Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Polymorphisms of the NLRC4 Gene are Associated with the Onset Age, Positive Rate of GADA and 2-h Postprandial C-Peptide in Patients with Type 1 Diabetes
Authors Xu L, Sun X, Xia Y, Luo S, Lin J, Xiao Y , Liu Y, Wang Y, Huang G, Li X, Xie Z, Zhou Z
Received 6 January 2020
Accepted for publication 15 February 2020
Published 19 March 2020 Volume 2020:13 Pages 811—818
DOI https://doi.org/10.2147/DMSO.S244882
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Linling Xu, 1, 2 Xiaoxiao Sun, 1, 2 Ying Xia, 1, 2 Shuoming Luo, 1, 2 Jian Lin, 1, 2 Yang Xiao, 1, 2 Yue Liu, 1, 2 Yanfei Wang, 1, 2 Gan Huang, 1, 2 Xia Li, 1, 2 Zhiguo Xie, 1, 2 Zhiguang Zhou 1, 2
1Department of Metabolism and Endocrinology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 2Key Laboratory of Diabetes Immunology, Central South University, Ministry of Education, National Clinical Research Center for Metabolic Diseases, Changsha, Hunan, People’s Republic of China
Correspondence: Zhiguang Zhou; Zhiguo Xie Email [email protected]; [email protected]
Purpose: The purpose of this study was to clarify the association between the NLRC4 gene and the susceptibility and clinical characteristics of type 1 diabetes (T1D) in a Chinese Han population.
Patients and Methods: A case-control study was performed in a Chinese Han population including 510 classical T1D patients and 531 healthy controls. rs212704 and rs385076 of the NLRC4 gene were genotyped by MassARRAY. The frequency distributions of alleles and genotypes of polymorphisms in the NLRC4 gene were compared by logistic regression and the chi-square test. The relationships between the polymorphisms of the NLRC4 gene and various clinical characteristics were analyzed by Kruskal–Wallis one-way ANOVA. The statistical power was calculated by Quanto software.
Results: 1) rs385076 of the NLRC4 gene was significantly correlated with the onset age of T1D patients and the positive rate of GADA. The relationship between rs212704 and 2-h postprandial C-peptide was statistically significant. 2) There was no significant difference in the frequency distributions of the genotypes and alleles of rs212704 and rs385076 between T1D patients and controls. 3) rs212704 and rs385076 were not correlated with T1D susceptibility under different genetic models.
Conclusion: rs212704 was associated with 2-h postprandial C-peptide, while rs385076 of the NLRC4 gene was associated with the onset age and positive rate of GADA in patients with T1D.
Keywords: type 1 diabetes, inflammasome, single nucleotide polymorphism
Introduction
Type 1 diabetes (T1D) was once thought to be an autoimmune disease that was simply caused by T lymphocyte-mediated self-attack on beta cells, but it is currently considered a complex disease owing to the interaction between environmental factors and genetic factors.1–3 The incidence and prevalence of T1D have been increasing worldwide by approximately 2–3% per year.4,5 Although the etiology of T1D remains obscure, genetic contribution certainly plays a pivotal role in the development of T1D. In the past few years, the human leukocyte antigen (HLA) region and more than 60 non-HLA loci have been identified by genome-wide association studies (GWAS), contributing to the susceptibility of T1D.6,7
Recent studies have found that both adaptive immunity and innate immunity are involved in the development of T1D.8 Innate immunity, a natural immune defense, provides a rapid anti–infective effect in all animals and plants. Pathogen-associated molecular patterns (PAMPs), such as viruses and bacteria, can be recognized and bound by pattern recognition receptors (PRRs) of the innate immune system to initiate the immune response. PRRs, which are mainly expressed on the surface of innate immune cells, are further divided into membrane-binding receptors such as Toll-like receptors and intracellular NOD-like receptors (NLRs).9 Once the NLR recognizes the corresponding ligand, it can assemble to form an inflammasome.
Inflammasomes are multiprotein complexes composed of receptors (mainly NLRs and AIM2 receptors), junction proteins (mainly ASCs) and effector proteins (mainly caspases) that initiate the inflammatory response. When NLRs sense PAMPs, caspase-1 is activated, thus promoting the maturation and secretion of IL-1β and IL-18 to play a proinflammatory role.10–12 There are five main types of inflammasomes that have been discovered, namely, the NLRP1 inflammasome, NLRP3 inflammasome, NLRC4 inflammasome, IPAF inflammasome and AIM2 inflammasome.
Recently, some evidence has shown that inflammasome-related genes play an indispensable role in autoimmune diseases,13 inflammatory diseases14,15 and metabolic diseases.16 The NLRP1 gene has been reported to be associated with vitiligo and systemic lupus erythematosus in Caucasian people,17,18 while polymorphisms of the NLRP3 gene have been reported to be correlated with rheumatoid arthritis (RA).19 As a kind of autoimmune disease, T1D has been reported to be associated with several inflammasome-related genes. It was reported that two SNPs in the NLRP3 gene were associated with T1D in the population of northeast Brazil, while polymorphisms in the NALP1 gene contributed to the susceptibility of T1D.20,21 We have reported in our previous study that the NLRP1 gene was associated with T1D in the Chinese Han population.22 NLRC4, an inflammasome that plays a pivotal role in the immune regulation of bacterial infections and autoinflammatory diseases, was associated with infantile enterocolitis and recurrent macrophage activation syndrome (MAS).23,24 Recently, it was found that polymorphisms in the NLRC4 gene were associated with glucose metabolism, which showed that there might be a relationship between the NLRC4 gene and diabetes.25 However, whether the NLRC4 gene is associated with susceptibility to T1D and whether it is involved in the pathogenesis of T1D has not been reported.
Therefore, this study selected polymorphisms of the NLRC4 gene to elucidate the association between polymorphisms of the NLRC4 gene and T1D in the Chinese Han population. In addition, this study also explored the association between polymorphisms of the NLRC4 gene and clinical characteristics in T1D patients.
Materials and Methods
Subjects
A total of 1041 Chinese Han participants were selected from the Second Xiangya Hospital of Central South University, including 510 T1D patients and 531 nondiabetic controls. The recruitment criteria for the case group were as follows: (1) complying with the WHO diabetes diagnostic criteria in 1999; (2) acute onset without obvious cause of diabetic ketosis or diabetic ketoacidosis; (3) dependent on insulin therapy within six months after disease diagnosis; and (4) at least one islet autoantibody is positive in serum, such as protein tyrosine phosphatase antibody (IA-2A), glutamic acid decarboxylase antibody (GADA) and zinc transporter 8 antibody (ZnT8A).26,27 The exclusion criteria were as follows: (1) secondary diabetes; (2) gestational diabetes or diabetes of special types; (3) combined with other types of autoimmune diseases; and (4) combined with malignant tumors. The recruitment criteria for the control group were fasting blood glucose <5.6 mmol/L and 2-h postprandial plasma glucose (PPG) < 7.8 mmol/L in the 75 g oral glucose tolerance test (OGTT), which were determined according to the definition of impaired fasting glucose(IFG) from WHO in 1999 and the International Diabetes Expert Committee in November 2003. People who have heart, brain, liver, kidney or other chronic diseases or combined with other types of autoimmune diseases are not included. Our research received approval from the Ethics Committee of the Second Xiangya Hospital of Central South University in accordance with the Declaration of Helsinki. All participants who were fully aware of this research signed informed consent.
Anthropometry and Biochemical Measurements
The demographic information, current medical history, diagnosis and treatment history, past medical history and family history of the participants were recorded. The height and weight of the subjects were obtained by the physician, and body mass index (BMI) was measured. Fasting blood glucose, postprandial blood glucose, high-density lipoprotein (HDL), low-density lipoprotein (LDL), cholesterol (TC), triglyceride (TG) and other biochemical indicators were obtained. Fasting C-peptide as well as 2-h postprandial C-peptide and glycated hemoglobin (HbA1c) were detected by the endocrine laboratory of Second Xiangya Hospital of Central South University using the chemiluminescence method (ADVIA Centaur XP Immunoassay System, Siemens, Germany) and automated liquid chromatography (HLC-723G8, Tosoh, Japan). ZnT8A, IA-2A and GADA were detected by the radioligand binding assay.28–30
Sample Collection
Each participant’s peripheral blood sample was collected in a tube containing ethylenediaminetetraacetic acid and stored at −80 °C. The GeneNode Genomic DNA Extraction Kit (Genenode Biotech Co. Ltd. (Beijing) was used to extract DNA according to the manufacturer’s instructions and then stored in a refrigerator at −80 °C after extraction.
Selection and Genotyping of SNPs
The selected SNPs of the NLRC4 gene were from recent literature reports related to other autoimmune diseases or inflammasome biomarker levels. At the same time, the minor allele frequency (MAF) must be more than 0.05 in the Asian population, and the selected SNPs were not in the same linkage region. Genotyping was performed using MassARRAY. PCR primers were designed by ADS2.0 software from Agena Bioscience and synthesized by BGI Corporation. The sequences of the primers are shown in Table 1.
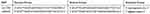 |
Table 1 The Primer Sequences of rs212704 and rs385076 in the NLRC4 Gene |
Statistical Analysis
SPSS 20.0 statistical software was used to analyze the data, and online software (http://ihg.gsf.de/cgi-bin/hw/hwa1.pl) was used to compare the genotype distributions for Hardy-Weinberg equilibrium (HWE) in controls. The frequencies of alleles and genotypes were compared by logistic regression and chi-square tests. The clinical characteristics of each genotype of two SNPs in the NLRC4 gene were compared using the Kruskal–Wallis H-test and chi-square test. Student’s t-test was used to analyze normally distributed data. P < 0.05 was considered statistically significant. The statistical power was calculated by Quanto software with the following parameters: an unmatched case-control study, an additive inheritance model, an expected OR value of 1.3, a sample size of 510 cases and 531 controls, a minor allele frequency of 0.48 of rs212704 and 0.46 of rs385076 and a prevalence of 1% of T1D in China.
Results
Anthropometry and Biochemical Characteristics of Cases and Controls
In this study, 510 patients with T1D and 531 nondiabetic controls were selected. The patients and controls were matched by sex(275/235 vs.273/258, p=0.418). The results of anthropometry and laboratory measurements in patients and controls have been summarized previously.22 The age and BMI of the T1D patients were significantly lower than those of the controls (P<0.001; P<0.001), while the fasting plasma glucose (FPG) and 2-h PPG values of the T1D patients were significantly higher.
Frequency Distributions of Alleles and Genotypes
The distributions of genotype frequencies for the controls were in HWE. The data are shown in Table 2. The distributions of alleles and genotype frequencies of the two SNPs between patients and healthy controls are shown in Tables 3 and 4. No significant difference was found in the frequency distributions of genotypes and alleles of rs212704 and rs385076 in the NLRC4 gene between T1D patients and healthy controls.
 |
Table 2 Hardy-Weinberg Equilibrium of NLRC4 Gene Polymorphisms |
 |
Table 3 Genotype and Allele Frequencies of rs212704 Between T1D Patients and Healthy Controls |
 |
Table 4 Genotype and Allele Frequencies of rs385076 Between T1D Patients and Healthy Controls |
Association Between Polymorphisms of the NLRC4 Gene and Susceptibility to T1D in Different Genetic Models
We found that rs212704 and rs385076 of the NLRC4 gene were not associated with T1D susceptibility in dominant, recessive, additive and overdominant models (see Table 5).
 |
Table 5 The Genetic Model of rs212704 and rs385076 Between T1D Patients and Healthy Controls |
The Clinical Characteristics of Patients with Different Genotypes of rs212704 and rs385076
We further analyzed the association between the clinical characteristics of T1D patients and genotypes of each SNP of the NLRC4 gene. The clinical characteristics of T1D patients with different rs212704 genotypes are shown in Table 6. There were significant differences in the 2-h postprandial C-peptide level among patients with different genotypes of rs212704 (P=0.003). Patients with the CC genotype had a lower 2-h postprandial C-peptide level than patients with the TC genotype of rs212704. The clinical characteristics of T1D patients with different genotypes of rs385076 are shown in Table 7. Marked differences were shown in onset age (P=0.031) and GADA antibody positive rate (P=0.041). Compared with patients in the CC genotype of rs385076, the TT genotype had a younger onset age and a lower GADA positive rate.
 |
Table 6 Clinical and Biochemical Characteristics of T1D Patients with Different Genotypes of rs212704 of the NLRC4 Gene |
 |
Table 7 Clinical and Biochemical Characteristics of T1D Patients with Different Genotypes of rs385076 of the NLRC4 Gene |
The Statistical Power of rs212704 and rs385076
From the data shown in Table 8, the statistical power of rs212704 in the additive model was 84.70%, while the statistical power of rs385076 was 84.72%. The results suggested that our research was well-powered to detect the association between the two SNPs of the NLRC4 gene and T1D.
 |
Table 8 The Statistical Power of rs212704 and rs385076 |
Discussion
This study was performed as a case-control study to clarify the potential relationship between T1D and polymorphisms of the NLRC4 gene in the Chinese Han population. Although no significant difference was found between susceptibility to T1D and rs212704 or rs385076 of the NLRC4 gene, these two SNPs were related to the clinical characteristics of T1D patients. rs212704 was associated with 2-h postprandial C-peptide, while rs385076 was correlated with the onset age of T1D and positive rate of GADA antibody.
As a multifactorial disease, T1D is caused by both genetic and environmental factors. The NLRC4 inflammasome is one of the major components of the NOD-like receptors. Considering the important role of the NLRC4 inflammasome in immune response and autoimmune diseases, this study selected polymorphisms of the NLRC4 gene to explore their potential relationship with T1D in the Chinese Han population.
In this study, the associations between rs212704 and rs385076 of the NLRC4 gene and T1D in a Chinese Han population were analyzed. rs212704 is an intronic variant that has been reported to be associated with lipid and glucose metabolism as well as specific microbial colonization in the lungs of patients with cystic fibrosis.31 Specific genotype of rs212704 in patients with cystic fibrosis showed a reduction in NLRC4 expression level in lung expectorates. The molecular analyses from a published article showed that rs385076 affected NLRC4 expression and was considered to be a protective factor against cardiovascular diseases.32 It was also reported that rs385076 has an association with lower levels of IL-18 and IL-6 in HIV patients with active tuberculosis in South Africa.33 Considering the potential influence of rs212704 and rs385076 on inflammation and immunity, we selected the two SNPs preferentially. Since most epidemiological studies on T1D report that the peak in the onset of T1D was close to puberty,34 we chose older individuals as controls to reduce their possibility of developing T1D thus makes our results more credible.
The results showed that none of these SNPs were associated with T1D susceptibility. Furthermore, the differences in clinical characteristics were analyzed from patients with different genotypes of rs212704 and rs385076. C-peptide is commonly measured in clinical practice as a marker of the function of islet cells. Persistent C-peptide secretion is associated with reduced development of complications of T1D, such as retinopathy, nephropathy, and hypoglycemia. From the results, rs212704 was associated with 2-h postprandial C-peptide. Patients with the CC genotype had a lower 2-h postprandial C-peptide than patients with the TC genotype, which indicated that patients with the CC genotype had worse islet function than the TC genotype in rs212704.
For rs385076 of the NLRC4 gene, the onset age of T1D patients was significantly different under different genotypes. The results showed that patients with the TT genotype had a younger onset age than those with the CC genotype. Patients with different onset ages of T1D have significant heterogeneity in clinical phenotype and immunogenetics. A recently published article showed that the risk of major vascular and microvascular complications in patients younger than 14 years old was relatively low, suggesting that lower age was a protective factor for microvascular complications.34 Therefore, we can speculate that patients with different genotypes of rs385076 may have corresponding differences in clinical phenotype. To investigate whether NLRC4 polymorphisms are associated with islet autoantibodies, we analyzed the differences in the islet autoantibody positive rate and titer among patients with different genotypes of two SNPs of the NLRC4 gene. Islet autoantibodies are biomarkers of islet β cell destruction as well as indicators of T1D diagnosis and prognosis. Previous studies have confirmed that there is a certain genetic susceptibility to the production of islet autoantibodies.35 We found that rs385076 of the NLRC4 gene was associated with the positive rate of GADA, and patients with the CC genotype had a higher positive rate. These results suggested that specific genotypes were associated with positive rates or titers of specific islet antibodies, and these antibodies may be produced through different immunological mechanisms.
These findings may help predict the islet function and clinical prognosis in T1D patients, and the identification of related genetic variants may provide individualized prevention and treatment for patients and individuals with high genetic risk of T1D in the future. Since the HLA gene was reported to contribute 50% susceptibility to T1D, which was also associated with the islet function of T1D patients, we will consider combining non-HLA genes such as the NLRC4 gene with the HLA gene to build a risk predictive model of islet function of T1D patients in the future.
This study was the first to examine the association between polymorphisms of the NLRC4 gene and T1D in the Chinese Han population. In consideration of the genetic heterogeneity of T1D, our samples consisting of 510 T1D patients and 531 controls were all from Hunan Province, China. In addition, we not only examined the association of the NLRC4 gene with T1D susceptibility but also analyzed the relationship between different genotypes and clinical phenotypes of T1D patients, laying the foundation for a more comprehensive study of the NLRC4 gene in T1D pathogenesis.
However, there were also some limitations in this study. First, we only analyzed two SNPs of the NLRC4 gene, which do not represent the whole gene. In future studies, we can consider including more polymorphisms of the NLRC4 gene and other inflammasome-related genes for further research. Although the correlations between NLRC4 polymorphisms and clinical characteristics of T1D were analyzed, the mechanisms of the association have not yet been explored. To clarify how genetic factors contribute to differences in clinical features in T1D patients, a broader and deeper functional study is needed, which would make it possible to discover new targets that can predict, intervene and treat T1D.
Conclusion
This study investigated the association between polymorphisms of the NLRC4 gene and T1D in a Chinese Han population. The results showed that rs212704 and rs385076 of the NLRC4 gene were not correlated with T1D susceptibility in the Chinese Han population. However, rs212704 was associated with 2-h postprandial C-peptide levels in T1D patients, while rs385076 was associated with the GADA positive rate and onset age. Further studies are needed to comprehensively clarify the mechanism of NLRC4 polymorphisms associated with the clinical characteristics.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81873634, 81400783), the National Key R&D Program of China (2016YFC1305000, 2016YFC1305001, 2018YFC1315603), and the Science and Technology Major Project of Hunan Province (2017SK1020). We would like to give our sincere appreciation for all the staff of the Department of Metabolism and Endocrinology of Second Xiangya Hospital for collecting the samples.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014;383(9911):69–82. doi:10.1016/S0140-6736(13)60591-7
2. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464(7293):1293–1300. doi:10.1038/nature08933
3. Xie Z, Chang C, Zhou Z. Molecular mechanisms in autoimmune type 1 diabetes: a critical review. Clin Rev Allergy Immunol. 2014;47(2):174–192. doi:10.1007/s12016-014-8422-2
4. Mayer-Davis EJ, Dabelea D, Lawrence JM. Incidence trends of type 1 and type 2 diabetes among youths, 2002–2012. N Engl J Med. 2017;377(3):301.
5. Patterson CC, GG D, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi:10.1016/S0140-6736(09)60568-7
6. Noble JA. Immunogenetics of type 1 diabetes: a comprehensive review. J Autoimmun. 2015;64:101–112. doi:10.1016/j.jaut.2015.07.014
7. Pociot F, Lernmark A. Genetic risk factors for type 1 diabetes. Lancet. 2016;387(10035):2331–2339. doi:10.1016/S0140-6736(16)30582-7
8. Lehuen A, Diana J, Zaccone P, Cooke A. Immune cell crosstalk in type 1 diabetes. Nat Rev Immunol. 2010;10(7):501–513. doi:10.1038/nri2787
9. Xie Z, Huang G, Wang Z, Luo S, Zheng P, Zhou Z. Epigenetic regulation of toll-like receptors and its roles in type 1 diabetes. J Mol Med (Berl). 2018;96(8):741–751. doi:10.1007/s00109-018-1660-7
10. Bauernfeind F, Ablasser A, Bartok E, et al. Inflammasomes: current understanding and open questions. Cell Mol Life Sci. 2011;68(5):765–783. doi:10.1007/s00018-010-0567-4
11. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi:10.1016/S1097-2765(02)00599-3
12. Sutterwala FS, Mijares LA, Li L, Ogura Y, Kazmierczak BI, Flavell RA. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. J Exp Med. 2007;204(13):3235–3245. doi:10.1084/jem.20071239
13. Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17(2):57–64.
14. Baroja-Mazo A, Martin-Sanchez F, Gomez AI, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15(8):738–748. doi:10.1038/ni.2919
15. Hoffman HM, Rosengren S, Boyle DL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364(9447):1779–1785. doi:10.1016/S0140-6736(04)17401-1
16. Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. doi:10.1038/nm.2279
17. Dieude P, Guedj M, Wipff J, et al. NLRP1 influences the systemic sclerosis phenotype: a new clue for the contribution of innate immunity in systemic sclerosis-related fibrosing alveolitis pathogenesis. Ann Rheum Dis. 2011;70(4):668–674. doi:10.1136/ard.2010.131243
18. Jin Y, Mailloux CM, Gowan K, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi:10.1056/NEJMoa061592
19. Addobbati C, da Cruz HLA, Adelino JE, et al. Polymorphisms and expression of inflammasome genes are associated with the development and severity of rheumatoid arthritis in Brazilian patients. Inflamm Res. 2018;67(3):255–264. doi:10.1007/s00011-017-1119-2
20. Magitta NF, Boe Wolff AS, Johansson S, et al. A coding polymorphism in NALP1 confers risk for autoimmune Addison’s disease and type 1 diabetes. Genes Immun. 2009;10(2):120–124. doi:10.1038/gene.2008.85
21. Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity. 2010;43(8):583–589. doi:10.3109/08916930903540432
22. Sun X, Xia Y, Liu Y, et al. Polymorphisms in NLRP1 gene are associated with type 1 diabetes. J Diabetes Res. 2019;2019:7405120. doi:10.1155/2019/7405120
23. Canna SW, de Jesus AA, Gouni S, et al. An activating NLRC4 inflammasome mutation causes autoinflammation with recurrent macrophage activation syndrome. Nat Genet. 2014;46(10):1140–1146. doi:10.1038/ng.3089
24. Romberg N, Al Moussawi K, Nelson-Williams C, et al. Mutation of NLRC4 causes a syndrome of enterocolitis and autoinflammation. Nat Genet. 2014;46(10):1135–1139. doi:10.1038/ng.3066
25. Gomes Torres A, Leite N, Tureck LV, et al. Association between Toll-like receptors (TLR) and NOD-like receptor (NLR) polymorphisms and lipid and glucose metabolism. Gene. 2019;685:211–221. doi:10.1016/j.gene.2018.11.065
26. Yang L, Luo S, Huang G, et al. The diagnostic value of zinc transporter 8 autoantibody (ZnT8A) for type 1 diabetes in Chinese. Diabetes Metab Res Rev. 2010;26(7):579–584. doi:10.1002/dmrr.v26:7
27. Yi B, Huang G, Zhou ZG. Current and future clinical applications of zinc transporter-8 in type 1 diabetes mellitus. Chin Med J (Engl). 2015;128(17):2387–2394. doi:10.4103/0366-6999.163389
28. Jin P, Huang G, Lin J, Luo S, Zhou Z. Epitope analysis of GAD65 autoantibodies in adult-onset type 1 diabetes and latent autoimmune diabetes in adults with thyroid autoimmunity. Acta Diabetol. 2011;48(2):149–155. doi:10.1007/s00592-010-0250-0
29. Jin P, Xiang B, Huang G, Zhou Z. The association of cytotoxic T-lymphocyte antigen-4 + 49A/G and CT60 polymorphisms with type 1 diabetes and latent autoimmune diabetes in Chinese adults. J Endocrinol Invest. 2015;38(2):149–154. doi:10.1007/s40618-014-0162-x
30. Xiang Y, Huang G, Shan Z, et al. Glutamic acid decarboxylase autoantibodies are dominant but insufficient to identify most Chinese with adult-onset non-insulin requiring autoimmune diabetes: LADA China study 5. Acta Diabetol. 2015;52(6):1121–1127. doi:10.1007/s00592-015-0799-8
31. Iannitti RG, Napolioni V, Oikonomou V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7:10791. doi:10.1038/ncomms10791
32. Zeller T, Haase T, Muller C, et al. Molecular characterization of the NLRC4 expression in relation to interleukin-18 levels. Circ Cardiovasc Genet. 2015;8(5):717–726. doi:10.1161/CIRCGENETICS.115.001079
33. Ravimohan S, Maenetje P, Auld SC, et al. A common NLRC4 gene variant associates with inflammation and pulmonary function in human immunodeficiency virus and tuberculosis. Clin Infect Dis. 2019. doi:10.1093/cid/ciz898
34. Weng J, Zhou Z, Guo L, et al. Incidence of type 1 diabetes in China, 2010-13: population based study. BMJ. 2018;360:j5295. doi:10.1136/bmj.j5295
35. Qu HQ, Polychronakos C. The effect of the MHC locus on autoantibodies in type 1 diabetes. J Med Genet. 2009;46(7):469–471. doi:10.1136/jmg.2009.066647
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
