research involving human subjects. A review of seventh revision. Journal of Nepal Health Research Council. 2019;17(4):548–52.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
