Back to Journals » Infection and Drug Resistance » Volume 16
Pneumothorax with Eosinophilia is an Important Diagnostic Clue for Distinguishing Paragonimiasis from Chronic Eosinophilic Pneumonia: A Case Report
Authors Sakakura S, Yamaguchi F , Abe T, Cho H, Shimizu S, Mase A, Shikama Y , Maruyama H
Received 22 December 2022
Accepted for publication 13 April 2023
Published 25 April 2023 Volume 2023:16 Pages 2429—2432
DOI https://doi.org/10.2147/IDR.S402392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Shunsuke Sakakura,1,* Fumihiro Yamaguchi,1,* Takashi Abe,1 Hidekazu Cho,1 Shohei Shimizu,1 Ayaka Mase,1 Yusuke Shikama,1 Haruhiko Maruyama2
1Department of Respiratory Medicine, Showa University Fujigaoka Hospital, Yokohama, Japan; 2Division of Parasitology, Department of Infectious Diseases, Graduate School of Medicine and Veterinary Medicine, University of Miyazaki, Miyazaki, Japan
*These authors contributed equally to this work
Correspondence: Fumihiro Yamaguchi, Department of Respiratory Medicine, Showa University Fujigaoka Hospital, 1-30 Fujigaoka, Aoba-ku, Yokohama, 227-8501, Japan, Tel +81-45-971-1151, Email [email protected]
Abstract: The Paragonimus westermani infection is a parasitic foodborne infection that induces systemic symptoms with eosinophilia in humans. Here, we described pneumothorax in addition to pulmonary opacities with eosinophilia in a man with a positive P. westermani serology. He was misdiagnosed with chronic eosinophilic pneumonia (CEP) during the initial phase. Paragonimiasis can share similar clinical findings with CEP in cases where the worm is confined to the lungs. The findings of the current study suggest that paragonimiasis and CEP can be distinguished from each other by the presence of various symptoms. Notably, eosinophilia with pneumothorax should be an important diagnostic factor for paragonimiasis.
Keywords: Paragonimus westermani, chronic eosinophilic pneumonia, paragonimiasis, pneumothorax
Introduction
Paragonimiasis is a typical parasitic zoonosis that is transmitted via oral ingestion of undercooked crustaceans, raw boar meat, or deer meat1 and induces various symptoms in humans. Prodromal symptoms such as abdominal pain, fever, and diarrhea appear a few days after oral ingestion, followed by respiratory manifestations and systemic eosinophilia after an incubation period of 4–16 weeks.2 Radiographic findings of paragonimiasis are diverse and the clinical course resembles that of chronic eosinophilic pneumonia (CEP) in some cases. CEP is a lung-limited disorder in which eosinophils infiltrate the pulmonary parenchyma within a few weeks or months. CEP has various respiratory symptoms and presents with eosinophilia both in the peripheral blood and bronchoalveolar lavage fluid (BALF).3 Patients with CEP usually require systemic corticosteroid treatment because the condition may be considered an immunologically mediated lung disease. Although some diagnostic criteria have been proposed,3,4 there are no global guidelines for the diagnosis and management of the disease. As expected, there are several differential diagnoses for CEP. These differential diagnoses include allergic bronchopulmonary aspergillosis, eosinophilic granulomatosis with polyangiitis, drug reactions, and parasitic infestations.3 However, it is difficult to distinguish them at the early phase of the disease, and the diagnosis may be overturned during the course of the disease as the above differential diagnoses have similar imaging and clinical findings. In this study, we present a case of eosinophilic pneumonia and pneumothorax in a patient with the Paragonimus westermani infection.
Case Report
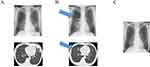
A 46-year-old Japanese man who was previously healthy and never a smoker presented with fever and left-sided chest pain for 3 weeks. There was no evidence of respiratory failure. The electrocardiogram was within the normal range and the cardiac ultrasound did not show any abnormalities. Computed tomography scan of the chest revealed airspace consolidation in the peripheral region of the upper lobe of his left lung (Figure 1A). No other abnormalities were observed in both lungs. Analysis of peripheral blood sample revealed elevated eosinophil counts of maximum 7017/μL (53%: normal range is <7%). Bronchoscopic examination revealed that lymphocytes and eosinophils were found with 73% and 14% in the BALF but no pathogens were observed. Eosinophilia in BALF was <25%; however, fractional exhaled nitric oxide was 54ppb, consistent with eosinophilic airway inflammation. The clinical symptom was present for at least 2 weeks and was confined to the lungs. No medications were administrated, and the imaging studies were consistent to CEP. Eventually, the patient was diagnosed with CEP3 and oral corticosteroids were initiated. However, right pneumothorax was detected 3 months after starting steroid therapy. Eosinophil levels in his peripheral blood reascended, chest pain recurred intermittently, and right pneumothorax was observed again (Figure 1B). A second round of detailed history taking revealed that the patient had consumed undercooked “Shanghai crab” 3 weeks before the first visit. No parasite eggs were detected in sputum and feces samples. The value of the serological test for P. westermani was increased according to microplate enzyme-linked immunosorbent assays as reported previously (Figure 2).1 After treatment with praziquantel (75 mg/kg/day) for 3 days, chest pain disappeared and eosinophil levels in peripheral blood dropped to the normal limit. The serological test 3 months after praziquantel administration revealed lower values (Figure 2). There was no recurrence of pneumothorax (Figure 1C). During the last follow-up, he was symptom-free.
Discussion
The patient in this case was initially misdiagnosed with CEP; however, he was finally diagnosed with paragonimiasis. Although the geographic distribution of Paragonimiasis is Orient, Africa and the Americas, most cases have been reported in Asia because of the eating habits. CEP presents mainly with cough, dyspnea, chest pain, and eosinophilia.3,4 By contrast, paragonimiasis induces systemic symptoms such as cough, sputum, chest pain, back pain, dyspnea, foul odors, subcutaneous nodules, rashes, abdominal pain, vomiting, diarrhea, headache, seizures, and eosinophilia.5–7 Patients with paragonimiasis who have low parasite burdens are usually asymptomatic.2,8 Paragonimiasis primarily targets the lungs and pleura for parasite infestation; however, atypical extrapulmonary migration of paragonimiasis occurs in the heart, liver, peritoneum, kidney, subcutaneous tissue, gut, and brain.2,6,9,10 Symptoms of paragonimiasis depend on the worm’s location. Therefore, the manifestation of the disease may be limited to the respiratory system, which is similar to the clinical presentation of CEP. Radiographic findings of paragonimiasis occasionally represent airspace consolidation, predominantly in the peripheral region,11 which is also a typical finding in CEP.3,12 The pathogenesis of paragonimiasis is eosinophilic inflammation around the body of the worm,10 which reveals a subpleural consolidation in the pulmonary parenchyma. Pleural effusion is the most common manifestation of paragonimiasis in imaging studies, followed by pneumothorax, and the presence of nodular opacities and airspace consolidation.5,6,10 The presence of pleural effusions is uncommon3 and pneumothorax has not yet been reported in CEP. The definitive diagnosis in the current case, which was made based on the pneumothorax that occurred during the disease, was paragonimiasis. Pneumothorax occurs when worm bodies shuttle into and out of the lung. Eosinophilia with a pleural effusion or pneumothorax can be an important diagnostic clue for distinguishing paragonimiasis from CEP.
In the present study, the diagnosis of the P. westermani infection was established based on a positive serological test performed using blood samples. There are more than 40 species of the culprit parasite, of which approximately 10 are known to cause infections in humans.2,5 In Japan, P. westermani and P. skrjabini miyazakii are the dominant pathogens.6,10 Normally, paragonimiasis is diagnosed by confirming the presence of specimens or eggs in sputum or feces samples; however, the detection of the P. westermani pathogen or its eggs is rare.6,7 Hence, serological testing was established as an alternative diagnostic method. Certain cases reported as classical “Löeffler’s syndrome” may be cases of parasitic infections, including Ancylostoma, Toxocara, Strongyloides, Microfilaria, and Paragonimus, that could not be diagnosed except via serological diagnostic methods. Family members or friends who have a dietary history similar to that of a patient with paragonimiasis should be recommended to seek medical attention regardless of whether they are symptomatic.
Conclusion
When encountering a patient with eosinophilic pneumonia, paragonimiasis should be considered in case of a pneumothorax.
Abbreviations
CEP, Chronic eosinophilic pneumonia; BALF, Bronchoalveolar lavage fluid; CT, Computed tomography.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
The study was approved by the Institutional Ethics Committee of Showa University (approval number 22-225-B).
Patient Consent for Publication
The patient provided written informed consent for publication of the case details and any accompanying images.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yoshida A, Matsuo K, Moribe J, et al. Venison, another source of Paragonimus westermani infection. Parasitol Int. 2016;65:607–612. doi:10.1016/j.parint.2016.09.009
2. Diaz JH. Paragonimiasis acquired in the United States: native and nonnative species. Clin Microbiol Rev. 2013;26:493–504. doi:10.1128/CMR.00103-12
3. Suzuki Y, Suda T. Eosinophilic pneumonia: a review of the previous literature, causes, diagnosis, and management. Allergol Int. 2019;68:413–419. doi:10.1016/j.alit.2019.05.006
4. Crowe M, Robinson D, Sagar M, et al. Chronic eosinophilic pneumonia: clinical perspectives. Ther Clin Risk Manag. 2019;15:397–403. doi:10.2147/TCRM.S157882
5. Ahn CS, Shin JW, Kim JG, et al. Spectrum of pleuropulmonary paragonimiasis: an analysis of 685 cases diagnosed over 22 years. J Infect. 2021;82:150–158. doi:10.1016/j.jinf.2020.09.037
6. Nagayasu E, Yoshida A, Hombu A, et al. Paragonimiasis in Japan: a twelve-year retrospective case review (2001–2012). Intern Med. 2015;54:179–186. doi:10.2169/internalmedicine.54.1733
7. Wang J, Luo W, Shen P, et al. Retrospective study of pleural parasitic infestations: a practical diagnostic approach. BMC Infect Dis. 2019;19:576. doi:10.1186/s12879-019-4179-9
8. Kwon YS, Lee HW, Kim HJ. Paragonimus westermani infection manifesting as a pulmonary cavity and adrenal gland mass: a case report. J Infect Chemother. 2019;25:200–203. doi:10.1016/j.jiac.2018.08.005
9. Singh TS, Sugiyama H, Rangsiruji A. Paragonimus & paragonimiasis in India. Indian J Med Res. 2012;136:192–204.
10. Yoshida A, Doanh PN, Maruyama H. Paragonimus and paragonimiasis in Asia: an update. Acta Trop. 2019;199:105074. doi:10.1016/j.actatropica.2019.105074
11. Jeong YJ, Kim KI, Seo IJ, et al. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiographics. 2007;27:617–637. doi:10.1148/rg.273065051
12. Pope-Harman AL, Davis WB, Allen ED, et al. Acute eosinophilic pneumonia. A summary of 15 cases and review of the literature. Medicine. 1996;75:334–342. doi:10.1097/00005792-199611000-00004
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.