Back to Journals » Journal of Inflammation Research » Volume 16
Polarization Behavior of Bone Macrophage as Well as Associated Osteoimmunity in Glucocorticoid-Induced Osteonecrosis of the Femoral Head
Authors Zhang Q , Sun W, Li T, Liu F
Received 29 December 2022
Accepted for publication 24 February 2023
Published 28 February 2023 Volume 2023:16 Pages 879—894
DOI https://doi.org/10.2147/JIR.S401968
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Adam D Bachstetter
Video abstract of "Polarization behavior of bone macrophage" [ID 401968].
Views: 74
Qingyu Zhang,1 Wei Sun,2,3 Tengqi Li,4,5 Fanxiao Liu1
1Department of Orthopaedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China; 2Department of Orthopaedic Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA; 3Centre for Osteonecrosis and Joint-Preserving & Reconstruction, Orthopaedic Department, China Japan Friendship Hospital, Beijing, 100029, People’s Republic of China; 4Department of Orthopedics, Peking University Shougang Hospital, Beijing, People’s Republic of China; 5Department of Orthopedics, Peking University China-Japan Friendship School of Clinical Medicine, Beijing, People’s Republic of China
Correspondence: Fanxiao Liu, Department of Orthopedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, Shandong, People’s Republic of China, Tel/Fax +86-531-68773195, Email [email protected]
Abstract: Glucocorticoid-induced osteonecrosis of the femoral head (GIONFH) is a disabling disease with high mortality in China but the detailed molecular and cellular mechanisms remain to be investigated. Macrophages are considered the key cells in osteoimmunology, and the cross-talk between bone macrophages and other cells in the microenvironment is involved in maintaining bone homeostasis. M1 polarized macrophages launch a chronic inflammatory response and secrete a broad spectrum of cytokines (eg, TNF-α, IL-6 and IL-1β) and chemokines to initiate a chronic inflammatory state in GIONFH. M2 macrophage is the alternatively activated anti-inflammatory type distributed mainly in the perivascular area of the necrotic femoral head. In the development of GIONFH, injured bone vascular endothelial cells and necrotic bone activate the TLR4/NF-κB signal pathway, promote dimerization of PKM2 and subsequently enhance the production of HIF-1, inducing metabolic transformation of macrophage to the M1 phenotype. Considering these findings, putative interventions by local chemokine regulation to correct the imbalance between M1/M2 polarized macrophages by switching macrophages to an M2 phenotype, or inhibiting the adoption of an M1 phenotype appear to be plausible regimens for preventing or intervening GIONFH in the early stage. However, these results were mainly obtained by in vitro tissue or experimental animal model. Further studies to completely elucidate the alterations of the M1/M2 macrophage polarization and functions of macrophages in glucocorticoid-induced osteonecrosis of the femoral head are imperative.
Keywords: osteonecrosis, macrophage polarization, osteoimmunity, osteoclast differentiation, osteogenesis, glucocorticoid
Introduction
Osteonecrosis of the femoral head (ONFH) is a debilitating disease characterized by the disrupted blood supply to a specific region of the femoral head which creates a hypoxic environment and leads to progressive deterioration of the hip joint.1 ONFH usually violates young and middle-aged subjects, leading to a high financial burden for individuals and health-care systems.2 Currently, the prevalence of non-traumatic ONFH is expected to keep rising because of heightened awareness and increased adjuvant therapy.1,2 Synthetic glucocorticoids (eg, dexamethasone, beclomethasone and prednisolone) have been widely used in the treatment of inflammatory, allergic and autoimmune diseases but also rank as the leading reason for non-traumatic ONFH in China, accounting for 26.35% of male cases and 55.75% of female cases.3 Glucocorticoid-induced ONFH (GIONFH) develops in 9–40% of patients receiving long-term therapy and it may also occur with short-term exposure to high doses or after intra-articular injection.4
A variety of hypotheses for the pathogenesis of GIONFH have been proposed, such as dysregulation of intraosseous hypertension, lipid metabolism disorder, intravascular coagulation, and arterial vasculitis, but the exact mechanism is yet to be confirmed.1 The primary histopathological character of ONFH is osteocyte apoptosis, which persists due to the anatomical unavailability of phagocytosis.4 Glucocorticoid excess results in bone marrow mesenchymal stem cells (BMSCs) and osteoblasts toward a higher rate of adipogenesis, retards osteocyte replacement and decreases bone remodeling.2 Meanwhile, glucocorticoids could damage the intraosseous microvasculature and improve the formation of osteoclasts.5 The cumulative and unrepairable bone defect uniquely breaks the mechanosensory function of the osteocyte-lacunar-canalicular system.4 Another distinctive feature of GIONFH is abnormal chronic inflammation.6 Acute inflammation is the first step in bone regeneration, but if the adverse stimuli persist and disrupt bone homeostasis, chronic inflammation occurs.7 In the context of chronic inflammation, the necrotic bone continuously undergoes fibrosis repair, which hinders the reconstruction of normal bone structure and function.8
Complex interactions existed between bone and immune cells (eg, macrophages, Th17 cells, and neutrophils).9 Immune cells and their products could influence the activities of osteoclasts, osteoblasts, bone mesenchymal stem cells (BMSCs) and osteocytes, therefore coupling immune and skeletal systems.6 The concept of “osteoimmunology” was first coined to underline the regulation of osteoclast activation by T lymphocytes, hinting that bone regeneration is not a straightforward process that simply involves bone formation and resorption.9 To prolong the lifetime of orthopedic implants and to accelerate fracture healing are the two clinical challenges to which immunomodulatory strategies were applied in orthopedics.10 Moreover, cell-based immunomodulation, biomaterials and immunomodulator delivery have been employed for osteolytic bone diseases, such as rheumatoid arthritis, osteoporosis and periodontitis.10 Among various immune cells, the macrophage is the main effector cell and sentinel of innate immunity, and the vital modulator of chronic inflammation.11 Macrophages are responsible for removing apoptotic or necroptotic cell debris but are also causally linked to GIONFH.11
As a cell population with plasticity and pluripotency, macrophages participate in a great deal of pathological and physiological processes such as maintaining osteogenesis-adipogenesis balance, tissue regeneration and inflammatory response.12 The phenotype and function of macrophages vary with the release of growth factors and cytokines in the local tissue microenvironment.13 During these processes, macrophages undergo reprogramming, allowing them to readily and rapidly adapt to the changing conditions within tissues. The role that macrophage plays in the progression of GIONFH has only been reported sporadically.12 A better understanding of the molecular and cellular mechanisms underlying bone macrophage activation could offer potential avenues to dealing with GIONFH.
Polarization of Bone Macrophages
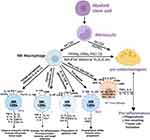
The bone-resident macrophage family encompasses bone marrow macrophage (erythroblastic island macrophage, hematopoietic stem cell niche macrophage), osteoclast and osteal macrophages.14 Osteal macrophages are the main population of bone-resident macrophages which support osteoblastic function and keep bone homeostasis.15,16 During the proliferation and differentiation of mononuclear phagocyte progenitor cells to osteal macrophages, macrophage colony-stimulating factor (M-CSF) is required (Figure 1).17
Macrophage polarization refers to the activation of macrophages, which are generally categorized into two directions according to the surface leukocyte differentiation antigen (cluster of differentiation, CD) and their functions, namely pro-inflammatory, classically activated type (M1) and anti-inflammatory, alternatively activated type (M2) (Figure 1).18,19 M1 macrophage polarization initiates when exposed to inflammatory molecules such as lipopolysaccharide (LPS), or Th1 cytokines (eg, IFN-γ, GM-CSF, and TNF-α).20 On the other hand, alternatively activated macrophages mainly polarize in response to Th2 cytokines such as IL-4, IL-10 and IL-13.21 During polarization, macrophages alter their shapes into flat-rounded (M1 type) or spindle-stretched (M2 type).22 As CD80/CD86 and CD206 are representative markers of M1 and M2 phenotypes, respectively, the average optical density of these molecules can reflect the number of M1 and M2 macrophages.23,24 Currently, the simplistic view of M1/M2 classification is not appropriate to describe the actual state of bone macrophage.25 More subtypes (M2a, M2b, M2c and M2d) of M2 macrophages have been derived depending on the inflammatory cues they encounter.25,26
Pro-inflammatory M1 macrophages are the principal producers of inflammatory cytokines (eg, IL-6, IL-1β and TNF-α) in a chronic inflammatory microenvironment and exert a pivotal role in antigen presentation and phagocytosis.27 M2 macrophage, on the contrary, mainly involves the resolution of inflammation, tissue regeneration, and remodeling.25,26 Maintenance of M1/M2 balance relies chiefly on metabolism due to that pro-inflammatory M1 macrophages are mainly supported by glycolysis and anti-inflammatory M2 macrophages utilize fatty acid oxidation (FAO).28 Metabolic reprogramming, especially glycolytic reprogramming, is widely identified in the polarization of macrophages.29
The sequence of events from the infiltration of pro-inflammatory macrophages followed by a phenotypic switch toward M2 macrophages is normal in the healing of bone gaps and inflammatory osteolysis.30,31 At about 21 days after fracture, M1 and M2 macrophages complete their mission and are no longer detectable in the microenvironment.32 However, if M1 macrophages fail to shift to the M2 phenotype, macrophage imbalance triggers chronic inflammation and induces tissue fibrosis, which is the main barrier to tissue regeneration and repair.30–33 Moreover, cross-talks between these activated macrophages and other bone cells perform essential functions in bone tissue homeostasis between bone resorption and formation, which may further the progression of GIONFH.
Interaction Between Bone Macrophage Polarization, Bone Homeostasis and Bone Repair
Polarized Macrophages and Osteoclasts Share a Common Source
Macrophage polarization and osteoclasts formation are two directions of macrophage/myeloid progenitors’ differentiation.34 The related regulators include peroxisome proliferator-activated receptor γ (PPARγ), estrogen-related receptor α (ERRα), PPARγ coactivator 1β (PGC-1β), recombinant human histidine NADH dehydrogenase Fe-S protein 4 (NDUFS4) and these associated with energy metabolism (Figure 1). Knockout of PPARγ in myeloid precursors can disrupt RANK-RANKL signal activation and inhibit osteoclasts’ differentiation.35 Meanwhile, PPARγ is a critical molecular inhibitor for the M1 polarization of macrophages.36 PGC-1β can induce osteoclasts differentiation and M2 macrophage polarization by advancing mitochondrial biogenesis and down-regulating pro-inflammatory cytokines.37 NDUFS4 is a nuclear-encoded accessory subunit of mitochondrial complex I, the first multisubunit enzyme complex of the mitochondrial respiratory chain.38 Further in-vivo experiments indicated that NDUFS4 has an autonomous role in activating M1 macrophage and contrarily, systemic NDUFS4 knockout or down-regulation inhibits osteoclastogenesis.39 These results prove a competitive relationship between M1 macrophage polarization and osteoclast differentiation, indicating that osteoclasts are a branch of the macrophage family.40
There is evidence that polarized macrophages could serve as candidates for the osteoclast progenitor.41 The polarization state of macrophages affects their ability to differentiate into osteoclasts, and produce pro-inflammatory cytokines and mediators. Cytokines secreted by M2 macrophages, such as IL-10 and IL-4, inhibit the expression of NFATc1, therefore suppressing the formation of osteoclasts.34 At the same time, the polarization of macrophages to the M1 phenotype leads to the release of multiple cytokines (eg, TNF-α, IL-6, IL-1, and IL-12) and chemokines (eg, CXCL2, CXCL8, and CXCL10), which will aid osteoclastogenesis.42,43
Macrophage Polarization and Osteogenesis
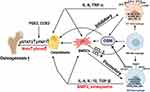
Increased osteocyte apoptosis and compromised osteogenesis is a crucial characteristic of GIONFH. The fibrovascular tissue form in the repair region that does not provide mechanical support comparable to cancellous bone, leading to mechanical fragility of the femoral head and the development of flattening and collapse. Both macrophages and BMSCs are essential sources of cytokines and chemokines, sharing complex interactions of signaling pathways.44 Traditionally, the M1 phenotype is well known for eliciting pro-inflammatory effects, which may cause bone resorption.45 However, in recent years, loads of studies have reported that the M0, M1 and M2 phenotypes are all beneficial for osteogenesis by activating the OSM signaling pathway, and the M1-type macrophages had the maximum effect on bone formation (Figure 2).46 OSM produced by macrophages could stimulate the mineralization activity and differentiation of osteoblasts by initiating STAT3 phosphorylation with the help of prostaglandin E2 (PGE2) and COX2.47,48 Xue et al49 unveiled that graphene oxide intervention enhanced the M1 polarization of RAW 264.7 cells, and the expression levels of OSM and BMP2, promoting the establishment of an immune microenvironment favorable for the osteogenic differentiation of BMSCs. In addition, M1 macrophages could secrete VEGF, TNF-α, and basic fibroblast growth factor, to initiate angiogenesis.50 Adding M2 anti-inflammatory macrophages to BMSCs could increase alkaline phosphatase (ALP) activity.44 M1 macrophages contribute to the early and middle osteogenesis of MSCs, while M2 macrophage co-culture can lead to increasing the matrix mineralization.14
Bone macrophages also interact with osteoblasts, which derive from BMSCs. In vivo, osteal macrophages occur in multiple stages of intramembranous ossification, constitute a unique canopy structure on mature osteoblasts and accelerate osteoblast differentiation, through the OSM-mediated tyrosine phosphorylation, and interplay between the STAT3 and Yes-associated protein 1 (YAP1).51 TNF-α released by activated macrophages can stimulate osteoblast chemotactic effect in vitro, and inhibit osteoblast differentiation in rheumatoid arthritis patients.52 Macrophage-derived BMP-2 is critical in inducing ossification through the Wnt and Wnt/LRP5 signaling cascade in osteoblasts.53 In addition to Wnt, the macrophage derived-BMP-2 can cause the dimerization of BMP-R and the phosphorylation of Smad proteins.54 Then, the phosphorylated molecules activate Runx2 to up-regulate osteoblast activity and differentiation.14
Bone Macrophage Polarization Impacted by Mesenchymal Stem Cells
BMSCs secrete CCL-2 and CCL-4, the main chemoattractants for monocytes and macrophages.55 In the co-culture model, MSCs significantly suppressed the production of LPS-induced pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) through iNOS and COX2-dependent pathways, and increased the secretion of IL-10 in macrophages by enhancing PGE2,56 which inhibit M1-macrophage polarization and induce M2 polarization.57 This MSC-mediated macrophage recruitment and macrophage phenotypic regulation may affect local inflammation and progression of GIONFH. Human umbilical cord mesenchymal stem cells (HUC-MSCs) might prevent necrosis and apoptosis of osteocytes by reducing M1 macrophage infiltration in the GIONFH model.58 Direct application of IL-4 could also encourage an immunomodulatory microenvironment that polarizes macrophages towards the M2 phenotype, and promote bone formation and MSC osteogenesis.59,60
Bone Macrophage Polarization in Bone Repair
Most previous research about bone repair focused on optimizing the osteogenic capacity of MSCs, biomaterials and bone graft, but neglected to research into the immune responses in local environment, which is key in maintaining skeletal health.61–63 Bone repair is a complex and dynamic process that involves the reciprocal effect of multiple types of cells and signaling pathways. Despite the benefit of M1 macrophages in osteogenesis and angiogenesis, the persistence of chronic inflammation involving M1 macrophages severely impairs bone repair.61 As pointed out, M2 macrophages co-culture can increase matrix mineralization of MSCs.14 However, it should be pointed out that M2 macrophages are able to perform a wide range of functions based on the subtypes, including immunomodulating, stimulating angiogenesis, promoting tumor progression, and coordinating tissue remodeling.25,26 Which M2 macrophage subtype is responsible for bone regeneration and remodeling is still being determined.
Immune Infiltration and Macrophage Distribution in GIONFH
Immune Infiltration Analysis in GIONFH
Inflammatory cytokines such as TNF-α, IL-6 and IL-1β were detected in synovial fluid and plasma of patients with GIONFH.64 During the progression of GIONFH, large numbers of chronic inflammatory cells may infiltrate into the peripheral blood and necrotic zone.65 Multiple studies66–68 evaluated immune infiltration in serum by analyzing GSE123568 which enrolled thirty patients with GIONFH and ten healthy controls, and found decreased infiltration of memory B cells and activated dendritic cells. It could be noticed that macrophages (M0, M1 and M2) were not significantly enriched in the GIONFH group. However, the condition of immune infiltration in the femoral head was not reported before.
In order to further clarify this question, Gene Expression Omnibus (GEO) repository (https://www.ncbi.nlm.nih.gov/geo/) was systematically searched to retrieve data expression profiles about the femoral head of GIONFH. Eventually, GSE26316, which involves three samples of GIONFH and three samples of healthy control, was included for analysis. The relative infiltration abundance of 22 immune cells in each sample was assessed using the CIBERSORT algorithm referring to the gene expression signature template provided on CIBERSORT official website. The parameter was set as perm=100 and QN=F. Pearson correlation analysis was utilized to appraise the associations among immune cells.
It was suggested that M0 macrophages, B cell memory, NK cell resting and M2 macrophages accounted for the majority of immune cell composition in bone tissue (Figure 3A). Pearson correlation analysis outlined a strong negative association between M0 macrophages and mast cells resting (r = 0.74). M1 macrophages were significantly correlated with mast cells activated (r = 1.00), while revealed strong negative correlations with mast cells resting (r = 0.72) (Figure 3B). Bone tissue in normal control had a high abundance of M0 macrophages and a lower number of M2 macrophages compared with the GIONFH group, although these differences did not achieve statistical significance (p = 0.148 and 0.580, respectively) (Figure 3C). Compared with healthy controls, T cells CD4 naive in GIONFH samples were relatively infiltrated less (p < 0.01) (Figure 3C). Given the findings of immune infiltration in serum and necrotic bone, it could be inferred that GIONFH is a local chronic inflammatory disease initiated by aggregated macrophages in the femoral head without an apparent impact on the polarization of peripheral macrophages.
However, the results of immune infiltration analysis should be interpreted cautiously. First, only three samples were enrolled and analyzed in each group. Secondly, in this profile, Rattus norvegicus was utilized to establish a GIONFH model by injecting methylprednisolone and endotoxin. In this animal model, extensive lesions, subchondral fracture and femoral head collapse are seldom observed. Therefore, these findings could only reflect the early-stage pathologic characteristics of GIONFH. To this end, the whole femoral head (avascular areas, perivascular area and cartilage) was isolated for RNA extraction and hybridization on Affymetrix microarrays. Further studies about immune infiltration during GIONFH should focus on a specific region of the femoral head.
Distribution of M1/M2 Polarized Macrophage in GIONFH
Kamal et al enrolled 39 patients diagnosed with aseptic ONFH for various risk factors (alcohol, alcohol and smoking, trauma and glucocorticoid), and noticed that macrophage is highlighted both in the area of necrosis and in the adjacent tissue (including cartilage).69 The highest number of macrophages was present in patients with GIONFH, and the lowest in patients with traumatic ONFH. However, few or no macrophages existed in the regenerative region with dense fibrous tissue.
In fact, during GIONFH, different phenotypes of macrophages do not form mutually exclusive populations but tend to coexist.70 An imbalance of M1/M2 macrophage polarization was observed in the necrotic femoral head. Adapala et al established an avascular ONFH piglet model by placing a suture ligature tightly around the femoral neck and transecting the ligamentum teres.71 After the flattening deformity of the necrotic femoral occurring at eight weeks, macrophages were mainly present around small blood vessels in the fibrovascular tissue. In the repair tissue, significantly increased M1 macrophage markers were detected, whereas the number of M2 macrophages remained stable. Meanwhile, a large amount of M1 macrophages infiltrated into the necrotic zone of GIONFH.65 Consistent results were obtained in studies using clinical samples. In the repair area of patients with progressive-stage GIONFH (Ficat Stage III), M1 macrophages were enriched in avascular regions (M1/M2 ratio ~12), while M2 macrophages were distributed mainly in the perivascular area (M1/M2 ratio ~0.05), manifesting that these cells arose from monocytes that migrated from the neovascularization.64 Blood vessels transport monocytes, oxygen and nutrients, which can lead macrophages to adopt and stay an M2 phenotype. In animal models of GIONFH, with the time going on after injection of methylprednisolone, significantly elevated levels of pro-inflammatory cytokines released by M1 polarized macrophages were identified in serum.72
The transition from the M1 to M2 phenotype is crucial in improving osteocyte survival and promoting the regeneration of injured tissue.73,74 As the GIONFH lesion advances from the progressive stage to the end stage (Ficat stage IV), macrophage infiltration in the reparative area as well as macrophage M1/M2 imbalance increased (M1/M2 ratio from 3 to 10), while the number of blood vessels, inflammatory cells and macrophages declined.64 Decreased level of M1 phenotypes is associated with the reduction of TNF-α and IL-1β.74 The neovascularization and M2 macrophages in the reparative area are insufficient to reconstruct the normal bone structure, which result in disease deterioration and poor prognosis.74 Currently, it is difficult to detect the phenotype of macrophages using flow cytometry as the inconvenience of extracting cells from the necrotic region and the low viability of the extracted cells. Most studies mentioned above used in situ staining or animal studies.
Mechanism Underlying the Macrophage Polarization Imbalance in GIONFH
Glucocorticoids Action on Bone Macrophage Polarization
Glucocorticoids act on nearly every component of the immune system but the functional aspects differ by cell type, which was systematically confirmed in the human system.75 While glucocorticoids are mainly inhibitable to cells of the adaptive immune system, especially T-cells, the influence of glucocorticoids on cells of the innate immune system is more complex.76 Overall, glucocorticoids activate and strengthen the innate immune system, provoking changes in macrophages by binding glucocorticoid receptors (GR), an intracellular receptor that acts as a transcription modulator.76 Glucocorticoids may affect the vascular migration of pro-inflammatory M1 phenotype macrophage to inflammatory regions.77 Dexamethasone treatment during monocyte-to-macrophage differentiation reduces the expression of adhesion molecules such as CD11b and CD18, leading to compromised lamellipodia formation and ineffective functional activities.77,78 These morphological changes are consistent with the repolarization of M1- into M2-like macrophages caused by metabolic transformation.78
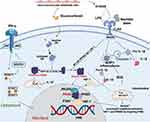
Glucocorticoids have been related to an M2c- like phenotype (Figure 1), which is considered deactivated macrophages with the capacity to speed tissue repair and wound healing, and is central to the erythroblastic island.79,80 Yang et al reiterated that during monocyte-macrophage differentiation, dexamethasone treatment significantly upregulated the M2-associated surface marker CD163.81 Glucocorticoids can increase a broad range of molecules associated with apoptotic cell clearance, including Axl, ADORA3, MFG-E8, C1q, thrombospondin-1 and MerTK, which is closely related to the induction of M2c polarization.82 This effect was IL-10 independent, partially achieved by inhibiting the production of the M2 isoform of Pyruvate Kinase (PKM2) (Figure 4).78,80,83 PKM2 is an enzyme catalyzing the last step of glycolysis which is upregulated in inflammation and tumors, but mainly in the enzymatically inactive dimeric form.83 The dimeric PKM2 could serve as a downstream target of LPS, translocate to the nucleus, and step up the production and stabilization of HIF-1α and IL-1β.84,85 Beclomethasone suppresses the induction of almost all pro-inflammatory M1 associated-genes, such as IL-6, TNF-α, IL-1b, IL-8 and MMP-9.86 In line with these data, many genes involved in glycolysis were inhibited by beclomethasone.86 These findings hint that the increased polarization of macrophage to the M1 phenotype in GIONFH was not realized by the direct action of glucocorticoid on the macrophage.
Necrotic Tissue and the Macrophage Polarization Imbalance in GIONFH
Multiple damage-associated molecular patterns (eg, Tenascin-C, biglycans and decorin) were present in necrotic tissues.87,88 These components of necrotic bone can stimulate pro-inflammatory responses from macrophages by activating a specific pattern recognition receptor Toll-like receptor 4 (TLR4), which is also a receptor of LPS and lipid A in Gram-negative bacteria (Figure 4). Binding to TLR4 induces dimerization of the TLR4-MD-2 complex, and subsequently activates Myd88-extracellular regulated kinase 1/2 (ERK1/2), Ik kinase (IKK)α-transcription factors nuclear factor-κB (NF-κB) pathway, which raises Th1 cytokines (IFN-γ and TNF-α) and HIF-1.71,89,90 Meanwhile, the increased content of the TCA cycle intermediate succinate could be transported from the mitochondria into the cytosol and impair the activity of prolyl hydroxylases (PDH), which further facilitates the stabilization and production of HIF-1α.91 This event induced by excessive succinate has been described as “pseudo hypoxia”.91 Necrotic bone alone could not trigger the phosphorylation of STAT1. However, the activation of the JAK1/2-STAT1 pathway by IFN-γ and subsequent phosphorylation of STAT1 have also been reported to correlate with the M1 macrophage polarization in GIONFH.92–94
TNF-α is a potent osteoclastogenic factor that simultaneously inhibits osteoblastogenesis.95 In the early stage of GIONFH, there was high TNF-α activity; and a large population of M1 macrophages infiltrated the necrotic zone.96 TNF-α, IL-6 and IL-1β could be transferred from the local lesion into the circulation and used as a hematological marker of the progressive stage ONFH patients.97 These cytokines disturb bone homeostasis and impair osteocyte survival. The pathologic repair process modulated by macrophages facilitates chronic inflammatory responses marked by tissue fibrosis instead of tissue regeneration, namely new bone formation.71
Bone Marrow Endothelial Cells (BMECs) and the Initiation of Macrophage Polarization in GIONFH
Necrotic bone and cytokines released by M1-polarized macrophages trigger a cascade of events to maintain the chronic inflammatory state and aggravate the apoptosis of osteocytes. However, the initiation of these events is not universally acknowledged. Bone microvessels are monolayer structures essential for bone formation and metabolism, and direct contact with ingredient in the blood.98 In our former study, it could be found that glucocorticoid intervention could induce apoptosis of the BMECs and enhance eleven cytokines in the released extracellular vesicles (Figure 5).98,99 Among them, S100A8 (Fold change = 56.23) is an atypical inflammatory cytokine that is most markedly elevated, which could also promote the M1 polarization of macrophage and the chronic inflammatory processes by activating the TLR4/NF-κB signal pathway according to published article.100 The glucocorticoid-induced injury of bone microvascular endothelial vessels and subsequently enhanced level of BMECs-derived cytokines may act as a prerequisite for the macrophage-induced chronic inflammation in GIONFH.
Bioactive Compounds and Molecular Targets Involving Macrophage Polarization in GIONFH
Multiple bioactive compounds such as Astragaloside IV (AS-IV) and curcumin have proved to intervene in macrophage polarization in GIONFH, which can be used as potential candidates for treating this disorder.72,74 Jiang et al confirmed that AS-IV, a drug derived from astragalus, could promote the repolarization of M1 macrophages into M2 macrophages.74 The cytotoxicity of the culture supernatant of M1-type macrophages was greatly attenuated by pretreatment of the macrophages with AS-IV. Concomitantly, osteocyte apoptosis was inhibited, and the proliferation was improved. Curcumin (C21H20O6) is extracted from turmeric and has long been employed in alleviating inflammatory disorders.72 Jin et al demonstrated that curcumin could prevent the progression of GIONFH by regulating immunity and suppressing inflammation.72 This was partially because of the reduced infiltration of M1‐type macrophages into the femoral heads by suppressing the JAK1/2‐STAT1 signaling pathway. In the in vitro study, when the concentration of curcumin was 12.5 or 25 μmol/L, M2 polarization of macrophage was boosted.
TLR4 is a potential target for treating GIONFH by restricting the recognition of DAMPs in necrotic bone. TAK242 acts as a selective inhibitor of the intracellular domain of TLR4, therefore blocking the activation of NF-κB and ligand-independent NF-κB activation resulting from over-expressed TLR4, and rescuing the bone loss and femoral head collapse during ONFH.101 Immunomodulatory bioactive components such as calycosin, resveratrol, catechin, emodin and allicin can intervene with TLR4/Myd88/NF-κB signal and prevent the M1 polarization of macrophage.102 Mir-335-5p, a microRNA (miRNA) well-recognized for the osteogenetic effect, was also able to down-regulate the expression of the TLR4 gene at the post-transcriptional level.103
Identification of immune cells and signaling molecules underlying M1 macrophage polarization presents evidence that PKM2 acts as another research hotspot for treating tumors and inflammatory diseases including GIONFH. As for PKM2, there was a dynamic equilibrium of an enzymatically inactive form (dimers and monomers) which can be further translocated into the nucleus and an enzymatically active state (tetramers) which stays in the cytosol to participate in glycolysis.85 Activation of PKM2 by TEPP-46 or DASA-58 could stimulate the formation of active tetramers, thereby boosting PKM2 enzymic activity and inhibiting LPS-induced production of HIF-1.85 Multiple bioactive compounds (eg, iminostilbene, shikonin and resveratrol) from fruit, vegetable and herb extracts could regulate PKM2, suppress macrophage inflammation and inhibit osteoclast differentiation.104,105 Besides, non-coding RNAs (miRNA, lncRNAs, and circRNA) targeting the PKM2 gene are also widely investigated in inflammatory conditions. Wade et al suggested a distinct loss of miR-125a in Psoriatic arthritis (PsA) synovial tissue, which induced metabolic reprogramming in endothelial cells towards increased expression of PKM2.106
Conclusion and Future Perspective
So far, most immunomodulatory regimens for bone repair and GIONFH have primarily focused on macrophages. The role of other immune cells, especially those involving adaptive immune, needs to be further explored.107 The evaluation index for restoring critical-sized femoral defects, such as bone volume fraction, bone mineral density, and tissue mineral density is demonstrated to be higher in athymic rats than in immunocompetent animals, hinting at the significance of the adaptive immune system.108 Neutrophils are the initial line of defense of the innate immune system. Besides generating pro-inflammatory cytokines, eliminating pathogens and clearing debris,109 neutrophils can actively coordinate the resolution of inflammation and regulate immune responses by interacting with cells of the innate and adaptive immune systems, thereby participating in bone repair and ONFH.110,111 After tissue injury, neutrophils immediately arrive at the site of the defect, recruiting BMSCs and macrophages.110 Inhibitory T cells can bind to osteoclast precursors to inhibit osteoclast activity, and the decreased number of inhibitory T cells is suboptimal to the prognosis of ONFH.112 For osteolytic disease, future studies also lie in understanding the roles of different T cell subpopulations in the context of disease progression.10 Lastly, dendritic cells have been shown to directly restrain osteoclast formation via secreting IFN -λ1 in inflammatory bone diseases, but its role in bone repair and GIONFH is still unknown.113
Limitations existed in current studies about macrophage polarization in GIONFH. First, technologies for immune infiltration of femoral head require the sacrifice of the animals or excision of femoral heads for end-point analysis, and are not suitable for real-time monitoring of the immune cell infiltration in situ. There remains a critical need to develop non-invasive cell imaging techniques (eg, two-photon microscopy, three-photon microscopy, transgenic reporter animal models and labeled probes) that enable efficient detection of immune cells in the bone niche. Second, only progressive and end-stage GIONFH specimens are available for clinical studies because of the occult nature of early ONFH. It was hard to extract cells from the necrotic femoral head. Lastly, the outcomes or pathogenesis of GIONFH in animals of different species and humans may be variable, while the animal models were more likely to be at an early stage which may not coincide with the results from a clinical perspective. Currently, rat models are considered desirable and suitable for studying molecular mechanisms of metabolic diseases. However, there is still a long way to go before advanced animal models can mimic the late-stage ONFH.
Patients with GIONFH are at high risk for subchondral fracture, and head collapse happens in approximately 90% of cases within five years after the diagnosis.114 For the side effects and limited efficiency of current therapeutics for GIONFH, more immunomodulatory strategies that favor bone regeneration and prevent disease progression in the early stage should be developed in the future. Compared to pill drug delivery, nanomaterial-mediated drug delivery is a viable plan. The problems limiting their use in clinical practice are to control the biodistribution of systemically administrated nanoparticles and improve the targeting efficiency to the disease sites.115,116 Exploring new immunomodulators for targets such as TLRs, PKM2 or HIF-1 signaling pathway is a necessity.
In a word, macrophage polarization and associated chronic inflammation play a critical role in the onset and progression of GIONFH. M1 macrophages generated after exposure to necrotic tissue could secrete a series of cytokines to facilitate the differentiation and maturation of osteoclasts. In the late stage of GIONFH, M2 macrophages resolve inflammation and facilitate tissue repair. In the development of GIONFH, injured bone vascular endothelial cells and necrotic bone activate the TLR4/NF-κB signal pathway, promote dimerization of PKM2 and subsequently enhance the production of HIF-1, inducing metabolic transformation of macrophage to the M1 phenotype. Putative interventions to correct the M1/M2 imbalance by using MSCs, chemokine and bioactive medicines (eg, AS-IV and curcumin) appear to be a plausible choice for treating GIONFH. However, there are still many controversies about the association between macrophage polarization, osteoclast differentiation, and osteogenesis of bone mesenchymal stem cells. The way forward is to fully elucidate the cellular and molecular mechanism underlying alterations of the polarization and functions of macrophages in the development of GIONFH.
Acknowledgments
This work was partially supported by the Young Scholars Program of Shandong Provincial Hospital, the Young Taishan Scholars Program of Shandong Province (Grant number tsqn201909183), the Academic Promotion Program of Shandong First Medical University (Grant number 2020RC008), and the Natural Science Foundation of Shandong Province (Grant numbers ZR2020QH072 and ZR2021QH307).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Q-Y, Li Z-R, Gao F-Q, Sun W. Pericollapse stage of osteonecrosis of the femoral head: a last chance for joint preservation. Chin Med J. 2018;131(21):2589–2598. doi:10.4103/0366-6999.244111
2. Liu LH, Li ZR, Sun W, et al. Reliability and repeatability of the China-japan friendship hospital typing classification for nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg Am. 2022;104:40–46. doi:10.2106/JBJS.20.00051
3. Zhao DW, Yu M, Hu K, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chin Med J. 2015;128:2843–2850. doi:10.4103/0366-6999.168017
4. Weinstein RS. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–190. doi:10.1007/s12020-011-9580-0
5. Lu Y, Yu Q, Guo W, et al. Effect of glucocorticoids on the function of microvascular endothelial cells in the human femoral head bone. Adv Clin Exp Med. 2020;29:345–353. doi:10.17219/acem/112602
6. Ma M, Tan Z, Li W, et al. Osteoimmunology and osteonecrosis of the femoral head. Bone Joint Res. 2022;11:26–28. doi:10.1302/2046-3758.111.BJR-2021-0467.R1
7. Loi F, Cordova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi:10.1016/j.bone.2016.02.020
8. Chen TT, Xiao F, Li N, et al. Inflammasome as an effective platform for fibrosis therapy. J Inflamm Res. 2021;14:1575–1590. doi:10.2147/JIR.S304180
9. Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19:626–642. doi:10.1038/s41577-019-0178-8
10. Su N, Villicana C, Yang F. Immunomodulatory strategies for bone regeneration: a review from the perspective of disease types. Biomaterials. 2022;286:121604. doi:10.1016/j.biomaterials.2022.121604
11. Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19(1):92. doi:10.3390/ijms19010092
12. Terkawi MA, Kadoya K, Takahashi D, et al. Identification of IL-27 as potent regulator of inflammatory osteolysis associated with vitamin E-blended ultra-high molecular weight polyethylene debris of orthopedic implants. Acta Biomater. 2019;89:242–251. doi:10.1016/j.actbio.2019.03.028
13. Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J Innate Immun. 2012;4:463–477. doi:10.1159/000336717
14. Chen K, Jiao Y, Liu L, et al. Communications between bone marrow macrophages and bone cells in bone remodeling. Front Cell Dev Biol. 2020;8:598263. doi:10.3389/fcell.2020.598263
15. Michalski MN, Mccauley LK. Macrophages and skeletal health. Pharmacol Ther. 2017;174:43–54. doi:10.1016/j.pharmthera.2017.02.017
16. Chang MK, Raggatt LJ, Alexander KA, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi:10.4049/jimmunol.181.2.1232
17. Jones CV, Ricardo SD. Macrophages and CSF-1: implications for development and beyond. Organogenesis. 2013;9:249–260. doi:10.4161/org.25676
18. Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi:10.1016/j.ejphar.2020.173090
19. Liu F, Dong J, Zhou D, Zhang Q. Identification of key candidate genes related to inflammatory osteolysis associated with vitamin E-blended UHMWPE debris of orthopedic implants by integrated bioinformatics analysis and experimental confirmation. J Inflamm Res. 2021;14:3537–3554. doi:10.2147/JIR.S320839
20. Shapouri-Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–6440. doi:10.1002/jcp.26429
21. Mantovani A, Biswas SK, Galdiero MR, et al. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi:10.1002/path.4133
22. Rostam HM, Reynolds PM, Alexander MR, et al. Image based Machine Learning for identification of macrophage subsets. Sci Rep. 2017;7:3521. doi:10.1038/s41598-017-03780-z
23. Watanabe S, Alexander M, Misharin AV, Budinger G. The role of macrophages in the resolution of inflammation. J Clin Invest. 2019;129:2619–2628. doi:10.1172/JCI124615
24. Zhou X, Li W, Wang S, et al. YAP aggravates inflammatory bowel disease by regulating M1/M2 macrophage polarization and gut microbial homeostasis. Cell Rep. 2019;27:1176–1189. doi:10.1016/j.celrep.2019.03.028
25. Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi:10.1155/2015/816460
26. Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi:10.1038/ni.1937
27. Zhang WJ, Chen SJ, Zhou SC, et al. Inflammasomes and Fibrosis. Front Immunol. 2021;12:643149. doi:10.3389/fimmu.2021.643149
28. Christofides A, Strauss L, Yeo A, et al. The complex role of tumor-infiltrating macrophages. Nat Immunol. 2022;23:1148–1156. doi:10.1038/s41590-022-01267-2
29. Marrocco A, Ortiz LA. Role of metabolic reprogramming in pro-inflammatory cytokine secretion from LPS or silica-activated macrophages. Front Immunol. 2022;13:936167. doi:10.3389/fimmu.2022.936167
30. Desgeorges T, Caratti G, Mounier R, et al. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front Immunol. 2019;10:1591. doi:10.3389/fimmu.2019.01591
31. Alhamdi JR, Peng T, Al-Naggar IM, et al. Controlled M1-to-M2 transition of aged macrophages by calcium phosphate coatings. Biomaterials. 2019;196:90–99. doi:10.1016/j.biomaterials.2018.07.012
32. Schlundt C, El KT, Serra A, et al. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78–89. doi:10.1016/j.bone.2015.10.019
33. Wang Z, He X, Tang B, et al. Polarization behavior of bone marrow-derived macrophages on charged P(VDF-TrFE) coatings. Biomater Sci. 2021;9:874–881. doi:10.1039/d0bm01604g
34. Yao Y, Cai X, Ren F, et al. The macrophage-osteoclast axis in osteoimmunity and osteo-related diseases. Front Immunol. 2021;12:664871. doi:10.3389/fimmu.2021.664871
35. Jin Z, Wei W, Huynh H, Wan Y. HDAC9 inhibits osteoclastogenesis via mutual suppression of PPARgamma/RANKL signaling. Mol Endocrinol. 2015;29:730–738. doi:10.1210/me.2014-1365
36. Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–1120. doi:10.1038/nature05894
37. Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi:10.1016/j.cmet.2006.05.011
38. Johnson SC, Kayser EB, Bornstein R, et al. Regional metabolic signatures in the Ndufs4(KO) mouse brain implicate defective glutamate/alpha-ketoglutarate metabolism in mitochondrial disease. Mol Genet Metab. 2020;130:118–132. doi:10.1016/j.ymgme.2020.03.007
39. Jin Z, Wei W, Yang M, et al. Mitochondrial complex I activity suppresses inflammation and enhances bone resorption by shifting macrophage-osteoclast polarization. Cell Metab. 2014;20:483–498. doi:10.1016/j.cmet.2014.07.011
40. Van den Bossche J, Baardman J, Otto NA, et al. Mitochondrial dysfunction prevents repolarization of inflammatory macrophages. Cell Rep. 2016;17:684–696. doi:10.1016/j.celrep.2016.09.008
41. Munoz J, Akhavan NS, Mullins AP, Arjmandi BH. Macrophage polarization and osteoporosis: a review. Nutrients. 2020;12. doi:10.3390/nu12102999
42. Sun Y, Li J, Xie X, et al. Macrophage-osteoclast associations: origin, polarization, and subgroups. Front Immunol. 2021;12:778078. doi:10.3389/fimmu.2021.778078
43. Kitami S, Tanaka H, Kawato T, et al. IL-17A suppresses the expression of bone resorption-related proteinases and osteoclast differentiation via IL-17RA or IL-17RC receptors in RAW264.7 cells. Biochimie. 2010;92:398–404. doi:10.1016/j.biochi.2009.12.011
44. Gao Q, Rhee C, Maruyama M, et al. The effects of macrophage phenotype on osteogenic differentiation of MSCs in the presence of polyethylene particles. Biomedicines. 2021;9. doi:10.3390/biomedicines9050499
45. Pajarinen J, Lin T, Gibon E, et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi:10.1016/j.biomaterials.2017.12.025
46. Lu LY, Loi F, Nathan K, et al. Pro-inflammatory M1 macrophages promote Osteogenesis by mesenchymal stem cells via the COX-2-prostaglandin E2 pathway. J Orthop Res. 2017;35:2378–2385. doi:10.1002/jor.23553
47. Sims NA, Quinn JM. Osteoimmunology: oncostatin M as a pleiotropic regulator of bone formation and resorption in health and disease. Bonekey Rep. 2014;3:527. doi:10.1038/bonekey.2014.22
48. Nicolaidou V, Wong MM, Redpath AN, et al. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS One. 2012;7:e39871. doi:10.1371/journal.pone.0039871
49. Xue D, Chen E, Zhong H, et al. Immunomodulatory properties of graphene oxide for osteogenesis and angiogenesis. Int J Nanomedicine. 2018;13:5799–5810. doi:10.2147/IJN.S170305
50. Spiller KL, Anfang RR, Spiller KJ, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 2014;35:4477–4488. doi:10.1016/j.biomaterials.2014.02.012
51. Wang J, Zheng Z, Huang B, et al. Osteal tissue macrophages are involved in endplate osteosclerosis through the OSM-STAT3/YAP1 signaling axis in modic changes. J Immunol. 2020;205:968–980. doi:10.4049/jimmunol.1901001
52. Sun W, Meednu N, Rosenberg A, et al. B cells inhibit bone formation in rheumatoid arthritis by suppressing osteoblast differentiation. Nat Commun. 2018;9:5127. doi:10.1038/s41467-018-07626-8
53. Jamalpoor Z, Asgari A, Lashkari MH, et al. Modulation of macrophage polarization for bone tissue engineering applications. Iran J Allergy Asthma Immunol. 2018;17:398–408.
54. Zou ML, Chen ZH, Teng YY, et al. The smad dependent TGF-beta and BMP signaling pathway in bone remodeling and therapies. Front Mol Biosci. 2021;8:593310. doi:10.3389/fmolb.2021.593310
55. Seebach E, Freischmidt H, Holschbach J, et al. Mesenchymal stroma cells trigger early attraction of M1 macrophages and endothelial cells into fibrin hydrogels, stimulating long bone healing without long-term engraftment. Acta Biomater. 2014;10:4730–4741. doi:10.1016/j.actbio.2014.07.017
56. Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. doi:10.1371/journal.pone.0009252
57. Cho DI, Kim MR, Jeong HY, et al. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. doi:10.1038/emm.2013.135
58. Tian G, Liu C, Gong Q, et al. Human umbilical cord mesenchymal stem cells improve the necrosis and osteocyte apoptosis in glucocorticoid-induced osteonecrosis of the femoral head model through reducing the macrophage polarization. Int J Stem Cells. 2022;15:195–202. doi:10.15283/ijsc21120
59. von Kaeppler EP, Wang Q, Raghu H, et al. Interleukin 4 promotes anti-inflammatory macrophages that clear cartilage debris and inhibits osteoclast development to protect against osteoarthritis. Clin Immunol. 2021;229:108784. doi:10.1016/j.clim.2021.108784
60. Mahon OR, Browe DC, Gonzalez-Fernandez T, et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials. 2020;239:119833. doi:10.1016/j.biomaterials.2020.119833
61. Wang Y, Zhang H, Hu Y, et al. Bone repair biomaterials: a perspective from immunomodulatory. Adv Funct Mater. 2022;2208639. doi:10.1002/adfm.202208639
62. Zhang H, Wu S, Chen W, et al. Bone/cartilage targeted hydrogel: strategies and applications. Bioact Mater. 2023;23:156–169. doi:10.1016/j.bioactmat.2022.10.028
63. Wu P, Liang Y, Sun G, et al. Engineering immune-responsive biomaterials for skin regeneration. Biomater Transl. 2021;2:61–71. doi:10.3877/cma.j.issn.2096-112X.2021.01.008
64. Tan Z, Wang Y, Chen Y, et al. The dynamic feature of macrophage M1/M2 imbalance facilitates the progression of non-traumatic osteonecrosis of the femoral head. Front Bioeng Biotechnol. 2022;10:912133. doi:10.3389/fbioe.2022.912133
65. Wu X, Xu W, Feng X, et al. TNF-A mediated inflammatory macrophage polarization contributes to the pathogenesis of steroid-induced osteonecrosis in mice. Int J Immunopathol Pharmacol. 2015;28:351–361. doi:10.1177/0394632015593228
66. Zhao J, Zhang X, Guan J, et al. Identification of key biomarkers in steroid-induced osteonecrosis of the femoral head and their correlation with immune infiltration by bioinformatics analysis. BMC Musculoskelet Disord. 2022;23:67. doi:10.1186/s12891-022-04994-7
67. Yu R, Zhang J, Zhuo Y, et al. ARG2, MAP4K5 and TSTA3 as diagnostic markers of steroid-induced osteonecrosis of the femoral head and their correlation with immune infiltration. Front Genet. 2021;12:691465. doi:10.3389/fgene.2021.691465
68. Wang B, Gong S, Shao W, et al. Comprehensive analysis of pivotal biomarkers, immune cell infiltration and therapeutic drugs for steroid-induced osteonecrosis of the femoral head. Bioengineered. 2021;12:5971–5984. doi:10.1080/21655979.2021.1972081
69. Kamal D, Trăistaru R, Kamal CK, et al. Macrophage response in patients diagnosed with aseptic necrosis of the femoral head presenting different risk factors. Rom J Morphol Embryol. 2015;56:163–168.
70. Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(LPS+) vs classically and M2(LPS-) vs alternatively activated macrophages. Front Immunol. 2019;10:1084. doi:10.3389/fimmu.2019.01084
71. Adapala NS, Yamaguchi R, Phipps M, et al. Necrotic bone stimulates proinflammatory responses in macrophages through the activation of toll-like receptor 4. Am J Pathol. 2016;186:2987–2999. doi:10.1016/j.ajpath.2016.06.024
72. Jin S, Meng C, He Y, et al. Curcumin prevents osteocyte apoptosis by inhibiting M1-type macrophage polarization in mice model of glucocorticoid-associated osteonecrosis of the femoral head. J Orthop Res. 2020;38:2020–2030. doi:10.1002/jor.24619
73. Sica A, Erreni M, Allavena P, Porta C. Macrophage polarization in pathology. Cell Mol Life Sci. 2015;72:4111–4126. doi:10.1007/s00018-015-1995-y
74. Jiang C, Zhou Z, Lin Y, et al. Astragaloside IV ameliorates steroid-induced osteonecrosis of the femoral head by repolarizing the phenotype of pro-inflammatory macrophages. Int Immunopharmacol. 2021;93:107345. doi:10.1016/j.intimp.2020.107345
75. Quatrini L, Ugolini S. New insights into the cell- and tissue-specificity of glucocorticoid actions. Cell Mol Immunol. 2021;18:269–278. doi:10.1038/s41423-020-00526-2
76. Ganio EA, Stanley N, Lindberg-Larsen V, et al. Preferential inhibition of adaptive immune system dynamics by glucocorticoids in patients after acute surgical trauma. Nat Commun. 2020;11:3737. doi:10.1038/s41467-020-17565-y
77. Rohwedder I, Kurz A, Pruenster M, et al. Src family kinase-mediated vesicle trafficking is critical for neutrophil basement membrane penetration. Haematologica. 2020;105:1845–1856. doi:10.3324/haematol.2019.225722
78. Luvanda MK, Posch W, Vosper J, et al. Dexamethasone promotes aspergillus fumigatus growth in macrophages by triggering M2 repolarization via targeting PKM2. J Fungi. 2021;7. doi:10.3390/jof7020070
79. Galuppo P, Vettorazzi S, Hovelmann J, et al. The glucocorticoid receptor in monocyte-derived macrophages is critical for cardiac infarct repair and remodeling. FASEB J. 2017;31:5122–5132. doi:10.1096/fj.201700317R
80. Diaz-Jimenez D, Kolb JP, Cidlowski JA. Glucocorticoids as regulators of macrophage-mediated tissue homeostasis. Front Immunol. 2021;12:669891. doi:10.3389/fimmu.2021.669891
81. Yang P, Zhang X, Lin Z, et al. Adoptive transfer of polarized M2c macrophages ameliorates acute rejection in rat liver transplantation. Am J Transl Res. 2020;12:2614–2626.
82. Zizzo G, Hilliard BA, Monestier M, Cohen PL. Efficient clearance of early apoptotic cells by human macrophages requires M2c polarization and MerTK induction. J Immunol. 2012;189:3508–3520. doi:10.4049/jimmunol.1200662
83. Kuppermann BD, Zacharias LC, Kenney MC. Steroid differentiation: the safety profile of various steroids on retinal cells in vitro and their implications for clinical use (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2014;112:116–141.
84. Finucane OM, Sugrue J, Rubio-Araiz A, et al. The NLRP3 inflammasome modulates glycolysis by increasing PFKFB3 in an IL-1beta-dependent manner in macrophages. Sci Rep. 2019;9:4034. doi:10.1038/s41598-019-40619-1
85. Palsson-Mcdermott EM, Curtis AM, Goel G, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab. 2015;21:65–80. doi:10.1016/j.cmet.2014.12.005
86. Xie Y, Tolmeijer S, Oskam JM, et al. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis Model Mech. 2019;12. doi:10.1242/dmm.037887
87. Midwood K, Sacre S, Piccinini AM, et al. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi:10.1038/nm.1987
88. Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115:2223–2233. doi:10.1172/JCI23755
89. Ciesielska A, Matyjek M, Kwiatkowska K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2021;78:1233–1261. doi:10.1007/s00018-020-03656-y
90. Pei J, Fan L, Nan K, et al. Excessive activation of TLR4/NF-kappaB interactively suppresses the canonical Wnt/beta-catenin pathway and induces SANFH in SD rats. Sci Rep. 2017;7:11928. doi:10.1038/s41598-017-12196-8
91. Selak MA, Armour SM, Mackenzie ED, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi:10.1016/j.ccr.2004.11.022
92. Zhou J, Zhang A, Fan L. HSPA12B secreted by tumor-associated endothelial cells might induce M2 polarization of macrophages via activating PI3K/Akt/mTOR signaling. Onco Targets Ther. 2020;13:9103–9111. doi:10.2147/OTT.S254985
93. Wei Y, Liang M, Xiong L, et al. PD-L1 induces macrophage polarization toward the M2 phenotype via Erk/Akt/mTOR. Exp Cell Res. 2021;402:112575. doi:10.1016/j.yexcr.2021.112575
94. Liang YB, Tang H, Chen ZB, et al. Downregulated SOCS1 expression activates the JAK1/STAT1 pathway and promotes polarization of macrophages into M1 type. Mol Med Rep. 2017;16:6405–6411. doi:10.3892/mmr.2017.7384
95. Marahleh A, Kitaura H, Ohori F, et al. TNF-alpha directly enhances osteocyte RANKL expression and promotes osteoclast formation. Front Immunol. 2019;10:2925. doi:10.3389/fimmu.2019.02925
96. Fang B, Wang D, Zheng J, et al. Involvement of tumor necrosis factor alpha in steroid-associated osteonecrosis of the femoral head: friend or foe? Stem Cell Res Ther. 2019;10:5. doi:10.1186/s13287-018-1112-x
97. Okazaki S, Nishitani Y, Nagoya S, et al. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology. 2008;48(3):227–232. doi:10.1093/rheumatology/ken462
98. Li T, Liu Y, Zhang Q, et al. A steroid‑induced osteonecrosis model established using an organ‑on‑a‑chip platform. Exp Ther Med. 2021;22(4):1070. doi:10.3892/etm.2021.10504
99. Zhang Q, Li T, Li Z, et al. Autocrine activity of extracellular vesicles induced by icariin and its effectiveness in glucocorticoid-induced injury of bone microvascular endothelial cells. Cells-Basel. 2022;11. doi:10.3390/cells11121921
100. Liu Y, Kong X, You Y, et al. S100A8-mediated NLRP3 inflammasome-dependent pyroptosis in macrophages facilitates liver fibrosis progression. Cells-Basel. 2022;11. doi:10.3390/cells11223579
101. Alqranei MS, Senbanjo LT, Aljohani H, et al. Lipopolysaccharide- TLR-4 axis regulates osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunology. 2021;22(1):23. doi:10.1186/s12865-021-00409-9
102. Zhu D, Yu H, Liu P, et al. Calycosin modulates inflammation via suppressing TLR4/NF-κB pathway and promotes bone formation to ameliorate glucocorticoid-induced osteonecrosis of the femoral head in rat. Phytother Res. 2021. doi:10.1002/ptr.7028
103. Lian J, Wu X, Liu Y, et al. Potential roles of miR-335-5p on pathogenesis of experimental periodontitis. J Periodontal Res. 2020;55(2):191–198. doi:10.1111/jre.12701
104. Liu T, Li S, Wu L, et al. experimental study of hepatocellular carcinoma treatment by shikonin through regulating PKM2. J Hepatocell Carcinoma. 2020;7:19–31. doi:10.2147/JHC.S237614
105. Lu S, Tian Y, Luo Y, et al. Iminostilbene, a novel small-molecule modulator of PKM2, suppresses macrophage inflammation in myocardial ischemia–reperfusion injury. J Adv Res. 2021;29:83–94. doi:10.4103/0366-6999.244111
106. Wade SM, Ohnesorge N, Mcloughlin H, et al. Dysregulated miR-125a promotes angiogenesis through enhanced glycolysis. Ebiomedicine. 2019;47:402–413. doi:10.1016/j.ebiom.2019.08.043
107. Liu H, Li D, Zhang Y, Li M. Inflammation, mesenchymal stem cells and bone regeneration. Histochem Cell Biol. 2018;149:393–404. doi:10.1007/s00418-018-1643-3
108. Garske DS, Schmidt-Bleek K, Ellinghaus A, et al. Alginate hydrogels for in vivo bone regeneration: the immune competence of the animal model matters. Tissue Eng Part A. 2020;26:852–862. doi:10.1089/ten.TEA.2019.0310
109. Zhao T, Chu Z, Ma J, Ouyang L. Immunomodulation effect of biomaterials on bone formation. J Funct Biomater. 2022;13. doi:10.3390/jfb13030103
110. Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129:2629–2639. doi:10.1172/JCI124616
111. Nonokawa M, Shimizu T, Yoshinari M, et al. Association of neutrophil extracellular traps with the development of idiopathic osteonecrosis of the femoral head. Am J Pathol. 2020;190:2282–2289. doi:10.1016/j.ajpath.2020.07.008
112. Ma J, Ge J, Gao F, et al. The role of immune regulatory cells in nontraumatic osteonecrosis of the femoral head: a retrospective clinical study. Biomed Res Int. 2019;2019:1302015. doi:10.1155/2019/1302015
113. Chen Y, Wang Y, Tang R, et al. Dendritic cells-derived interferon-lambda1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis. Cell Death Dis. 2020;11:414. doi:10.1038/s41419-020-2612-z
114. Li S, Liu Y, Zhou G, et al. Pre-collapse femoral head necrosis treated by Hip abduction: a computational biomechanical analysis. Health Inf Sci Syst. 2022;10:8. doi:10.1007/s13755-022-00175-x
115. Xue X, Hu Y, Deng Y, Su J. Recent advances in design of functional biocompatible hydrogels for bone tissue engineering. Adv Funct Mater. 2021;31:2009432.
116. Steijvers E, Ghei A, Xia Z. Manufacturing artificial bone allografts: a perspective. Biomater Transl. 2022;3:65–80. doi:10.12336/biomatertransl.2022.01.007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.