Back to Journals » Infection and Drug Resistance » Volume 15
Pneumocystis jirovecii and Mycobacterium tuberculosis Pulmonary Coinfection in an HIV-Seronegative Patient: A Case Report and Literature Review
Received 9 April 2022
Accepted for publication 21 July 2022
Published 30 July 2022 Volume 2022:15 Pages 4149—4154
DOI https://doi.org/10.2147/IDR.S370023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Shanchen Wei, Lianjun Lin
Geriatric Department, Peking University First Hospital, Beijing, 100034, People’s Republic of China
Correspondence: Lianjun Lin, Geriatric Department, Peking University First Hospital, Xicheng District, Beijing, 100034, People’s Republic of China, Email [email protected]
Background: Coinfection with Pneumocystis jirovecii and Mycobacterium tuberculosis is rare in HIV-seronegative patients. Because it is associated with unknown morbidity and a high mortality rate especially in patients with immunosuppression, health care practitioners should have a high index of suspicion when dealing with such patients.
Case Presentation: A 66-year-old man with glucocorticoid therapy for 9 years had a fever after getting a cold and developed respiratory failure rapidly within 3 days. He was given trimethoprim-sulfamethoxazole empirically before Pneumocystis pneumonia (PCP) was confirmed with the presence of cysts in the sputum. Although there was a partial improvement of symptoms, an area of consolidation on the left upper lung lobe gradually enlarged. Bronchoscopy was performed 3 times and Mycobacterium tuberculosis infection was finally diagnosed. For 1 years, he was treated with standard antituberculosis agents, and his psychological well-being was managed using traditional Chinese medicine techniques. After 3 years of follow-up, his outcome was very good.
Conclusion: HIV-seronegative patients on long-term glucocorticoid therapy in areas with a high incidence of Mycobacterium tuberculosis may be co-infected with Pneumocystis jirovecii. When opportunistic infections are suspected, diagnostic procedures including invasive ones should be performed as soon as possible and appropriate interventions need to be carried out promptly.
Keywords: Mycobacterium tuberculosis, Pneumocystis jirovecii, PCP, HIV-seronegative, Immunosuppressed, corticosteroids
Background
Pneumocystis jirovecii and Mycobacterium tuberculosis are common opportunistic pulmonary pathogens in patients infected with human immunodeficiency virus (HIV). However, co-infection is extremely rare in HIV-seronegative patients;1 while once occurred, it is severe and has a higher mortality rate.2 Early diagnosis is difficult and delays in antibiotic treatment are more common in HIV-negative patients, which may lead to a poor prognosis.3,4 Currently, there are no accurate diagnostic tools, so being aware of the possibility of coinfection and rapidly performing diagnostic procedures that may include invasive ones are key to improving clinical outcomes.
Case Presentation
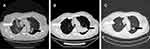
A 66-year-old man presented and was admitted to the hospital with fever for 3 days and dyspnea for 1 day. The patient had been diagnosed with malignant pemphigus vulgaris and put on prednisolone, methylprednisolone and other immunosuppressive drugs for the past 9 years. On admission, he experienced respiratory failure. The absolute count of regulatory T lymphocytes was 156.67 cells/uL. The lactate dehydrogenase increased to 457 IU/L. The chest computed tomography (CT) scan revealed multiple flaky, fuzzy, and high-density opacities in both lungs (Figure 1A). The patient had been on steroids for a long time. He had obvious hypoxia and had never had any other respiratory symptoms or other infections. On respiratory examination, the patient did not have many rales in the lungs. The lung CT scan showed interstitial changes. PCP, usually treated with sulfonamides, was considered a possible etiology because of his immunocompromised state. Caspofungin is a good treatment of choice for Aspergillus infection, which could not be excluded. For other atypical pathogens, moxifloxacin should be added. Thus he was given trimethoprim-sulfamethoxazole (3 tablets, twice daily for 3 weeks), caspofungin (loading dose, 70mg infused over 1 hour; maintenance dose, 50 mg daily infused over 1 hour for 2 weeks), steroids, and moxifloxacin. Consequently, his respiratory function improved, and his body temperature dropped. PCP was confirmed 4 days later when cysts were found in the sputum (Supplementary Material).
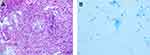
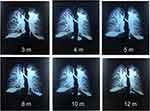
Curiously, the follow-up chest CT suggested an enlarged consolidation in the left upper lobe (Figure 1A–C). The patient was admitted to the hospital again due to cough, expectoration, and intermittent fever. Bronchoscopy was performed for 3 times. Smears and culture of the bronchial brush, tracheal secretions, and bronchoalveolar lavage fluid (BALF) were performed. Lung tissue was taken for culture and pathological examination, and tuberculosis was diagnosed (Figure 2). Antituberculosis treatment (isoniazid 300 mg once daily, rifapentine 600 mg twice weekly, ethambutol 750 mg once daily, and pyrazinamide 500 mg thrice daily) was given for 2 months and later ethambutol and pyrazinamide were excluded. Antituberculosis treatment lasted for 1 year. Simultaneously, traditional Chinese medicine techniques were used to manage the patient’s psychological well-being and improve mental state. After 3 years of follow-up, the clinical outcome was good (Figure 3). The patient’s complete course is shown in Figure 4.
Discussion and Conclusion
We performed a PubMed literature review of HIV-seronegative patients with PCP and tuberculosis (TB) coinfection using the keywords “tuberculosis” and “pneumocystis pneumonia”. From 2000 to 2021, a total of 17 cases including our case were reported. We divided these 17 reported cases based on the cause of the disease into the following categories: steroid therapy (alone or with chemotherapy) (8 cases), CD4+ lymphocytopenia (3 cases), malnutrition (1 case), visceral leishmaniasis (1 case), alcohol-related cirrhosis (1 case), and pancytopenia (1 case). There were 2 cases with no obvious cause (Figure 5). Based on these categories, steroid therapy appears to be the major predisposing factor for PCP and TB coinfection.
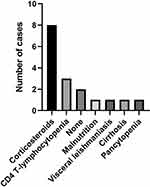 |
Figure 5 Distribution of predisposing factors for TB and PCP coinfection in cases reported in literature. |
Glucocorticoids have a variety of anti-inflammatory and immunosuppressive effects on virtually all immune cells, and their exact effects depend on the state of cell differentiation and activation.5 Glucocorticoid use increases the risk of new infections and reactivation of chronic infections, which influences the vaccines that are recommended.6 Observational studies from the “real world” consistently show a dose-dependent increase in the risk of severe infections and opportunistic infections such as TB and PCP.7 A Mayo Clinic study of 116 patients with PCP (without HIV) from 1985 to 1991 found that 105 patients (91%) received corticosteroids for multiple indications within 1 month of a PCP diagnosis. They had a median prednisone equivalent (PEQ) dose of 30 mg/day.8 In a case-control study, the adjusted odds ratio of TB was 2.8 (95% confidence interval [CI], 1.0–7.9) for less than a 15 mg daily dose of prednisone and 7.7 (95% CI, 2.8–21.4) for more than a 15 mg daily dose of prednisone.9 Vaccination and screening strategies should be used to mitigate this risk in patients who are starting chronic steroid therapy.6 Trimethoprim-sulfamethoxazole is an effective PCP prophylactic agent with a low risk of adverse effects.7 The tuberculin skin test (TST) or the interferon-gamma release assay (IGRA) can be used to screen and identify patients at a higher risk of active TB and treat them appropriately.10,11
In our case, we suspected that the patient had PCP and treated them accordingly before we received the sputum results. Bronchoscopy was done to diagnose TB because other non-invasive diagnostic procedures were not useful. Antituberculosis drug treatment was carried out in time. The patient was followed up for 3 years after discharge and his prognosis was good.
In conclusion, co-infection with Pneumocystis jirovecii and Mycobacterium tuberculosis may exist in HIV-seronegative patients on long-term glucocorticoid therapy. When it is suspected, diagnostic procedures including invasive ones should be done as soon as possible and appropriate therapeutic interventions need to be given promptly.
Abbreviations
CT, computed tomography; HIV, human immunodeficiency virus; PCP, pneumocystis pneumonia; TB, tuberculosis.
Data Sharing Statement
All data are presented in this manuscript and can be accessed through the corresponding author on request.
Ethics Approval and Consent to Participate
This study was approved by the institutional review boards of Peking University First Hospital (2021keyan111). The patient had given his written, informed consent to the use of his data for research.
Consent to Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Funding
This work was supported by National Key R&D Program of China (2020YFC2005401), Xicheng financial, scientific and technological project (XCSTS-SD2021-02), and Project funded by Baidu Fund of Peking University (2020BD045).
Disclosure
The authors declare that they have no competing interests.
References
1. Mongardon N, Bruneel F, Henry-Lagarrigue M, Legriel S, Azarian R, Bedos JP. Pneumonia involving Mycobacterium tuberculosis and Pneumocystis jiroveci in HIV-seronegative patients. Eur J Intern Med. 2008;19(7):e70–e72. doi:10.1016/j.ejim.2008.04.004
2. Suk CW, Bai KJ, Yu MC, Hu TY. Coinfection of Pneumocystis jiroveci pneumonia and pulmonary tuberculosis in a non-HIV-infected patient. J Microbiol Immunol Infect. 2015;48(6):711–712. doi:10.1016/j.jmii.2014.07.001
3. Li MC, Lee NY, Lee CC, Lee HC, Chang CM, Ko WC. Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J Microbiol Immunol Infect. 2014;47(1):42–47. doi:10.1016/j.jmii.2012.08.024
4. World Health Organization. Global Tuberculosis Report. World Health Organization; 2019.
5. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi:10.1016/j.mce.2010.04.005
6. Youssef J, Novosad SA, Winthrop KL. Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am. 2016;42(1):157–176. doi:10.1016/j.rdc.2015.08.004
7. Caplan A, Fett N, Rosenbach M, Werth VP, Micheletti RG. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: infectious complications and vaccination recommendations. J Am Acad Dermatol. 2017;76(2):191–198. doi:10.1016/j.jaad.2016.02.1240
8. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13. doi:10.4065/71.1.5
9. Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Rheum. 2006;55(1):19–26. doi:10.1002/art.21705
10. Abubakar I, Stagg HR, Whitworth H, Lalvani A. How should I interpret an interferon gamma release assay result for tuberculosis infection? Thorax. 2013;68(3):298–301. doi:10.1136/thoraxjnl-2013-203247
11. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev. 2014;27(1):3–20. doi:10.1128/CMR.00034-13
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.