Back to Journals » International Journal of Nanomedicine » Volume 19
Polymeric Nanohydrogel in Topical Drug Delivery System
Authors Yuniarsih N, Chaerunisaa AY, Elamin KM , Wathoni N
Received 21 October 2023
Accepted for publication 15 February 2024
Published 15 March 2024 Volume 2024:19 Pages 2733—2754
DOI https://doi.org/10.2147/IJN.S442123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kamakhya Misra
Nia Yuniarsih,1,2,* Anis Yohana Chaerunisaa,1,* Khaled M Elamin,3,* Nasrul Wathoni1,*
1Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia; 2Department of Pharmaceutical Technology, Faculty of Pharmacy, Universitas Buana Perjuangan Karawang, Karawang, 41361, Indonesia; 3Graduate School of Pharmaceutical Sciences, Kumamoto University, Kumamoto, 862-0973, Japan
*These authors contributed equally to this work
Correspondence: Nasrul Wathoni, Department of Pharmaceutics and Pharmaceutical Technology, Faculty of Pharmacy, Universitas Padjadjaran, Sumedang, 45363, Indonesia, Tel +62-22-842-888-888, Email [email protected]
Abstract: Nanohydrogels (NH) are biodegradable polymers that have been extensively studied and utilized for various biomedical applications. Drugs in a topical medication are absorbed via the skin and carried to the intended location, where they are metabolized and eliminated from the body. With a focus on their pertinent contemporary treatments, this review aims to give a complete overview of recent advances in the creation and application of polymer NH in biomedicine. We will explore the key features that have driven advances in nanotechnology and discuss the significance of nanohydrogel-based formulations as vehicles for delivering therapeutic agents topically. The review will also cover the latest findings and references from the literature to support the advancements in nanotechnological technology related to the preparation and application of NH. In addition, we will also discuss the unique properties and potential applications of NH as drug delivery systems (DDS) for skin applications, underscoring their potential for effective topical therapeutic delivery. The challenge lies in efficiently delivering drugs through the skin’s barrier to specific areas with high control. Environmentally sensitive systems, like polymer-based NH, show promise in treating dermatological conditions. Polymers are pivotal in developing these drug delivery systems, with NH offering advantages such as versatile drug loading, controlled release, and enhanced skin penetration.
Keywords: nanohydrogels, topical drug delivery system, polymers, mechanisms of drug release, topical biomedical applications
Introduction
The current topical therapies for treating skin inflammation are limited, necessitating the development of a drug delivery system with controlled release. This technique aims to achieve more effective results in reducing pain, inflammation, ensuring greater safety, and preventing adverse reactions.1 By using such a delivery system, therapeutic agents can be transported to the deeper layers of the epidermis and dermis, maximizing their therapeutic efficacy.2 To enhance topical drug delivery, formulations that increase skin permeability and penetrate the skin using various mechanisms are being explored. A potentially effective strategy entails the utilization of nanoparticle systems to enhance the permeability of the skin and facilitate the deposition of the encapsulated pharmacological agents. However, studies have revealed that these nanoparticles often do not penetrate the stratum corneum (SC) but instead accumulate in the SC layer, gradually releasing the encapsulated drug into the upper epidermis. From there, the drug passively diffuses into the underlying skin layers.3 Although recent research has shown that nanoparticles can transport active pharmaceutical ingredients (APIs) through hair follicles and into the skin layers, the total amount reaching the dermal sites remains limited.3,4 As the newest drug delivery systems, various nanotechnology techniques have been introduced, including protein-based nanoparticles, lipid-based nanoparticles, nanoemulsions, nanocrystals, nanodiamonds, carbon nanotubes, nanosuspensions, and NH. These technologies exhibit considerable significant effect for enhancing drug delivery through the skin and represent attractive avenues for further exploration and development in the field of topical drug delivery.5
Nano-based systems have the capability to deliver active compounds at minimal concentrations and target them to specific sites of action. This is achieved by utilizing suitable drug carriers, often composed of polymers and other excipients. However, the success of DDS relies on various factors, such as the characteristics of the drug and polymer, the type of dosage form, and the route of administration Polymer nanoparticles are composed of biodegradable and biocompatible polymers that are non-toxic, making the selection of the appropriate polymer crucial.6 The chosen polymer should be able to form synergistic combinations, significantly enhancing the retention of cells in local tissues. While cells are regenerating and new tissue is being created, it should biodegrade gradually to avoid any unwanted consequences that might result from lingering traces.1 For the treatment of diseases, nanomedicine, pharmaceuticals, and bio-nanotechnology have all turned their focus to polymer-based DDS.7,8 DDS has the potential to treat a wide range of ailments, from those amenable to topical treatment to more complicated conditions, with fewer adverse effects than traditional drug administration methods.9 Based on polymer classification, several polymers have been reported as natural-based, synthetic, or hybrid combinations. Extensive research has been conducted on natural-based polymers because of their notable biodistribution, non-cytotoxic characteristics, biodegradability, and ease of excretion.10 Multiple investigations showed that by forming cross-linked networks using synthetic and hybrid polymers, the structure and drug release behavior of natural polymers might be altered.11 However, synthetic polymers lack immunological recognition for natural extracellular matrix proteins while offering great stability, maintaining the nanohydrogel structure, and well-behaved drug release qualities.12,13 Several researchers have paired bioactive probes with synthetic polymers, while yet others have mixed bioactive probes with natural and synthetic polymers.14 Numerous studies have focused on nanohydrogels, one of the most promising polymeric DDS, to investigate innovative formulation methodologies and uses of nanocarriers with enhanced therapeutic benefits.15,16
The main purpose of this review is to provide an in-depth overview of recent advancements in the production of polymer NH and their applications in biomedicine, with a particular focus on the use of these materials in topical treatment. In addition, we reviewed the essential characteristics that have accelerated advancements in nanotechnology and emphasized the important role of NH as carriers for delivering therapeutic agents via topical administration. Then, we integrated the latest findings and sources from the literature to support the advancements in nanotechnology related to NH. The discussion also addressed the distinctive features of NH, emphasizing their capacity for effective management of topical treatments. Furthermore, this review investigated the advancements achieved in polymer nanohybrids (NH) and their use in the field of biomedicine, with a special emphasis on understanding the possible advantages and applications of NH in topical therapy. Through the integration of recent research findings and a thorough analysis of the distinct attributes of nanohydrogels, we may gain valuable understanding regarding their capacity to improve drug delivery systems for topical treatments.
Polymeric Nanohydrogels
When it comes to the development of intelligent systems for skin applications, polymer-based NH have been characterized the most often as potential candidates. The polymeric channel system that makes up the core of a hydrogel may be created either by the physical or chemical cross-linking of homopolymers or copolymers; this kind of cross-linking causes the hydrogel to expand when it is exposed to an aqueous environment.17,18 Materials having viscoelastic qualities and with hydrophilic polymeric networks within the sub-micron size range are referred to as polymer NH. These materials are exploited as nanocarriers in the process of drug delivery.19 The kind and quantity of the polymer building blocks that are used in the production of viscoplastic nanogels determines the qualities that are shown by the finished product.20 Raw materials sourced from either naturally occurring or synthetically manufactured polymers, or a mix of the two, are used in the manufacturing process of NH. The processing of natural materials or the synthesis of primary components from oil, gas, and other sources may result in the production of a type of polymers known as synthetic polymers. These polymers are characterized by their long molecular chains and organic linkages.21 NH made of synthetic polymers provide an alternative and a number of benefits to traditional polymer networks.22 In spite of the absence of any intrinsic bioactivity, they are well-known for the regulated structure and favorable mechanical qualities that they possess.1,11 There are several advantages associated with the use of scaffolds in tissue engineering. One notable advantage is the ability to achieve a controlled structure, which allows for precise manipulation and arrangement of the scaffold. Additionally, scaffolds possess desirable mechanical properties, ensuring their stability and support for tissue growth.23 Another advantage is their biocompatibility, indicating that they are well-tolerated by living organisms and do not elicit adverse reactions. Furthermore, scaffold design may be easily controlled, enabling the customization of the scaffold’s physical characteristics to suit specific tissue engineering. There are several drawbacks associated with this phenomenon. One limitation of synthetic polymers is their general lack of innate biological activity, which can restrict their capacity to induce certain cellular responses. Natural polymers are commonly employed in the synthesis of NH possessing bioactive characteristics. Several natural polymers, such as hyaluronic acid, chitosan, alginate, and dextran, have been extensively used as biomaterials in the development of nanoparticles for cancer therapy.24,25 These natural polymers possess several desirable characteristics, such as biocompatibility, biodegradability, non-cytotoxicity, and non-immunogenicity, making them highly suitable for various biomedical applications.21,26 The preclinical trials that developed a chitosan-based pH-responsive biodegradable nanohydrogel showed the integrity of the skin layer when compared with other conventional melanoma formulations.27,28 The use of traditional hydrogels faces certain limitations, such as their macroscopic dimensions and rapid drug elution from the swollen hydrogel matrix. NH have emerged as a viable answer to these problems. NH are a kind of hydrogel with nanoscale structures created by chemically or physically connected swellable polymer networks, and their diameters range from around 1 to 1000 nm. NH can keep a lot of water without becoming mushy or losing their shape, despite their little size.29,30 They offer several advantages for drug delivery such as, active ingredient protection,31,32 biocompatibility,7,33 versatile drug formulation,13,33 stimuli-responsive properties,34 preventing reticuloendothelial invasion, fast response to external stimuli, enhanced bioavailability.33 Therefore, NH offer a range of advantages that make them promising candidates for drug delivery systems, particularly in topical therapy and targeted drug delivery applications. Furthermore, it can provide a moist effect on the wound area, reducing swelling and speeding up the wound healing process. Nanohydrogel can also reduce pain around the wound and improve patient comfort.35 Their unique properties, high biocompatibility, and stimuli-responsive behavior contribute to their potential in advancing drug delivery techniques. There are certain drawbacks associated with the use of NH, including tissue toxicity and the development of strong polymerization reactions. The polymerization processes utilized in the synthesis of NH can exhibit considerable severity, hence posing potential obstacles in relation to scalability and safety considerations.33,34
Topical Nanohydrogel Drug Delivery System
In topical drug delivery systems, the skin serves as one of the primary and accessible organs for drug administration.36 However, the skin is a highly effective barrier that restricts the penetration of most drugs used for therapeutic purposes.37 Only a few drugs have the ability to penetrate the skin significantly. As a result, most topical dosage forms available on the market today have poor penetration capabilities, leading to limited therapeutic benefits.38 The difficulties of achieving adequate medication absorption via the skin using topical formulations emphasize the need for novel drug delivery technologies, such as NH. Because of their controlled release, increased penetration, and protection of the loaded pharmaceuticals, NH have the potential to improve drug delivery via the skin. Effective topical medication delivery and tailored therapy are made possible by NH because they overcome the drawbacks of conventional topical formulations.39 A total of 207 patients were randomized in a clinical trial for the treatment of acne vulgaris of the face. The results suggested that with the tretinoin 0.025% nanohydrogel formulation, reductions in total (72.9% vs 65.0%; p = 0.03) and inflammatory (78.1% vs 66.9%; p = 0.02) acne lesions were reported to be significantly greater with the nanohydrogel formulation as compared to the conventional gel formulation. Local adverse events were significantly less (p = 0.04) in the nanohydrogel group (13.3%) as compared to the conventional gel group (24.7%).40 A total of 33 patients were treated using the polyacrylate-based nanoydrogel and 37 patients using the amorphous hydrogel. The estimated total direct costs per patient and per 14 days of therapy were €306 for both treatment groups. With the PA-based nanohydrogel, 2.5 additional days with wounds covered >50% with granulation tissues were gained within 14 days of leg ulcer care compared to the comparator.
However, it is essential to consider the potential disadvantages, such as tissue toxicity and the harshness of polymerization reactions, when designing nanohydrogel-based DDS for topical applications. Proper formulation design and safety assessments are crucial to overcome these challenges and ensure the successful translation of NH into topical therapeutic applications.41
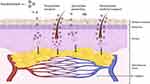
The skin is composed of three layers: the SC, the epidermis, and the dermis. The SC, often known as the SC, is responsible for the most important function of safeguarding the underlying structures. However, due to the presence of a strong barrier provided by the SC, the penetration of the majority of active APIs through the skin is restricted.37 This includes large, hydrophilic API molecules, such as proteins, peptides, nucleotides, and oligonucleotides.42 There are two primary ways that are being examined in order to circumvent this barrier and make it easier for drugs to be delivered to the deeper layers of the skin or into the systemic circulation. These are the transepidermal route and the transappendageal route. The transepidermal pathway is equivalent to drug penetration via the corneocytes, which is referred to as transcellular drug penetration, or through the bilipid layer that occurs in between the corneocytes, which is referred to as intercellular drug penetration. Drug penetration across the SC usually occurs via the intercellular route as the drug has to pass through only one type of structure, namely the bilipid layer as compared to the corneocyte repeat layer and the bilipid layer in the transcellular route. Usually, drug penetration across the SC predominantly occurs via the intercellular route. This is because the drug only needs to pass through one type of structure, the bilipid layer, as compared to dealing with both the corneocyte repeat layer and the bilipid layer in the transcellular route.43 The transappendageal route of administration is suitable for drugs that meet these criteria and can facilitate their penetration through sweat glands or hair follicles. This route is preferred for high molecular weight molecules and substances, including vesicular and nanoparticles. Drugs with certain physicochemical and biological parameters, including an MW of less than 500 Da, water solubility of more than 100 µg/mL and a log P (lipophilicity) value between 1 and 3.5, small doses of drug (<10mg/day) and lower boiling points (<200°C) correspond to the transappendageal route of administration.38
Understanding these routes of drug penetration through the skin is essential for designing effective topical drug delivery systems, including NH, to enhance drug absorption and achieve the desired therapeutic effects. Topical drug delivery aims to deliver drugs to various layers of the skin for local effects, providing several distinct advantages: elimination of systemic drug delivery, minimization of side effects, and lower dose requirements.2 On the other hand, topical drug delivery (TDD) aims to penetrate the drug through the skin to reach the systemic circulation in sufficient amounts to produce a therapeutic effect.3,44 Unlike topical drug delivery, where the goal is to target local tissues, transdermal delivery is designed to deliver drugs systemically, bypassing the first-pass metabolism that occurs with oral administration. In the administration of topical drugs, Fick’s second law governs drug absorption in the SC45 Fick’s second law describes the diffusion of a substance (in this case, the drug) through a medium (the SC) over time. To improve medication formulations and achieve the intended therapeutic effects, knowing how quickly and how much a drug is absorbed via the skin is crucial information. Understanding the principles of drug diffusion through the skin is crucial to the design and development of effective topical drug delivery systems.
In topical drug delivery, API must penetrate various layers of the skin, which consist of hydrophilic and lipophilic domains. For topical administration, the objective is to target local tissues, and for transdermal delivery, the goal is to achieve systemic circulation. First, the medicine is released from the topical formulation, and then it enters the SC and diffuses there, then it enters the viable epidermis and diffuses there, and finally it enters the dermis.7 The overall process of drug penetration through the skin involves complex interactions between the drug, the skin’s barrier properties, and the DDS (see in Figure 1). Understanding these stages of API release in topical drug delivery is crucial for designing effective formulations that optimize drug delivery and achieve the desired therapeutic outcomes.
Methods of Topical Nanohydrogel Preparation
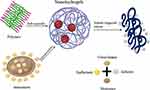
These different methods of nanohydrogel preparation offer versatility in designing nanohydrogel-based drug delivery systems. Depending on the specific application and desired properties of the NH, researchers can choose the most suitable method to achieve effective drug delivery and therapeutic outcomes. Figure 2 shows methods in topical preparation:1,15,33,46
Concomitant Polymerization and Cross-Linking
Nanohydrogel can be synthesized by having polymerization and cross-linking carried out simultaneously. Since most of the monomers and cross-linking agents used to prepare nanogels are water-soluble, the polymerization reaction is generally carried out in aqueous media.
Separate Polymerization and Cross-Linking
The polymer is first formed, followed by cross-linking between the polymer molecular chains to produce the nanogel. This method is very suitable for the preparation of nanogels based on natural polymers. Based on the mechanism for making nanogels, this method can consist of various types: precipitation/crosslinking, emulsification/crosslinking, self-assembly/crosslinking, and micro-template forming/crosslinking.
Covalent Conjugation
This method involves the assembly of acrylic groups with enzymes and the copolymerization of these groups with acrylamide in either a solution or an inverse microemulsion. The outcome is the production of hydrogels that are nano-sized. The inclusion of hydrophobic molecules into nonpolar regions leads to the creation of hydrophobic chains.
Self-Assembly
The method involves the integration of components into nanohydrogel structures, which offers several benefits like minimum thermodynamics, flexibility, simplicity, and affordability. Molecules are characterized by their ability to diffuse and interact with other molecules through non-covalent, hydrophobic, or electrostatic forces.
API Release Mechanisms from Nanohydrogel
The API’s active pharmaceutical ingredient loaded into the nanohydrogel can be released through three mechanisms:18,27 Controlled diffusion, or the release of drug molecules, relies on the structure and morphology of the polymer. The rate and extent of drug release are influenced by the diffusion properties of the polymer matrix. Chemically controlled degradation. The release depends on the rate of bond degradation in the polymer, which leads to polymer degradation and dissolution. In hydrophilic polymers, erosion occurs throughout the polymer matrix, while in hydrophobic polymers, erosion occurs primarily on the surface of the polymer matrix. Swelling control and drug release occur when the polymer matrix swells and the glass transition temperature are lowered due to the relaxation of the molecular chains.
These three mechanisms play a crucial role in the controlled and targeted release of drugs from NH. Understanding the API release mechanisms is essential for designing NH with the desired drug delivery profiles for specific therapeutic applications.
Polymeric Topical NH
DDS, like modified-release formulations, have been made with polymers made from both natural and man-made materials (see Table 1). Polymer gels can be categorized based on their diameter size, either as nano-sized or micro-sized gels. These nano- and micro-sized gels can be prepared by controlling the gelling crosslinking reactions used in hydrogel formulations.21 Nanomaterials have the capability to enable controlled drug release and enhance the stability of therapeutic agents, opening up new possibilities for disease treatment. These polymers may either expand (as a result of water absorption) or dissolve in water because they include polar functional groups including carboxyl, hydroxyl, and amino groups. This property distinguishes them from other types of polymers.47,48
 |
Table 1 Most Widely Used Polymers for Topical Nanohydrogel Design and Manufacture |
Synthetic Polymers
Emulsification is a process that can be utilized to produce polymeric NH. This can be accomplished by many methods such as emulsification solvent evaporation or solvent-emulsion diffusion.49,55,76 Modifying PEG polymers: Modifying PEG polymers can enhance their properties for biomedical applications. However, single PEGs may have limitations like low antimicrobial ability and volume expansion.77,78 Polyvinyl Alcohol (PVA) is a widely recognized hydrophilic polymer that shows remarkable biocompatibility, and its properties can be enhanced by combining it with natural polymers.58,62 Polyethylene Glycol (PEG) polymers have gained significant attention in the field of biomedical applications due to their remarkable biocompatibility and flexibility in manipulating their scaffold architecture. However, it is crucial to recognize that PEG may have significant limits, including a relatively low effectiveness against germs and the possibility of volume expansion. In addition to PEG, carbopol is a synthetic polymer composed of acrylic acid monomer with a high molecular weight. The capacity to absorb and retain water results in the development of enlarged NH.79,80 Synthetic polymers play a significant role in the preparation of NH for topical applications. By carefully selecting and modifying these polymers, researchers can design NH with specific properties that meet the requirements of targeted drug delivery and biomedical applications.
Combination Polymers
The improvement of biological properties can be achieved by combining synthetic polymers with natural polymers. One potential approach to enhance the biological characteristics of polyvinyl alcohol (PVA) involves the utilization of cross-linking agents in conjunction with the incorporation of natural polymers.58,62 PLGA-based NH can be used for combination therapy, where multiple drugs or therapeutic agents are encapsulated and released simultaneously, allowing for synergistic effects in cancer treatment. Poly(D, L-lactide-co-glycolide) (PLGA) is a synthetic biodegradable polymer that has been extensively studied and utilized for biomedical applications, including in the treatment of melanoma and other cancers. PLGA is a copolymer of lactic acid and glycolic acid, and its properties can be tailored by adjusting the ratio of lactic acid to glycolic acid as well as the molecular weight of the polymer.57 PLGA NH have shown great promise in melanoma treatment and other cancer therapies. Ongoing research and advancements in nanotechnology are continuously improving the performance and effectiveness of PLGA-based drug delivery systems, providing new hope for better cancer treatments in the future.
Natural Polymers
Hyaluronic acid (HA) is a highly hydrophilic polymer and a major component of the extracellular matrix. In the context of cancer treatment, HA has shown promise due to its ability to target and inhibit overexpressed hyperpigmented components of the extracellular matrix. Studies have shown that HA can target cancer cells by binding to CD44 receptor on their surface. This causes cancer cells to take up HA-based NH, which kill cancer cells.81 Natural polymers like hyaluronic acid hold great potential in nanohydrogel fabrication for cancer treatment and other biomedical applications. Researchers are actively exploring ways to overcome the challenges associated with natural polymers and improve their stability, mechanical properties, and synthesis methods to maximize their effectiveness in drug delivery and other therapeutic approaches. Bacterial-Derived Polymers for Biomedical Applications: Bacterial-derived polymers have gained increasing attention as potential substitutes for conventional polymeric materials used in biomedical applications. These microbial polymers offer several advantages, including non-toxicity, non-immunogenicity, biocompatibility, biodegradability, and competitive processing costs. These characteristics make them ideal candidates for various medical bio-applications.82 The fermentation of bacteria using genetically modified strains is required for the manufacturing of polymers generated from bacteria. This method, which has become a feasible and promising alternative owing to its cost efficiency, includes the fermentation of bacteria. Gelatin is a collagen (COL) product that has only been partly hydrolyzed. The processing of collagen has a substantial influence on the properties of gelatin, including the molecular weight and the isoelectric point of the substance. Collagen may be converted into gelatin by first undergoing pre-treatment with acids, bases, or enzymes, which breaks the bonds that keep the collagen structure stable Gelatin can then be extracted from the collagen. The gelatins that are produced are separated into two distinct types: type A gelatin, which has an isoelectric point in the range of 8–9; type B gelatin, which was produced by treating collagen with a base and has an isoelectric point in the range of 4–5; and type C gelatin, which was produced by treating collagen with an alkaline solution.83 Gelatin has specific properties, and its behavior can be modulated based on its type. The presence of amines in the lysine side chains allows gelatin to attach to the carboxyl groups of surface tissue molecules, making it suitable for various applications in biomedical and pharmaceutical formulations.82,84 As research progresses, microbial-derived polymers like gelatin and other bacterial-derived materials are likely to find even broader use in biomedical applications, as they offer a combination of desirable properties that make them highly attractive for medical bio-applications.
Chitosan-based nanohydrogel: Chitosan is made by partially deacetylating chitin, which is a naturally occurring amino polysaccharide that can be found in many places.85 The nanohydrogel that is based on chitosan is made up of units that are connected to N-acetyl-D-glucosamine and -(1,4) D-glucosamine.86 Chitosan polymers are well known for their biocompatibility and biodegradability, making them attractive candidates for biomedical applications. Advantages of Chitosan-Based Nanohydrogel: biocompatibility and biodistribution, amphiphilic nature, versatility, degradation and properties, processing conditions.87 Chitosan-based NH have shown great promise in various biomedical applications, including drug delivery systems, wound dressings, tissue engineering, and regenerative medicine. Their biocompatibility, ease of modification, and tunable properties make them a valuable material for designing targeted and controlled DDS and other therapeutic applications. Researchers continue to explore and optimize the properties of chitosan-based NH to enhance their performance in various medical and pharmaceutical applications.
Alginate-Based Nanohydrogel: Alginate is a biopolymer obtained from brown algae and is widely used in various biomedical applications. The vast majority of its chemical composition is comprised of two distinct units, namely -L-guluronic acid and -D-mannuronic acid, which are connected to one another by a 1.4-glycosidic bond.88 The advantages associated with alginate-based NH are their biocompatibility, porosity and water retention capabilities, viscosity, and ability to undergo ionic gel formation.25,89 The challenges associated with Alginate-Based Nanohydrogel are closely related to the process of ionic crosslinking and degradation, which has garnered significant attention within the scientific community. In addition, the degradation mechanism of alginate-based NH formed through ionic crosslinking can be described as the unregulated dissolution of the polymer matrix caused by ion depletion.90 To address the aforementioned issues, alginates are frequently utilized in conjunction with other polymers to achieve synergistic effects, hence augmenting their mechanical and biological properties as individual polymers.91 Alginate-based NH have found applications in tissue engineering, drug delivery, wound dressings, and regenerative medicine. Alginate-based NH have a lot of potential to improve biomedical approaches and DDS if the problems are solved by making the right changes and combining them with other polymers.
The cellulose-based nanohydrogel is derived from biopolymers sourced from plants and microbes, which undergo polymerization of D-type glucose monosaccharides. Cellulose-based nanohydrogel has garnered considerable interest as a prospective biomaterial for adsorption, exhibiting numerous advantages in comparison to traditional synthetic adsorbents.92 The utilization of cellulose-based nanohydrogel has several notable advantages. Firstly, it is a cost-effective and readily available material due to its abundance. Additionally, it exhibits biocompatibility and biodegradability, making it suitable for various biomedical applications. Moreover, cellulose-based nanohydrogel is non-toxic, ensuring its safety for use in biological systems. Furthermore, it demonstrates thermal and chemical stability, enhancing its durability under different environmental conditions. Lastly, this nanohydrogel possesses an exceptional adsorption capacity, enabling efficient removal of various substances.93 The fundamental limitation of the nanohydrogel system derived from cellulose is its inability to be utilized in its natural form, mostly due to the abundance of hydroxyl groups present. Nevertheless, the presence of hydroxyl groups, as well as other functional groups like carboxyl and aldehyde groups, enables the formation of cellulose-based hydrogel through several chemical processes.92,93 Cellulose-based NH find applications in various fields, including wastewater treatment, drug delivery, and tissue engineering. The ability to functionalize cellulose enables the development of tailored NH with specific properties, further expanding their potential applications in different industries.
Applications Topical NH in Drug Delivery
DDS for NH in various topical therapies are shown in Table 2.
 |
Table 2 Applications Topical in Drug Delivery |
According to the information in Table 2, these NH offer several advantages for delivering:
Anti-Bacterial Agents
Higher permeability, polymer-based NH show improved permeability, which means they can more effectively penetrate the skin and deliver the active anti-bacterial substances to the targeted areas. Longer release of active substances, these NH exhibit sustained release properties, allowing for a prolonged and controlled delivery of the anti-bacterial agents over time. This sustained release can enhance the effectiveness of the treatment and reduce the need for frequent applications. Enhanced carrier properties, polymer-based NH act as carriers for the anti-bacterial agents, which helps protect the active substances, enhances their stability, and ensures controlled release at the desired site. Stronger antibacterial activity, the combination of improved permeability, longer release, and efficient carrier properties results in a stronger antibacterial activity, making the treatment more effective in combating bacterial infections.32,53,76
Anti-Psoriasis
The NH possess a size that is considered favorable for topical treatments, as it facilitates easy penetration into the skin and enables targeted administration of the anti-psoriasis drugs. pH-dependent swelling refers to a phenomenon in which the rate of swelling of an ingredient is dependent upon the pH level of the surrounding skin. This pH responsiveness can help improve the release of the active substances at the desired site, enhancing their therapeutic efficacy. Effective SC permeation is designed to effectively permeate through the SC, which is the outermost layer of the skin and is typically a major barrier for drug delivery. This quality allows the anti-psoriasis drugs to penetrate the skin to where they are most effective. The NH have improved retention in the skin’s outermost layers (the epidermis and dermis) than conventional hydrogels. This extended retention allows for a more sustained and targeted release of the anti-psoriasis agents, resulting in improved therapeutic effects. Micellar NH for curcumin delivery: micellar NH are used to deliver curcumin, a natural anti-inflammatory agent. The NH help dissolve curcumin, protect it from degradation due to chemical and light exposure, and release it slowly in the form of curcumin-containing micelles. This controlled release mechanism enhances the effectiveness of curcumin for psoriasis treatment. In combination therapy, NH containing both Imiquimod and curcumin are used for anti-psoriasis treatment. This combination therapy shows controlled release of both agents, and their synergistic effects make them more effective together than when used individually.48,55,75
Androgenic Alopecia
The nanohydrogel exhibits a substantial API concentration, with a content of 91.25 ± 0.9%, indicating a significant proportion of the active pharmaceutical ingredient (API). This high API content ensures that a significant amount of the therapeutic agent is present in the formulation, which is essential for effective treatment. Desired physicochemical properties, the nanohydrogel exhibits desired physicochemical properties, which may include stability, biocompatibility, and suitable particle size for topical application. These properties are crucial for ensuring the nanohydrogel’s effectiveness as a drug delivery system. With high cumulative drug release, the cumulative percent drug release from the nanohydrogel over 24 hours is reported to be 758.52 ± 1.49 µg/mL. This indicates that a substantial amount of the API is released from the nanohydrogel within the first 24 hours of application, making it a suitable vehicle for therapy. The high drug release suggests that the nanohydrogel can deliver a sufficient amount of the drug to the target site for effective treatment of androgenic alopecia.94
Anti-Inflammatory
Research evaluation revealed that NH incorporating extracts from cinnamon (Cinnamomum zeylanicum) and clove (Syzygium aromaticum) exhibited notable anti-inflammatory and anti-nociceptive properties. The NH demonstrated a notable reduction in leg edema during the foot edema test, as well as a decrease in nociception observed in both the hot plate and formalin test.82 These results suggest that the NH containing cinnamon and clove extracts can effectively reduce inflammation and pain. Curcumin nanohydrogel containing curcumin and based on glycerol and phosphatidylcholine exhibited linear growth in enthalpy concerning the concentration of the gelling agent. This formulation showed greater anti-inflammatory activity than the positive control, indicating that it can be a potent anti-inflammatory agent.49 Resveratrol and chia seed oil, NH containing resveratrol and chia seed oil demonstrated potential as a drug delivery system for anti-inflammatory agents. These NH effectively crossed the SC barrier to deliver the active substances to their targets. In in vivo studies, they were found to be effective in reducing local macrophage activity and decreasing the level of proinflammatory mediators.77 Piroxicam nanohydrogel delivery was used to deliver piroxicam, an anti-inflammatory drug. These NH showed increased retention of piroxicam in the epidermis, slow absorption, and penetration of the SC, leading to gradual distribution in the epidermis.90 This controlled release mechanism suggests that NH can be used to provide sustained anti-inflammatory effects. Overall, the use of polymer-based NH in anti-inflammatory therapy offers advantages such as targeted drug delivery, sustained release, and enhanced therapeutic effects, making them promising candidates for topical treatments to reduce inflammation and associated pain.
Antiparasitic
The use of nanoemulsion-based NH in antiparasitic therapy has shown significant improvements in potency, stability, and ease of topical application. These NH have demonstrated enhanced leishmanicidal effects, particularly against L. major and L. tropica parasites. In experimental tests, these NH reduced the viability of these parasites to 0%, indicating their strong antiparasitic activity. The NH with a nanometric mesh structure have an additional advantage in preventing the entry of environmental pathogens into the lesion, reducing the risk of secondary infection. This protective barrier provided by the NH can enhance the overall therapeutic efficacy of antiparasitic treatments.80 Overall, nanoemulsion-based NH offer a promising approach in antiparasitic therapy, providing enhanced effectiveness against parasites and minimizing the risk of secondary infections, thereby improving patient outcomes in parasitic infections.
Actinic Keratosis
In the treatment of actinic keratosis, the encapsulation of 5-fluorouracil (5-FU) with lipid vesicles in nanohydrogel tissue systems has shown promising results. This encapsulation technique enhances the local action of the drug on the skin, as it allows for targeted delivery of 5-FU to the affected area. By delivering the drug directly to the site of action, the nanohydrogel system reduces systemic toxicity, minimizing the risk of unwanted side effects in other parts of the body. Moreover, the use of lipid vesicles in the nanohydrogel system improves drug retention in the skin epithelial cells. This sustained release of 5-FU allows for prolonged therapeutic effects, increasing the effectiveness of the treatment for actinic keratosis.78 In general, the encapsulation of 5-FU with lipid vesicles in nanohydrogel tissue systems offers a valuable approach in the management of actinic keratosis. It enhances the drug’s local action, reduces systemic toxicity, and improves drug retention in the skin, making it a promising option for treating this skin condition.
Wound Treatment
In wound treatment, NH offer several benefits that aid in the healing process and promote better wound recovery. The use of Asiatic acid/ZnO/CuO NH has been studied, and their surface morphology shows porosity, which helps in swelling and slow release of active ingredients into the wound system. Additionally, the incorporation of nanoparticles, such as ZnO and CuO, enhances the mechanical strength of the nanohydrogel and provides antibacterial properties, which can help prevent infection and promote wound healing.88 NH used for wound treatment have been found to demonstrate controlled and prolonged drug release. This sustained release of active ingredients helps in accelerating cell migration and promoting dermal fibroblast activity, which are essential for the wound healing process. The nanohydrogel’s ability to influence the expression of genes related to wound healing further contributes to faster and more complete skin recovery.85,89,92,93 Furthermore, the incorporation of the cesium salt of heteropolyphosphotungstic acid in NH has shown biocidal activity at an acidic pH that is non-irritating to human skin. This property helps in inhibiting bacterial growth in the wound area without causing irritation.92 In vivo studies have also revealed that NH used for wound treatment exhibit antioxidant and anti-inflammatory properties, which can further aid in reducing inflammation and promoting the healing process.50 Overall, NH used in wound treatment have demonstrated significant potential in providing controlled drug release, promoting cell migration, and influencing wound healing genes, resulting in improved wound healing quality and better outcomes for patients with various types of wounds.
Dermatophytosis
NH have been investigated for their potential to optimize the administration of antifungal drugs and treat dermatophytosis, a fungal infection of the skin caused by dermatophytes. NH were the subject of one study that looked at their potential use in the treatment of tinea (ringworm) and athlete’s foot (tinea pedis). The sonication method was employed to generate NH, with the preparation time and liquid/solid lipid ratio being modified to get the desired results in terms of nanohydrogel characteristics. Research showed that the zeta potential and effective particle size of the NH were significantly affected by the sonication period and the ratio of liquid to solid lipids. Particle stability and penetration into NH are both affected by particle size and zeta potential, which measures the surface charge of particles. Compared to a positive control (likely referring to a conventional topical antifungal treatment), the NH showed promising results in terms of their zeta potential and particle size. These optimized properties suggest that the NH may have improved drug delivery capabilities, which could lead to enhanced antifungal activity against dermatophytes.95 Patients with dermatophytosis, such as ringworm and athlete’s foot, may benefit from using NH for medication delivery since this may lead to more successful therapy. NH for the treatment of dermatophytosis: further research and clinical investigations are required to determine their effectiveness and safety.
Arthritis
In the context of arthritis treatment, NH have shown promising results in terms of drug release, tissue penetration, and anti-arthritic activity in preclinical studies. One study looked at the possibility of using NH to transport the anti-inflammatory drug diacerein, which is often used to treat rheumatoid arthritis. The NH demonstrated adequate drug release at 24 hours in vitro, indicating that they can release the drug over a sustained period.58 Additionally, ex vivo experiments showed good tissue penetration, suggesting that the NH can effectively reach the target site of inflammation. In animal models of arthritis induced by Freund’s Complete Adjuvant (FCA), the NH exhibited significant anti-arthritic activity. The in vivo results in arthritic rats showed a reduction in the concentration of pro-inflammatory cytokines such as TNF-α, IL-1β, and RF compared to using pure diacerein gel.58 This indicates that the NH have the potential to modulate the inflammatory response associated with arthritis and provide better therapeutic outcomes. Similar positive findings were found in a separate investigation investigating the use of NH for the delivery of berberine chloride. In rat models of arthritis, the nanohydrogel formulation containing berberine chloride reduced leg swelling by up to 24.4% after 12 hours. This suggests that the NH can effectively deliver the drug and alleviate arthritis-related symptoms.57 These findings indicate that NH have the potential to enhance the delivery and efficacy of drugs for arthritis treatment. They can provide sustained drug release, improve tissue penetration, and exhibit significant anti-inflammatory effects in preclinical models. NH may be an effective and safe therapy for arthritis, but further study is needed to confirm this.
Antifungal
The use of NH for antifungal applications has shown promising results in terms of drug release, permeation, and antifungal activity. NH have been found to exhibit a slower and controlled drug release profile, which is beneficial for antifungal therapy as it allows for sustained drug delivery to the target site over an extended period. This controlled release can help maintain effective drug concentrations at the infection site, leading to improved treatment outcomes. In addition, NH have shown significantly increased permeation compared to conventional formulations. This enhanced permeation allows the antifungal drug to penetrate deeper into the affected tissues, reaching the site of infection more effectively. As a result, NH can improve the distribution of the antifungal agent and enhance its overall antifungal activity. Safety is a crucial aspect of any pharmaceutical formulation, and NH have demonstrated safety in irritation studies. This indicates that the NH are well tolerated and do not cause significant irritation or adverse reactions when applied to the skin. Moreover, the antifungal efficacy of NH has been found to be higher than that of control formulations. This suggests that NH can effectively combat fungal infections and provide better outcomes in terms of inhibiting fungal growth and promoting healing.51,79 Overall, the use of NH for antifungal applications offers several advantages, including controlled drug release, improved permeation, safety, and enhanced antifungal activity. These results suggest that NH may be a useful strategy for combating fungal infections. To confirm effectiveness and safety of NH in antifungal treatment for human patients, further research and clinical investigations are required.
Antiaging
NH designed for antiaging applications exhibit a strong and porous macrostructure, which is advantageous for topical formulations targeting aging skin. The porous nature of the nanohydrogel allows for better absorption and retention of active antiaging ingredients, enhancing the overall efficacy of the formulation. The addition of jojoba oil to the nanohydrogel formulation can lead to changes in the morphology of the nanohydrogel. Jojoba oil is known for its moisturizing and skin-nourishing properties, and its incorporation into the nanohydrogel can further enhance its antiaging effects. The combination of jojoba oil with the nanohydrogel matrix may lead to better compatibility and improved skin penetration, resulting in more effective delivery of the active antiaging compounds. Skin permeation studies have demonstrated that NH designed for antiaging purposes exhibit increasing and controlled permeation through the skin. This controlled permeation is essential for antiaging formulations as it allows for a sustained release of the active ingredients, ensuring their continuous presence in the skin for extended periods. The gradual release of antiaging agents can lead to better absorption and long-lasting effects, promoting collagen synthesis, reducing wrinkles, and improving skin elasticity.81 Overall, NH formulated for antiaging applications offer several benefits, including their strong and porous structure, the addition of beneficial ingredients like jojoba oil, and controlled skin permeation. These features contribute to the potential effectiveness of NH in addressing various signs of aging, making them promising candidates for antiaging topical treatments. NH have been shown to have anti-aging characteristics, however further research and clinical tests are required to determine whether they are safe for human consumption.
Additives
In the context of drug delivery to the skin, additives can improve the overall efficacy of the nanohydrogel by facilitating the penetration of active APIs into the skin layers. The presence of certain additives can create a lipophilic environment within the nanohydrogel, which is particularly beneficial for drugs that have lipophilic properties. This lipophilic environment facilitates the solubilization of lipophilic medicines, allowing them to be efficiently absorbed by the deeper skin layers after penetrating the SC. Furthermore, the inclusion of specific additives can improve the drug release and permeation profiles of NH. Controlled and prolonged medication release may result from the additives’ effect on the nanohydrogel’s structure and content. The controlled release profile lessens the possibility of overexposure or quick removal of the medication from the skin by releasing the drug at a pace that corresponds to the targeted therapeutic impact. Moreover, some additives can act as penetration enhancers, facilitating the transport of drugs across the skin barrier and increasing their bioavailability. These enhancers can temporarily alter the skin’s lipid structure, creating micro-channels or pores that enable deeper drug penetration. The use of polymer combinations in nanohydrogel formulations can also be advantageous. Polymer combinations offer synergistic effects, enhancing the overall stability, mechanical properties, and drug-loading capacity of the nanohydrogel. Additionally, in certain cases, the presence of specific polymer combinations can facilitate the formation of NH without the need for additional cross-linking agents, simplifying the preparation process.84,86 In brief, the incorporation of additives, particularly those that promote lipophilic environments, controlled drug release, and improved permeation profiles, can significantly enhance the performance of NH as DDS for skin applications. By carefully selecting appropriate additives and optimizing their concentrations, researchers can design NH with superior drug delivery capabilities, making them promising candidates for topical drug delivery and related therapeutic applications that play a crucial role in enhancing the performance and properties of NH for drug delivery applications. By incorporating specific additives, NH can achieve improved drug penetration into the skin, enhanced drug release, and better permeation profiles, making them optimal systems for delivering drugs into the skin.
Skin Cancer
In the context of skin cancer, NH have shown promising potential as DDS for the treatment of skin cancer. Two specific examples involve the use of 5-fluorouracil (5-FU) and green tea leaf nanohydrogel for anti-cancer therapy. Several studies have demonstrated that 5-FU can be incorporated into NH to improve its stability and enhance its retention in the skin layer. Compared to a positive control, the nanohydrogel formulation of 5-FU showed similar stability, indicating that the drug remains effective. However, its retention in the skin layer was found to be 4–5 times higher, likely due to the nanohydrogel’s ability to loosen the epidermal horny layer. This enhanced retention can lead to a prolonged and sustained release of 5-FU, allowing for better drug penetration and treatment efficacy. Moreover, histopathological evaluation of the nanohydrogel demonstrated cationically charged chitin interactions without causing inflammation, indicating its biocompatibility and potential for skin cancer treatment.87 Green tea leaf nanohydrogel has been shown to possess desirable characteristics such as pH, viscosity, spreadability, and permeability, making it a promising candidate for anti-cancer therapy. In in vivo animal models, the green tea leaf nanohydrogel exhibited effectiveness in inhibiting cancer growth. This indicates that the nanohydrogel formulation can deliver the active compounds from green tea leaves effectively to the target site, potentially leading to positive therapeutic outcomes in skin cancer treatment.96 Furthermore, another approach involves the use of thermosensitive NH for delivering anti-cancer drugs like paclitaxel and temozolomide. The nanohydrogel formulations of these drugs are thermosensitive, meaning they respond to changes in temperature. The presence of an anhydrous absorbent ointment base allows for slow and controlled release of the drugs over an extended period. This controlled release profile enables longer penetration of the drugs into the skin, facilitating effective drug delivery and potentially improving their anti-cancer activity.62 Overall, NH show promise in enhancing drug stability, improving drug retention in the skin, and promoting effective drug delivery for the treatment of skin cancer. These advancements in nanotechnology hold significant potential for developing more targeted and efficient therapies for skin cancer patients. However, further research and clinical studies are needed to validate and optimize these nanohydrogel-based DDS for skin cancer treatment.
Hyperpigmentation
In the context of hyperpigmentation therapy, NH have demonstrated promising potential for effective topical delivery of active compounds to melanocytes. In vivo studies have shown that these nanohydrogel formulations achieve an ideal balance between permeability and minimized epidermal diffusion, allowing targeted delivery to the melanocytes while reducing unwanted spread to other skin layers. One of the key mechanisms of action in hyperpigmentation therapy involves the inhibition of tyrosinase activity and melanin production. Tyrosinase is an enzyme involved in the production of melanin, which is responsible for skin pigmentation. By inhibiting tyrosinase activity, the formation of melanin is reduced, leading to lightening or fading of hyperpigmented areas. The nanohydrogel-based formulations have been shown to effectively inhibit tyrosinase activity and melanin production, particularly under conditions of UV B radiation. This is crucial in hyperpigmentation therapy, as UV B radiation can trigger melanin production and worsen hyperpigmentation. By targeting tyrosinase and melanin production, the nanohydrogel formulations offer a potential solution for managing hyperpigmentation.61 Furthermore, the localized and controlled drug release characteristics of NH can contribute to the therapeutic efficacy of hyperpigmentation therapy. The NH allow for sustained release of active compounds, ensuring prolonged exposure to the target cells, such as melanocytes, and maximizing the treatment’s effectiveness. Overall, NH hold promise as a novel approach for hyperpigmentation therapy. Their ability to deliver active compounds efficiently to melanocytes, inhibit tyrosinase activity, and minimize epidermal diffusion makes them a potential candidate for treating hyperpigmentation disorders. However, like any emerging therapy, further research, and clinical trials are required to fully validate and optimize the use of NH in hyperpigmentation treatment.
Challenges and Future Perspectives
The future perspective of NH in drug delivery is highly promising and holds great potential for addressing various clinical challenges associated with topical therapy and conventional drug formulations. Nanosystems, including NH, offer the advantage of deeper tissue penetration, allowing for targeted treatment of deeper layers of the skin, even in hair follicles.38,73,98
NH represent a novel drug delivery technology that can overcome issues such as drug resistance, toxicity-related concerns, and targeted cellular absorption. They can be designed to respond to specific physiological cues of the skin, such as pH or temperature gradients in healthy or diseased skin. However, researchers should explore a wider range of stimuli, such as the redox potential of skin cells, to enhance the versatility and effectiveness of NH for different skin conditions and pathologies. The lack of stability testing in published studies might hinder the general development of nanohydrogel. Therefore, it is crucial for researchers to carry out stability investigations, including both short-term and long-term timeframes.
To ensure the clinical applicability of NH, considerations about toxicity, biocompatibility, and biodegradability are of utmost importance. Natural polymers offer an interesting avenue to address these concerns, and their modification to be responsive to pH or temperature could provide additional benefits. NH offer several unique features, including stability, biocompatibility, and stimulus responsiveness, making them a promising choice for drug delivery. Their capacity to transport various biomolecules with different dimensions and high encapsulation efficiency enhances their potential as effective nanocarriers. However, while preclinical data is encouraging, more conclusive studies are needed to validate the potential of NH through in vitro and in vivo evaluations. Researchers should address concerns related to toxicity, immunogenicity, pharmacokinetic differences between animal and human models, and regulatory challenges to pave the way for successful clinical translation. Apart from that, future challenges for large-scale production will likely experience obstacles due to quite expensive equipment and high production and quality control costs.
Hybridizing natural and synthetic polymers to form NH is a common approach that offers the advantages of both polymer types while compensating for their individual limitations. Future research should focus on shifting from preclinical investigations to clinical trials to gain a better understanding of nanohydrogel efficacy and safety in human subjects. NH hold tremendous promise as the next generation of DDS for topical therapy and beyond. Their potential to improve quality of life, address clinical challenges, and enhance treatment outcomes makes them a topic of great interest for future research and clinical application. As scientists continue to explore and optimize NH, nanotechnology is expected to play an increasingly prominent role in medical applications.
Conclusion
Recent advancements in topical drug delivery have deepened our understanding of skin structure and permeation pathways, highlighting the need for targeted and noninvasive therapeutic strategies. The challenge lies in efficiently delivering drugs through the skin’s barrier to specific areas with high control. Environmentally sensitive systems, like polymer-based NH, show promise in treating dermatological conditions. Polymers are pivotal in developing these drug delivery systems, with NH offering advantages such as versatile drug loading, controlled release, and enhanced skin penetration. Many nanohydrogel formulations have proven to be more effective than traditional dosage forms. However, further research is needed to optimize nanohydrogel performance for topical drug delivery. Focus areas include improving drug loading, release kinetics, skin penetration, and overall therapeutic efficacy, such as long-term efficacy and tolerability. Analytical methods, including in vitro, ex vivo, and in vivo studies, are crucial in demonstrating NH’s effectiveness and safety. The potential of NH as a drug delivery platform for topical applications is significant, and ongoing innovation holds promise for improving treatment outcomes in various skin disorders. Continued research and development could position NH as an essential component of the pharmaceutical sciences. Therefore, it is important to overcome these issues in future studies, which can eventually optimize the existing NH and contribute to the clinical approval of these products.
Acknowledgments
We would like to thank The Rector of Universitas Padjadjaran for the APC.
Funding
This study was funded by the review article grant 2024 from Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jiménez-Rosado P, Romero M. Drug transport pathways across skin. Polymers. 2022;2022:3023.
2. Shah P, Singh M. Enhanced skin permeation using polyarginine modified nanostructured lipid carriers. J Control Release. 2012;161(3). 735–745. doi:10.1016/j.jconrel.2012.05.011
3. Shah PP, Desai PR, Patel AR, Singh MS. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials. 2012;33(5):1607–1617. doi:10.1016/j.biomaterials.2011.11.011
4. Somagoni J, Boakye CHA, Godugu C, et al. Nanomiemgel - A novel drug delivery system for topical application - In vitro and in vivo evaluation. PLoS One. 2014;9(12). doi:10.1371/journal.pone.0115952
5. Chouhan C, Rajput RPS, Sahu R, Verma P, Sahu S. An updated review on nanoparticle based approach for nanogel drug delivery system. J Drug Delivery Ther. 2020;10(5–s):254–266. doi:10.22270/jddt.v10i5-s.4465
6. Joglekar M, Trewyn BG. Polymer-based stimuli-responsive nanosystems for biomedical applications. Biotechnol J. 2013;8(8):931–945. doi:10.1002/biot.201300073
7. Parhi R, Sahoo SK, Das A. Applications of polysaccharides in topical and transdermal drug delivery: A recent update of literature Braz. J. Pharm. Sci. 2023;58(e20802):1–38. doi:10.1590/s2175-97902022e20802
8. Wang W, Lu KJ, Yu CH, Huang QL, Du YZ. Nano-DDS in wound treatment and skin regeneration. J Nanobiotechnology. 2019;17(1). doi:10.1186/s12951-019-0514-y
9. Bashir MH, Korany NS, Farag DBE, et al. Polymeric nanocomposite hydrogel scaffolds in craniofacial bone regeneration: a comprehensive review. Biomolecules. 2023;13(2). doi:10.3390/biom13020205
10. Álvarez-Bautista A, Duarte CMM, Mendizábal E, Katime I. Controlled delivery of drugs through smart pH-sensitive NH for anti-cancer therapies: synthesis, drug release and cellular studies. Des Monomers Polym. 2016;19(4):319–329. doi:10.1080/15685551.2016.1152542
11. Quazi MZ, Park N. NH: advanced polymeric nanomaterials in the era of nanotechnology for robust functionalization and cumulative applications. Int J Mol Sci. 2022;23(4). doi:10.3390/ijms23041943
12. Asadi H, Rostamizadeh K, Salari D, Hamidi M. Preparation and characterization of tri-block poly(lactide)-poly(ethylene glycol)-poly(lactide) nanogels for controlled release of naltrexone. Int J Pharm. 2011;416(1):356–364. doi:10.1016/j.ijpharm.2011.06.035
13. Suhandi C, Wilar G, Lesmana R, et al. Propolis-based nanostructured lipid carriers for α-mangostin delivery: formulation, characterization and in vitro antioxidant activity evaluation. Molecules. 2023;28(16):6057. doi:10.3390/molecules28166057
14. Lu S, Lu S, Fan X, et al. Synthesis of gelatin-based dual-targeted nanoparticles of betulinic acid for antitumor therapy. ACS Appl Bio Mater. 2020;3(6):3518–3525. doi:10.1021/acsabm.9b01204
15. Khoee S. Nanogels: Chemical Approaches to Preparation. Vol. 27. CRC Press; 2016.
16. Zhang S, Ul M SSA, Basharat K, et al. Silk-based nano-hydrogels for futuristic biomedical applications. J Drug Deliv Sci Technol. 2022:72. doi:10.1016/j.jddst.2022.103385
17. Sood N, Bhardwaj A, Mehta S, Mehta A. Stimuli-responsive hydrogels in drug delivery and tissue engineering. Drug Deliv. 2016;23(3):758–780. doi:10.3109/10717544.2014.940091
18. Soni KS, Desale SS, Bronich TK. Nanogels: an overview of properties, biomedical applications and obstacles to clinical translation. J Control Release. 2016;240:109–126. doi:10.1016/j.jconrel.2015.11.009
19. Hajebi S, Rabiee N, Bagherzadeh M, et al. Stimulus-responsive polymeric nanogels as smart drug delivery systems. Acta Biomater. 2019;92:1–18. doi:10.1016/j.actbio.2019.05.018
20. Abd El-Rehim HA, Hegazy ESA, Hamed AA, Swilem AE. Controlling the size and swellability of stimuli-responsive polyvinylpyrrolidone-poly(acrylic acid) nanogels synthesized by gamma radiation-induced template polymerization. Eur Polym J. 2013;49(3):601–612. doi:10.1016/j.eurpolymj.2012.12.002
21. Sosnik A, Seremeta KP. Polymeric hydrogels as technology platform for drug delivery applications. Gels. 2017;3(3). doi:10.3390/gels3030025
22. Kousalová J, Etrych T. Polymeric nanogels as drug delivery systems. Physiol Res. 2018;67:s305–s317. doi:10.33549/physiolres.933979
23. Suhandi C, Mohammed AFA, Wilar G, El-Rayyes A, Wathoni N. Effectiveness of mesenchymal stem cell secretome on wound healing a systematic review and meta-analysis tissue. Eng Regen Med. 2023;20(7):1053–1062. doi:10.1007/s13770-023-00570-9
24. Thummarati P, Suksiriworapong J, Sakchaisri K, Junyaprasert VB. Effect of chemical linkers of curcumin conjugated hyaluronic acid on nanoparticle properties and in vitro performances in various cancer cells. J Drug Deliv Sci Technol. 2021;61. doi:10.1016/j.jddst.2021.102323
25. Sohail R, Abbas SR. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int J Biol Macromol. 2020;153:36–45. doi:10.1016/j.ijbiomac.2020.02.191
26. Saroia J, Yanen W, Wei Q, Zhang K, Lu T, Zhang B. A review on biocompatibility nature of hydrogels with 3D printing techniques, tissue engineering application and its future prospective. Bio-Design Manuf. 2018;1(4):265–279. doi:10.1007/s42242-018-0029-7
27. Zschocke I, Mrowietz U, Lotzin A, Karakasili E, Reich K. Assessing adherence factors in patients under topical treatment: development of the Topical Therapy Adherence Questionnaire (TTAQ). Arch Dermatol Res. 2014;306(3):287–297. doi:10.1007/s00403-014-1446-x
28. Makhathini SS, Mdanda S, Kondiah PJ, et al. Biomedicine innovations and its nanohydrogel classifications. Pharmaceutics. 2022;14(12). doi:10.3390/pharmaceutics14122839
29. Setia A, Ahuja P. Organic Materials as Smart Nanocarriers for Drug Delivery. Elsevier; 2018:293–368. doi:10.1016/B978-0-12-813663-8.00008-7
30. Zhang X, Wei P, Yang Z, et al. Current progress and outlook of nano-based hydrogel dressings for wound healing. Pharmaceutics. 2023;15(1). doi:10.3390/pharmaceutics15010068
31. Dalwadi C, Patel G Send orders for reprints to [email protected] recent patents application of nh in drug delivery systems: recent patents review. vol 9; 2015.
32. Li C, Obireddy SR, Lai WF. Preparation and use of nanogels as carriers of drugs. Drug Deliv. 2021;28(1):1594–1602. doi:10.1080/10717544.2021.1955042
33. Qureshi MA, Khatoon F. Different types of smart nanogel for targeted delivery. J Sci Adv Mater Devices. 2019;4(2):201–212. doi:10.1016/j.jsamd.2019.04.004
34. Yuniarsih N, Hidayah H, Gunarti NS, et al. Evaluation of wound-healing activity of hydrogel extract of sansevieria trifasciata leaves (Asparagaceae). Adv Pharmacol Pharm Sci. 2023;2023. doi:10.1155/2023/7680518
35. Roberts MS, Mohammed Y, Pastore MN, et al. Topical and cutaneous delivery using nanosystems. J Control Release. 2017;247:86–105. doi:10.1016/j.jconrel.2016.12.022
36. Galliano MF, Tfayli A, Dauskardt RH, et al. Comprehensive characterization of the structure and properties of human SC relating to barrier function and skin hydration: modulation by a moisturizer formulation. Exp Dermatol. 2021;30(9):1352–1357. doi:10.1111/exd.14331
37. Goyal R, Macri LK, Kaplan HM, Mbbc F, Kohn J. Nanoparticles and Nanofibers for Topical Drug Delivery; 2015. Availabe from: http://www.elsevier.com/open-access/userlicense/1.0/.
38. Chandrashekha BS, Anitha M, Ruparelia M, et al. Tretinoin nanogel 0.025% versus conventional gel 0.025% in patients with acne vulgaris: a randomized, active controlled, multicentre, parallel group, Phase IV clinical trial. J Clin Diagn Res. 2015;9(1):WC04–WC09. doi:10.7860/JCDR/2015/10663.5469
39. Kaspar D, Linder J, Zöllner P, Simon U, Smola H. Economic benefit of a polyacrylate-based hydrogel compared to an amorphous hydrogel in wound bed preparation of venous leg ulcers. Chronic Wound Care Manag Res. 2015;63. doi:10.2147/cwcmr.s78580
40. Kaur L, Jain SK, Manhas RK, Sharma D. Nanoethosomal formulation for skin targeting of amphotericin B: an in vitro and in vivo assessment. J Liposome Res. 2015;25(4):294–307. doi:10.3109/08982104.2014.995670
41. Vyumvuhore R, Tfayli A, Biniek K, et al. The relationship between water loss, mechanical stress, and molecular structure of human SC ex vivo. J Biophotonics. 2015;8(3):217–225. doi:10.1002/jbio.201300169
42. Roberts MS, Cheruvu HS, Mangion SE, et al. Topical drug delivery: history, percutaneous absorption, and product development. Adv Drug Deliv Rev. 2021:177. doi:10.1016/j.addr.2021.113929
43. Raza K, Kumar M, Kumar P, et al. Topical delivery of aceclofenac: challenges and promises of novel drug delivery systems. Biomed Res Int. 2014;2014. doi:10.1155/2014/406731
44. Raina N, Rani R, Thakur VK, Gupta M. New insights in topical drug delivery for skin disorders: from a nanotechnological perspective. ACS Omega. 2023;8(22):19145–19167. doi:10.1021/acsomega.2c08016
45. Teng Y, Li S, Tang H, Tao X, Fan Y, Huang Y. Medical applications of hydrogels in skin infections: a review. Infect Drug Resist. 2023;16:391–401. doi:10.2147/IDR.S396990
46. Radulescu DM, Neacsu IA, Grumezescu AM, Andronescu E. New insights of scaffolds based on hydrogels in tissue engineering. Polymers. 2022;14(4). doi:10.3390/polym14040799
47. Bustamante-Torres M, Romero-Fierro D, Arcentales-Vera B, Palomino K, Magaña H, Bucio E. Hydrogels classification according to the physical or chemical interactions and as stimuli-sensitive materials. Gels. 2021;7(4). doi:10.3390/gels7040182
48. Panthi VK, Jha SK, Pangeni R, Paudel KR. Formulation and development of adapalene topical nanohydrogel using different surfactants and cosurfactants for antiacne activity: in vitro and ex vivo evaluation. J Nanomater. 2022;2022. doi:10.1155/2022/6889293
49. Algahtani MS, Ahmad MZ, Nourein IH, Ahmad J. Co-delivery of imiquimod and curcumin by nanoemugel for improved topical delivery and reduced psoriasis-like skin lesions. Biomolecules. 2020;10(7):1–19. doi:10.3390/biom10070968
50. Andrabi SM, Majumder S, Gupta KC, Kumar A. Dextran based amphiphilic nano-hybrid hydrogel system incorporated with curcumin and cerium oxide nanoparticles for wound healing. Colloids Surf B Biointerfaces. 2020;195. doi:10.1016/j.colsurfb.2020.111263
51. Zhou L, Zhao X, Li M, et al. Antibacterial and wound healing–promoting effect of sponge-like chitosan-loaded silver nanoparticles biosynthesized by iturin. Int J Biol Macromol. 2021;181:1183–1195. doi:10.1016/j.ijbiomac.2021.04.119
52. Piva RH, Rocha MC, Piva DH, Imasato H, Malavazi I, Rodrigues-Filho UP. Acidic dressing based on agarose/Cs2.5H0.5PW12O40 nanocomposite for infection control in wound care. ACS Appl Mater Interfaces. 2018;10(37):30963–30972. doi:10.1021/acsami.8b09066
53. Alshehri S, Imam SS. Formulation and evaluation of butenafine loaded PLGA-nanoparticulate laden chitosan nano gel. Drug Deliv. 2021;28(1):2348–2360. doi:10.1080/10717544.2021.1995078
54. Liu Y, Han Y, Zhu T, et al. Targeting delivery and minimizing epidermal diffusion of tranexamic acid by hyaluronic acid-coated liposome nanogels for topical hyperpigmentation treatment. Drug Deliv. 2021;28(1):2100–2107. doi:10.1080/10717544.2021.1983081
55. Qureshi M, Qadir A, Aqil M, et al. Berberine loaded dermal quality by design adapted chemically engineered lipid nano-constructs-gel formulation for the treatment of skin acne. J Drug Deliv Sci Technol. 2021:66. doi:10.1016/j.jddst.2021.102805
56. Stocke NA, Zhang X, Hilt JZ, DeRouchey JE. Transport in PEG-based hydrogels: role of water content at synthesis and crosslinker molecular weight. Macromol Chem Phys. 2017;218(3). doi:10.1002/macp.201600340
57. Puri V, Froelich A, Shah P, Pringle S, Chen K, Michniak-Kohn B. Quality by design guided development of polymeric nanospheres of terbinafine hydrochloride for topical treatment of onychomycosis using a nano-gel formulation. Pharmaceutics. 2022;14(10). doi:10.3390/pharmaceutics14102170
58. Afzal O, Altamimi ASA, Alamri MA, et al. Resveratrol-loaded chia seed oil-based nanogel as an anti-inflammatory in adjuvant-induced arthritis. Gels. 2023;9(2). doi:10.3390/gels9020131
59. Sabel-Grau T, Tyushina A, Babalik C, Lensen MC. UV-VIS curable PEG hydrogels for biomedical applications with multifunctionality. Gels. 2022;8(3). doi:10.3390/gels8030164
60. Ismail SH, Hamdy A, Ismail TA, Mahboub HH, Mahmoud WH, Daoush WM. Synthesis and characterization of antibacterial carbopol/ZnO hybrid nanoparticles Gel. Crystals. 2021;11(9). doi:10.3390/cryst11091092
61. Sindhu RK, Gupta R, Wadhera G, Kumar P. Modern herbal nanogels: formulation, delivery methods, and applications. Gels. 2022;8(2). doi:10.3390/gels8020097
62. Rajagopalan R, Jain SK, Kaul A, Trivedi P. Biodistribution and pharmacokinetic studies on topically-delivered technetium-99m-labeled 5-FU nanogel formulation for management of pre-cancerous skin lesions. Trop J Pharm Res. 2019;18(9):1977–1983. doi:10.4314/tjpr.v18i9.28
63. Yang L, Zhang L, Hu J, Wang W, Liu X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with miRNA-laden nanoparticles for chronic diabetic wound treatment. Int J Biol Macromol. 2021;166:166–178. doi:10.1016/j.ijbiomac.2020.10.129
64. Ghosh S, Lahiri D, Nag M, et al. Bacterial biopolymer: its role in pathogenesis to effective biomaterials. Polymers. 2021;13:8. doi:10.3390/polym13081242
65. Mahmood A, Patel D, Hickson B, Desrochers J, Hu X. Recent progress in biopolymer-based hydrogel materials for biomedical applications. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031415
66. Salahuddin B, Wang S, Sangian D, Aziz S, Gu Q. Hybrid gelatin hydrogels in nanomedicine applications. ACS Appl Bio Mater. 2021;4(4):2886–2906. doi:10.1021/acsabm.0c01630
67. Águila-Almanza E, Low SS, Hernández-Cocoletzi H, et al. Facile and green approach in managing sand crab carapace biowaste for obtention of high deacetylation percentage chitosan. J Environ Chem Eng. 2021;9(3). doi:10.1016/j.jece.2021.105229
68. Luckanagul JA, Pitakchatwong C, Ratnatilaka Na Bhuket P, et al. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr Polym. 2018;181:1119–1127. doi:10.1016/j.carbpol.2017.11.027
69. Sánchez-Cid P, Jiménez-Rosado M, Alonso-González M, Romero A, Perez-Puyana V. Applied rheology as tool for the assessment of chitosan hydrogels for regenerative medicine. Polymers. 2021;13(13). doi:10.3390/polym13132189
70. Liu W, Madry H, Cucchiarini M. Application of alginate hydrogels for next‐generation articular cartilage regeneration. Int J Mol Sci. 2022;23(3). doi:10.3390/ijms23031147
71. Zhang H, Cheng J, Ao Q, et al. Marine drugs preparation of alginate-based biomaterials and their applications in biomedicine; 2021.
72. Kharkar PM, Kiick KL, Kloxin AM. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem Soc Rev. 2013;42(17):7335–7372. doi:10.1039/c3cs60040h
73. Ahmad Raus R, Wan Nawawi WMF, Nasaruddin RR. Alginate and alginate composites for biomedical applications. Asian J Pharm Sci. 2021;16(3):280–306. doi:10.1016/j.ajps.2020.10.001
74. Bao Y, He J, Song K, Guo J, Zhou X, Liu S. Functionalization and antibacterial applications of cellulose-based composite hydrogels. Polymers. 2022;14(4). doi:10.3390/polym14040769
75. Kundu R, Mahada P, Chhirang B, Das B. Cellulose hydrogels: green and sustainable soft biomaterials. Curr Res Green Sustain Chem. 2022;5. doi:10.1016/j.crgsc.2021.100252
76. Filippone A, Consoli GML, Granata G, et al. Topical delivery of curcumin by choline-calix[4]arene-based nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int J Mol Sci. 2020;21(14):1–15. doi:10.3390/ijms21145053
77. Ermawati DE, Yugatama A, Ramadhani BR, Pertiwi I, Rosikhoh A, Novachiria SR. Stability And Antibacterial Activity Test Of Nanosilver Biosynthetic Hydrogel. Int J Appl Pharm. 2022;14(2):221–226. doi:10.22159/ijap.2022v14i2.43584
78. González-Ortega LA, Acosta-Osorio AA, Grube-Pagola P, et al. Anti-inflammatory activity of curcumin in gel carriers on mice with atrial edema. J Oleo Sci. 2020;69(2):123–131. doi:10.5650/jos.ess19212
79. Kesharwani D, Das Paul S, Paliwal R, Satapathy T. Exploring potential of diacerin nanogel for topical application in arthritis: formulation development, QbD based optimization and pre-clinical evaluation. Colloids Surf B Biointerfaces. 2023;223. doi:10.1016/j.colsurfb.2023.113160
80. Rahman S, Haque TN, Sugandhi VV, Saraswat AL, Xin X, Cho H. Topical cream carrying drug-loaded nanogels for melanoma treatment. Pharm Res. 2023. doi:10.1007/s11095-023-03506-z
81. Ghanbariasad A, Amoozegar F, Rahmani M, Zarenezhad E, Osanloo M. Impregnated nanofibrous mat with nanogel of citrus sinensis essential oil as a new type of dressing in cutaneous leishmaniasis. Biointerface Res Appl Chem. 2021;11(4):11066–11076. doi:10.33263/BRIAC114.1106611076
82. Elkomy MH, Alruwaili NK, Elmowafy M, et al. Surface-modified bilosomes nanogel bearing a natural plant alkaloid for safe management of rheumatoid arthritis inflammation. Pharmaceutics. 2022;14(3). doi:10.3390/pharmaceutics14030563
83. Basto R, Andrade R, Nunes C, Lima SAC, Reis S pharmaceutics topical delivery of niacinamide to skin using hybrid nanogels enhances photoprotection effect. 2021.
84. Esmaeili F, Zahmatkeshan M, Yousefpoor Y, Alipanah H, Safari E, Osanloo M. Anti-inflammatory and anti-nociceptive effects of Cinnamon and Clove essential oils nanogels: an in vivo study. BMC Complement Med Ther. 2022;22(1). doi:10.1186/s12906-022-03619-9
85. Pan X, Kong D, Wang W, et al. Synthetic polymeric antibacterial hydrogel for methicillin-resistant staphylococcus aureus-infected wound healing: nanoantimicrobial self-assembly, drug- And cytokine-free strategy. ACS Nano. 2020;14(10):12905–12917. doi:10.1021/acsnano.0c03855
86. Cirri M, Nerli G, Mennini N, Maestrelli F, Mura P. Development and characterization of cyclodextrin-based nanogels as a new ibuprofen cutaneous delivery system. Pharmaceutics. 2022;14(12). doi:10.3390/pharmaceutics14122567
87. Loo Y, Wong YC, Cai EZ, et al. Ultrashort peptide nanofibrous hydrogels for the acceleration of healing of burn wounds. Biomaterials. 2014;35(17):4805–4814. doi:10.1016/j.biomaterials.2014.02.047
88. Cai XJ, Mesquida P, Jones SA. Investigating the ability of nanoparticle-loaded hydroxypropyl methylcellulose and xanthan gum gels to enhance drug penetration into the skin. Int J Pharm. 2016;513(1–2):302–308. doi:10.1016/j.ijpharm.2016.08.055
89. Sabitha M, Sanoj Rejinold N, Nair A, Lakshmanan VK, Nair SV, Jayakumar R. Development and evaluation of 5-fluorouracil loaded chitin nanogels for treatment of skin cancer. Carbohydr Polym. 2013;91(1):48–57. doi:10.1016/j.carbpol.2012.07.060
90. Thanusha AV, Dinda AK, Koul V. Evaluation of nano hydrogel composite based on gelatin/HA/CS suffused with Asiatic acid/ZnO and CuO nanoparticles for second degree burns. Mater Sci Eng C. 2018;89:378–386. doi:10.1016/j.msec.2018.03.034
91. Manconi M, Manca ML, Caddeo C, et al. Preparation of gellan-cholesterol NH embedding baicalin and evaluation of their wound healing activity. Eur J Pharm Biopharm. 2018;127:244–249. doi:10.1016/j.ejpb.2018.02.015
92. Musazzi UM, Cencetti C, Franzé S, et al. Gellan NH: novel nanodelivery systems for cutaneous administration of piroxicam. Mol Pharm. 2018;15(3):1028–1036. doi:10.1021/acs.molpharmaceut.7b00926
93. Xi Loh EY, Fauzi MB, Ng MH, et al. Cellular and molecular interaction of human dermal fibroblasts with bacterial nanocellulose composite hydrogel for tissue regeneration. ACS Appl Mater Interfaces. 2018;10(46):39532–39543. doi:10.1021/acsami.8b16645
94. Sanganabhatla D, Sunder RS. A novel nanogel formulation of finasteride for topical treatment of androgenetic alopecia: design, characterization and in vitro evaluation. Int J Appl Pharm. 2021;13(4):228–240. doi:10.22159/ijap.2021v13i4.41599
95. Ahmed OAA, Rizq WY. Finasteride nano-transferosomal gel formula for management of androgenetic alopecia: ex vivo investigational approach. Drug Des Devel Ther. 2018;12:2259–2265. doi:10.2147/DDDT.S171888
96. Singh S, Sonia S. Formulation development and investigations on therapeutic potential of nanogel from beta vulgaris l. extract in testosterone-induced alopecia. Biomed Res Int. 2023;2023. doi:10.1155/2023/1777631
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.