Back to Journals » Vascular Health and Risk Management » Volume 19
Platelet, Neutrophil and Lymphocyte Quantitative Abnormalities in Patients with Heart Failure: A Retrospective Study
Authors Getawa S , Bayleyegn B
Received 25 October 2022
Accepted for publication 1 February 2023
Published 5 February 2023 Volume 2023:19 Pages 69—78
DOI https://doi.org/10.2147/VHRM.S394765
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Daniel Duprez
Solomon Getawa, Biruk Bayleyegn
Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Correspondence: Solomon Getawa, Department of Hematology and Immunohematology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, P.O.Box: 196, Gondar, Ethiopia, Tel +251-914-665-736, Email [email protected]
Background: Heart failure pathophysiology and its clinical symptoms are characterized by inflammation. Elevated levels of leukocyte subpopulations are a well-known indicator of inflammation and play a predictive role in determining the prognosis of patients with cardiovascular diseases. Besides, platelets are essential mediators of inflammation, especially when they interact with leukocytes. Platelet synthesis, activation, and function are all impacted by heart failure. Thus, the study was aimed at determining the magnitude of platelet, neutrophil, and lymphocyte abnormalities in patients with heart failure.
Methods: A retrospective cross-sectional study was conducted from June to July 2022 at the University of Gondar comprehensive specialized hospital. A total of 245 medical records of heart failure patients were included. Data regarding socio-demographic, clinical, and some hematological and biochemical parameters were collected from medical records. Data was entered into Epi-Data 4.6.0.2 and then exported to Stata 11.0 statistical software for analysis. A binary logistic regression analysis with its odds ratio was calculated to identify factors associated with the outcome variables. P-value < 0.05 was considered statistically significant.
Results: The most frequent leukocyte abnormality among adults with heart failure was neutrophilia, which was detected in 17.55% (95% CI: 13.26– 22.87). Besides, lymphocytosis was observed in 10.20% (95% CI: 6.97– 14.70) of patients. The magnitude of thrombocytopenia and thrombocytosis among patients with heart failure was 12.24% (95% CI: 8.67– 17.01%) and 2.86% (95% CI: 1.36– 5.90%), respectively. Only being female was significantly associated with neutrophilia in patients with heart failure (AOR = 2.33; 95% CI: 1.05– 5.16). However, none of the variables were significantly associated with platelet and lymphocyte abnormalities.
Conclusion: Neutrophilia, lymphocytosis, and thrombocytopenia are the common leukocyte and platelet abnormalities in heart failure patients. Therefore, early detection and management of the underlying causes of those abnormalities may be important to improve patients’ outcomes and prevent further complications.
Keywords: heart failure, platelet, neutrophil, lymphocyte, neutrophilia, lymphocytosis, thrombocytopenia
Introduction
Heart failure, the end stage of all types of cardiovascular disease (CVD), has become a major public health challenge in terms of incidence, prevalence, morbidity, and mortality globally, particularly in low- and middle-income countries in recent years.1–3 The comprehensive epidemiological study found that the pooled prevalence of CVD in Ethiopia was 5%, with a prevalence range between 1% and 20%.4
Previous studies have proven that an inflammatory process could be an important part of the etiology of heart failure.5 This could also show that various systemic inflammation markers, such as tumour necrosis factor, interleukin-6, C-reactive protein, and erythrocyte sedimentation rate, are elevated and associated with an increased risk of heart failure.6 Leukocyte concentration in blood is also another classical biomarker of inflammation, and the amount of inflammation may be directly related to the increased incidence of heart failure and hospitalization.7,8 Based on this promising future, current evidence indicates that hematological parameters of three cell lines, including leukocytes, red blood cells, and platelets, as well as their indices, have emerged as potential preclinical indicators for identifying individuals at risk of developing heart failure.9
Increased numbers of leukocytes and their subtypes, including neutrophils, basophils, and monocytes/macrophages, have been associated with strong and independent predictors of increased risk of coronary heart disease and death.7,10 More specifically, increased neutrophil and decreased lymphocyte counts were common findings in patients with heart failure and are independently and significantly associated with a poor prognosis for heart failure.9,11,12
Moreover, heart failure affects platelet production, activation, and functions.13 Although the utility of hematological parameters as potential risk factors for CVD has long been debated, platelet count, platelet distribution width (PDW), and mean platelet volume (MPV) continue to play an important role as potential contributing risk factors for CVD today.14 A study conducted by Polat et al showed that decreased platelet and lymphocyte counts were found to be independently correlated with one-year mortality in heart failure and reduced ejection fraction (HFrEF).15 However, in contrast to this, an increase in circulating platelet counts has been reported as an indicator of a poor prognostic marker in cardiovascular disease. This is because the release of various mediators during the inflammatory response, as well as increased platelet counts in the circulation, cause thrombosis-related complications in patients with congestive heart failure (CHF).16 Therefore, this study was aimed at determining the magnitude of different leukocyte and platelet count abnormalities and their associated factors among patients with heart failure.
Methods and Materials
Study Setting
The current study was conducted at the University of Gondar comprehensive specialized hospital which is located in Gondar town, Amhara regional state, and 738 km from Addis Ababa and 175 km from Bahir Dar. Gondar is situated at latitude and longitude 12°36′N and 37°28′E, and its elevation is 2133 meters above sea level. More than seven million residents of the central Gondar zone as well as those of other areas are served by this hospital. The hospital offers services like antenatal care, delivery, postnatal care, laboratory, pharmacy, maternity and neonatal care, internal medicine, pathology, surgery, dermatology, and other services.17
Study Design and Period
A retrospective cross-sectional study was conducted from June to July 2022.
Population
Heart failure patients who had been registered in the chronic follow-up clinic at the University of Gondar comprehensive specialized hospital were taken as a source population. Heart failure patients who were enrolled in the chronic follow-up clinic during the study period and who had complete data on essential variables in their medical records were included in the study population.
Eligibility Criteria
All adult patients with proven cases of acute or chronic heart failure who had complete data in their medical records and regular follow-up at the chronic follow-up clinic of the University of Gondar comprehensive specialized hospital during the study period were reviewed. The study excluded heart failure patients whose medical records revealed confirmed hematological disorders, transfusion histories, vitamin and iron supplementation, or incomplete information on the study variables.
Sample Size Determination
Due to the retrospective, cross-sectional design of the study, the sample size calculation was not appropriate. A total of 245 heart failure patients who had complete follow-up data on essential variables in their medical records were included in this study.
Operational Definitions
Platelet abnormalities such as thrombocytopenia and thrombocytosis were defined as the platelet count below 150,000/μL and above 450,000/μL, respectively.18 Total neutrophil count below 1500 cells/μL and lymphocyte count below 600 cells/μL, respectively, are used to define neutropenia and lymphopenia, whereas neutrophilia and lymphocytosis are defined as neutrophil counts >7000 cells/l and lymphocyte counts >4500 cells/l, respectively.19 Hypoglycaemia is defined as a blood glucose level of ≤70 mg/dL.20 Besides, biochemical parameters including serum glutamic pyruvic transaminase (SGPT), serum glutamic-oxaloacetic transaminase (SGOT) and creatinine level were defined based on their reference range.21
Data Extraction
Data on socio-demographic and clinical factors were gathered from the medical records of patients and entered into a data extraction form. Information on hematology laboratory results, such as platelet count, mean platelet volume, absolute and relative counts of neutrophils and lymphocytes, and biochemical parameters, such as serum creatinine, fasting blood glucose (FBS) level, SGPT and SGOT, and creatinine, was gathered from the patients’ medical records.
Data Quality Assurance
The accuracy of the data was guaranteed by properly extracting it from the patient’s medical records. Besides, training was provided for the data collectors.
Data Analysis and Interpretation
The data was entered into Epi-Data version 4.6.0.2 software and then exported to Stata 11.0 statistical software (Stata Corp, College Station, TX, USA) for analysis. To summarize the characteristics of the study participant’s descriptive statistics were performed. Factors associated with platelet, neutrophil and lymphocyte abnormalities in heart failure patients were assessed by performing binary logistic regression analyses. Crude odds ratio (COR) and adjusted odds ratio (AOR) with a 95% confidence interval (CI) were calculated to determine the strength of the association of independent variables with the outcome variables. A p-value of <0.05 was considered statistically significant.
Ethical Consideration
Before beginning the actual data collection, the study protocol was reviewed and approved by the ethical review committee of the School of Biomedical Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. Besides, the study was conducted per the declaration of Helsinki. The chief executive and clinical director of the hospital provided a letter of approval to conduct this study. The ethical review committee waived the requirement for patient consent because the data were obtained from medical records. Through the use of codes in an anonymous data collection method, data confidentiality was guaranteed.
Results
Socio-Demographic and Clinical Characteristics of Heart Failure Patients
A total of 245 heart failure patients’ medical records were reviewed for the current study. The mean ± standard deviation (SD) age of the study participants was 55.8±16.9 years. The majority of the patients, 62% (152) were women. More than half of the participants, 145 (59.2%) were from urban areas. Ischemic heart disease (IHD) was the most common cause of heart failure. Nearly half (46.9%) of the heart failure patients were in class II according to the NYHA functional class (Table 1). Besides, nearly all (93.5%) of the study participants had developed one or more comorbidities, with hypertensive diseases being the most common (66.81%) (Figure 1). Regarding their treatment regimens, the majority of patients used beta-blockers, Lasix (58.37%), and angiotensin-converting enzyme inhibitors; (50.62%) Enalapril (Figure 2).
 |
Table 1 Socio-Demographic and Clinical Characteristics of the Heart Failure Patients at the University of Gondar Comprehensive Specialized Hospital, 2022 |
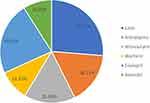 |
Figure 2 Major medication received by heart failure patients at the University of Gondar comprehensive specialized hospital. |
Hematological and Biochemical Findings
In the current study, some hematological and biochemical parameters of heart failure patients were collected from their medical records. Accordingly, the platelet count of patients with heart failure ranges from 29.0 to 780.0×103 cells/μL with a mean of 239.06±91.25×103 cells/μL. The neutrophil and lymphocyte count of the study participants were 5.28±2.64×103 cells/μL and 2.65±1.67×103 cells/μL, respectively. Besides, the mean level of FBS and creatinine was 117.26±36.26 mg/dL and 0.78±0.45 mg/dL, respectively (Table 2).
 |
Table 2 Some Hematogical and Biochemical Parameters of Adults with Heart Failure Attending at the University of Gondar Comprehensive Specialized Hospital |
Prevalence of Platelet, Neutrophil and Lymphocyte Abnormalities
In the current study, the most frequent white cell abnormalities among adults with heart failure were neutrophilia, which was detected in 17.55% (95% CI: 13.26–22.87). Besides, lymphocytosis was observed in 10.20% (95% CI: 6.97–14.70) of adult patients with heart failure. The prevalence of thrombocytopenia and thrombocytosis among patients with heart failure was 12.24% (95% CI: 8.67–17.01%) and 2.86% (95% CI: 1.36–5.90), respectively (Figure 3).
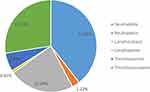 |
Figure 3 Platelet, neutrophil and lymphocyte abnormalities among patients with heart failure attending at the university of Gondar comprehensive specialized hospital. |
Factors Associated with Platelet and Neutrophil Abnormalities
In this study, to assess factors associated with neutrophilia, we performed both univariate and multivariate logistic regression analysis. The sex of study participants, age, duration, comorbidity, elevated levels of SGPT, SGOT, and creatinine all have p values of 0.2 during univariate logistic regression analysis. Then, those variables were re-analysed in final multivariable logistic regression models to control confounders and only being female (AOR = 2.33 (95% CI: 1.05–5.16)) was significantly associated with neutrophilia in patients with heart failure (Table 3). Besides, we did both univariate and multivariate logistic regression models to find out the possible factors associated with thrombocytopenia and lymphocytosis. However, none of the variables were significantly associated with platelet and lymphocyte abnormalities.
 |
Table 3 Logistic Regression Analysis of Factors Associated with Neutrophilia Among Patients with Heart Failure at the University of Gondar Comprehensive Specialized Hospital |
Discussion
Since inflammatory processes can be closely related to the pathophysiology of heart failure, treating inflammation is currently thought to be a novel therapeutic target for the treatment of heart failure.22 Elevated levels of leukocyte subpopulations are a well-established risk factor and prognostic indicator for individuals with cardiovascular diseases and a classic indication of acute or chronic systemic inflammation.7 Additionally, platelets play a crucial role in inflammation as mediators, particularly when they interact with leukocytes and endothelial cells.23,24 Moreover, platelet-to-monocyte and platelet-to-lymphocyte ratios have been considered as potential biomarkers to evaluate systemic inflammation in a variety of clinical situations.25,26 Therefore, the current study was aimed to assess the magnitude of platelet, neutrophil and lymphocyte abnormalities in patients with heart failure.
In the current study, the frequent white cell abnormalities among adults with heart failure were neutrophilia which was detected in 17.55% (95% CI: 13.26–22.87) of patients. The study’s findings were consistent with previous studies on heart failure patients by Jan et al,9 Ahmad et al,8 and Bajaj et al.27 Additionally, a meta-analysis study by Wheeler et al demonstrated the link between an increase in neutrophil counts and coronary heart disorders.28 Patients with ischemic heart failure also noted a rise in neutrophil count.29 A community-based study by Arruda-Olson et al revealed that neutrophilia can also predict the incidence of mortality and heart failure after myocardial infarction.30 The possibility is that this is because neutrophils are the first leukocytes to invade the damaged myocardium.31 Numerous proteolytic enzymes, including elastase32 and myeloperoxidase33 are released by activated neutrophils and have the potential to cause tissue damage. The production of cytokines during an inflammatory response may encourage demargination of intravascular neutrophils and accelerate the discharge of neutrophils from the bone marrow.34 Besides, evidences showed that the lifespan of neutrophils is also extended in congestive heart failure35 and coronary plaques.36 Being female sex was 2.33 times more likely to have high neutrophil count in patients with heart failure (AOR=2.33 (95% CI: 1.05–5.16)). The finding of the study was comparable with a community-based study done by Adelaide et al in Minnesota.30
High lymphocyte count was observed in 10.20% (95% CI: 6.97–14.70) of patients with heart failure. According to earlier research, myocardial injury activates the immune system, which then causes an infiltration into the heart tissue.37 These immune cells help the myocardium’s regeneration signals and removal of necrotic material. Cardiomyocyte dysfunction, apoptosis, and fibrosis may be brought on by immune cells that are overactive in the heart tissue.38 On the other hand, the magnitude of lymphopenia was very low (0.41%) in the current study. Previous studies confirm that low proportion of lymphocytes has been associated with, poor prognosis, worse outcomes and higher mortality in heart failure patients.39,40 The rise in cortisol during a stress response, which results in a drop in the relative concentration of lymphocytes, may be the cause of the lymphopenia occurrence.41 Other potential causes of peripheral lymphopenia in heart failure patients include the relocation of lymphocytes from peripheral blood to other locations, decreased production, and faster cell death due to apoptosis through down-modulation of Bcl-2 gene product.42,43
Thrombocytopenia was the common platelet abnormalities among patients with heart failure as compared to thrombocytosis (12.24% vs 2.86%) in the current study. The finding of the study was similar previous studies conducted in New York,44 Romania,13) and Gondar; Ethiopia.45 The common cause of thrombocytopenia in various cardiovascular diseases is related to an abnormal immune response brought on by drug therapy, such as intravenous blood thinner (heparin), diuretics to treat high blood pressure or symptoms of congestive heart failure, anti-diabetic medications to manage diabetes, or blood clotting medications (antiplatelet drugs).46 Thrombocytopenia in heart failure patients with reduced and perceived ejection fraction is an independent predictor of mortality and can be used as a prognostic biomarker to assess patient prognosis.13,44 However, high platelet counts brought on by an inflammatory response and platelet activation47 have been reported and linked to poor outcomes in heart disease.48 Additionally, higher levels of platelet activation and enhanced cytokine and catecholamine production have been linked to severe heart failure.48
The current study has limitations and readers should consider the following limitations. Due to the retrospective nature of the study, some essential clinical variables, including left ventricle ejection fraction and behavioural characteristics, are not analysed and discussed, which may potentially limit the finding. Besides, the prognostic role of such platelet and leukocyte parameters to predict diseases prognosis, treatment outcome and incidence of mortality were not assessed. Moreover, the long-term impact of platelet, neutrophil, and lymphocyte abnormalities in those heart failure patients is not explained.
Conclusion
The current study confirms that neutrophilia, followed by lymphocytosis, are the most common leukocyte abnormalities in patients with heart failure. Besides, thrombocytopenia was identified as the major platelet abnormality in the current study. Female sex was significantly associated with neutrophilia in patients with heart failure. However, none of the variables were significantly associated with platelet and lymphocyte abnormalities. Thus, counting, early screening and management of such platelet and leukocyte abnormalities might be important to prevent further complications and improve the outcomes of heart failure patients. Moreover, a large-scale prospective study is required to determine the long-term impact of such abnormalities in patients with heart failure.
Abbreviations
AOR, Adjusted Odds Ratio; CHF, Congestive Heart Failure; CI, Confidence Interval; COR, Crude Odds Ratio; CRD, Chronic Renal Disease; CRVHD, Chronic Valvular Heart Disease; CVD, Cardiovascular Disease; FBS, Fasting Blood Glucose; NYHA, New York Heart Association; RHD, Rheumatic Heart Disease; WHO, World Health Organization.
Data Sharing Statement
The datasets generated and/or analysed during the study are available within the manuscript and its supporting information.
Acknowledgments
We would like to acknowledge staff members of University of Gondar comprehensive specialized hospital chronic illness clinic for their cooperation during data collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Naik N, Narula J. Heart failure in low-income and middle-income countries: failing REPORT card grades. Lancet Global Health. 2020;8(3):e318. doi:10.1016/S2214-109X(20)30028-0
2. Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–1690. doi:10.1093/eurjpc/zwaa147
3. Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. Eur J Heart Fail. 2020;22(8):1342–1356. doi:10.1002/ejhf.1858
4. Angaw DA, Ali R, Tadele A, Shumet S. The prevalence of cardiovascular disease in Ethiopia: a systematic review and meta-analysis of institutional and community-based studies. BMC Cardiovasc Disord. 2021;21(1):1–9. doi:10.1186/s12872-020-01828-z
5. Zhu Z, Zhou S. Leukocyte count and the risk of adverse outcomes in patients with HFpEF. BMC Cardiovasc Disord. 2021;21(1):1–9. doi:10.1186/s12872-021-02142-y
6. Kalogeropoulos A, Georgiopoulou V, Psaty BM, et al. Inflammatory markers and incident heart failure risk in older adults: the Health ABC (Health, Aging, and Body Composition) study. J Am Coll Cardiol. 2010;55(19):2129–2137. doi:10.1016/j.jacc.2009.12.045
7. Engström G, Melander O, Hedblad B. Leukocyte count and incidence of hospitalizations due to heart failure. Circ Heart Fail. 2009;2(3):217–222. doi:10.1161/CIRCHEARTFAILURE.108.827071
8. Ahmad A, Alharbi MN, Aldhabaan OA, et al. Prevalence and risk factors of leukocytosis among heart failure patients. Int J Med Dev Countries. 2019;3(5):468–473. doi:10.24911/IJMDC.51-1547423021
9. Jan AF, Habib S, Naseeb K, Khatri MA, Zaman KS. High total leukocyte count and heart failure after myocardial infarction. Pakistan Heart J. 2011;44:1–2.
10. Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Texas Heart Institute J. 2013;40(1):17.
11. Núñez J, Miñana G, Bodí V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–3233. doi:10.2174/092986711796391633
12. Bian C, Wu Y, Shi Y, et al. Predictive value of the relative lymphocyte count in coronary heart disease. Heart Vessels. 2010;25(6):469–473. doi:10.1007/s00380-010-0010-7
13. Delcea C, Buzea C, Daha I, et al. P751 Low platelets in heart failure: small cells, important impact on all-cause long-term mortality. Eur Heart J. 2019;40(Supplement_1):ehz747. doi:10.1093/eurheartj/ehz747.0353
14. Lassale C, Curtis A, Abete I, van der Schouw Y, Verschuren W, Lu Y. Elements of the complete blood count associated with cardiovascular disease incidence: findings from the EPIC-NL cohort study. Sci Rep. 2018;8(1):1–11. doi:10.1038/s41598-018-21661-x
15. Polat N, Yildiz A, Bilik MZ, et al. The importance of hematologic indices in the risk stratification of patients with acute decompensated systolic heart failure. Turk Kardiyol Dern Ars. 2015;43(2):157–165. doi:10.5543/tkda.2015.76281
16. Bao K, Huang H, Huang G, et al. Platelet-to-hemoglobin ratio as a valuable predictor of long-term all-cause mortality in coronary artery disease patients with congestive heart failure. BMC Cardiovasc Disord. 2021;21(1):1–9. doi:10.1186/s12872-021-02423-6
17. Kassa E, Enawgaw B, Gelaw A, Gelaw B. Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar Hospital, Northwest Ethiopia. BMC Hematol. 2016;16(1):1–11. doi:10.1186/s12878-015-0037-1
18. Erkurt MA, Kaya E, Berber I, Koroglu M, Kuku I. Thrombocytopenia in adults. J Hematol. 2012;1(2–3):44–53.
19. Tefferi A, Hanson CA, Inwards DJ. How to Interpret and Pursue an Abnormal Complete Blood Cell Count in Adults. Elsevier; 2005.
20. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36(5):1384–1395. doi:10.2337/dc12-2480
21. Bisetegn H, Feleke DG, Debash H, Erkihun Y, Ebrahim H. Hematological and Biochemical changes in Schistosoma mansoni infected patients at Haik Primary Hospital, North-East Ethiopia: a comparative cross-sectional study. PLoS Negl Trop Dis. 2022;16(8):e0010728. doi:10.1371/journal.pntd.0010728
22. Glezeva N, Voon V, Watson C, et al. Exaggerated inflammation and monocytosis associate with diastolic dysfunction in heart failure with preserved ejection fraction: evidence of M2 macrophage activation in disease pathogenesis. J Card Fail. 2015;21(2):167–177. doi:10.1016/j.cardfail.2014.11.004
23. Glezeva N, Gilmer JF, Watson CJ, Ledwidge M. A central role for monocyte–platelet interactions in heart failure. J Cardiovasc Pharmacol Ther. 2016;21(3):245–261. doi:10.1177/1074248415609436
24. Davì G, Patrono C. Platelet activation and atherothrombosis. N Eng J Med. 2007;357(24):2482–2494. doi:10.1056/NEJMra071014
25. Balta S, Ozturk C. The platelet-lymphocyte ratio: a simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets. 2015;26(7):680–681. doi:10.3109/09537104.2014.979340
26. Uçar FM, Açar B, Gul M, Özeke Ö, Aydogdu S. The association between platelet/lymphocyte ratio and coronary artery disease severity in asymptomatic low ejection fraction patients. Korean Circ J. 2016;46(6):821–826. doi:10.4070/kcj.2016.46.6.821
27. Bajaj NS, Kalra R, Gupta K, et al. Leucocyte count predicts cardiovascular risk in heart failure with preserved ejection fraction: insights from TOPCAT Americas. ESC Heart Failure. 2020;7(4):1676–1687. doi:10.1002/ehf2.12724
28. Wheeler JG, Mussolino ME, Gillum RF, Danesh J. Associations between differential leucocyte count and incident coronary heart disease: 1764 incident cases from seven prospective studies of 30 374 individuals. Eur Heart J. 2004;25(15):1287–1292. doi:10.1016/j.ehj.2004.05.002
29. Kain V, Halade GV. Role of neutrophils in ischemic heart failure. Pharmacol Ther. 2020;205:107424. doi:10.1016/j.pharmthera.2019.107424
30. Arruda-Olson AM, Reeder GS, Bell MR, Weston SA, Roger VL. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes. 2009;2(6):656–662. doi:10.1161/CIRCOUTCOMES.108.831024
31. Russell CJ, Exley AR, Ritchie AJ. Widespread coronary inflammation in unstable angina. N Eng J Med. 2003;348(19):1931. doi:10.1056/NEJM200305083481919
32. Dinerman JL, Mehta JL, Saldeen TG, et al. Increased neutrophil elastase release in unstable angina pectoris and acute myocardial infarction. J Am Coll Cardiol. 1990;15(7):1559–1563. doi:10.1016/0735-1097(90)92826-N
33. Biasucci LM, D’Onofrio G, Liuzzo G, et al. Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996;27(3):611–616. doi:10.1016/0735-1097(95)00524-2
34. Naruko T, Ueda M, Haze K, et al. Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002;106(23):2894–2900. doi:10.1161/01.CIR.0000042674.89762.20
35. Tracchi I, Ghigliotti G, Mura M, et al. Increased neutrophil lifespan in patients with congestive heart failure. Eur J Heart Fail. 2009;11(4):378–385. doi:10.1093/eurjhf/hfp031
36. Narducci ML, Grasselli A, Biasucci LM, et al. High telomerase activity in neutrophils from unstable coronary plaques. J Am Coll Cardiol. 2007;50(25):2369–2374. doi:10.1016/j.jacc.2007.08.048
37. Yan X, Anzai A, Katsumata Y, et al. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J Mol Cell Cardiol. 2013;62:24–35. doi:10.1016/j.yjmcc.2013.04.023
38. Frangogiannis NG. Regulation of the inflammatory response in cardiac repair. Circ Res. 2012;110(1):159–173. doi:10.1161/CIRCRESAHA.111.243162
39. Rudiger A, Burckhardt OA, Harpes P, Müller SA, Follath F. The relative lymphocyte count on hospital admission is a risk factor for long-term mortality in patients with acute heart failure. Am J Emerg Med. 2006;24(4):451–454. doi:10.1016/j.ajem.2005.10.010
40. Vaduganathan M, Ambrosy AP, Greene SJ, et al. Predictive value of low relative lymphocyte count in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial. Circ Heart Fail. 2012;5(6):750–758. doi:10.1161/CIRCHEARTFAILURE.112.970525
41. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97(1):19–22. doi:10.1161/01.CIR.97.1.19
42. Chao DT, Korsmeyer SJ. BCL-2 family: regulators of cell death. Annu Rev Immunol. 1998;1:16.
43. Strasser A, Harris AW, Huang D, Krammer PH, Cory S. Bcl‐2 and Fas/APO‐1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14(24):6136–6147. doi:10.1002/j.1460-2075.1995.tb00304.x
44. Mojadidi MK, Galeas JN, Goodman-Meza D, et al. Thrombocytopaenia as a prognostic indicator in heart failure with reduced ejection fraction. Heart Lung Circ. 2016;25(6):568–575. doi:10.1016/j.hlc.2015.11.010
45. Aynalem M, Adane T, Getawa S. Magnitude of Coagulation Abnormalities and Associated Factors Among Patients with Heart Diseases at the University of Gondar Comprehensive Specialized Hospital. Vasc Health Risk Manag. 2022;18:617. doi:10.2147/VHRM.S371912
46. Gregg D, Goldschmidt-Clermont PJ. Platelets and cardiovascular disease. Circulation. 2003;108(13):e88–e90. doi:10.1161/01.CIR.0000086897.15588.4B
47. Klinger MH, Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22(9):913–922. doi:10.1089/10799900260286623
48. Chung I, Lip GY. Platelets and heart failure. Eur Heart J. 2006;27(22):2623–2631. doi:10.1093/eurheartj/ehl305
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

