Back to Journals » International Journal of General Medicine » Volume 12
Platelet-Derived Microparticles are an Important Biomarker in Patients with Cancer-Associated Thrombosis
Authors Yamanaka Y, Sawai Y, Nomura S
Received 26 October 2019
Accepted for publication 19 December 2019
Published 3 January 2020 Volume 2019:12 Pages 491—497
DOI https://doi.org/10.2147/IJGM.S236166
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Yuta Yamanaka, Yusuke Sawai, Shosaku Nomura
First Department of Internal Medicine, Kansai Medical University, Hirakata, Japan
Correspondence: Shosaku Nomura
First Department of Internal Medicine, Kansai Medical University, 2-3-1 Shinmachi, Hirakata, Osaka 573-1191, Japan
Tel + 81 724 45 1000
Fax + 81 725 32 1113
Email [email protected]
Background: Platelet-derived microparticles (PDMPs) that ultimately cause vascular complications might be used as a tool to assess thrombotic areas. We identified PDMPs, high-mobility group box-1 (HMGB1) and soluble endothelial protein C receptor (sEPCR) as useful prognosis indicators for cancer-related thrombosis (CAT) to evaluate the utility of PDMPs in cancer patients.
Methods: We investigated 232 cancer patients: 24 (10.3%) had thrombotic complications within 6 months after their first examination. Levels of PDMP and biomarkers were measured by enzyme-linked immunosorbent assay.
Results: The levels of PDMPs, HMGB1 and sEPCR were higher in cancer patients compared with controls. In particular, these levels were significantly elevated in lung cancer patients compared with controls, and all were higher in CAT-positive patients compared with CAT-negative patients. In particular, PDMP levels in CAT-positive patients were significantly elevated compared with CAT-negative patients. PDMP levels were significantly lower in patients who lived for more than 901 days after their first examination compared with previous data. PDMP levels were positively correlated with HMGB1, and caused the dose-dependent elevation of PDMPs in vitro using platelet-rich plasma from healthy persons.
Conclusion: The combined increase in PDMP and HMGB1 levels might be related to CAT in cancer patients. Therefore, coagulatory dysfunction may result from increased levels of these biomarkers and contribute to the poor prognosis of cancer patients.
Keywords: PDMP, CAT, HMGB1, sEPCR, cancer patients
Introduction
Hypercoagulability occurs in many cancer patients.1,2 This is based on “Virchow’s triad“ including hemodynamic disruption, intrinsic hypercoagulability, and endothelial dysfunction. The risk of thrombosis and the solidification asthenia state in cancer patients has led to an increased death rate.2,3 When thrombosis occurs in conjunction with cancer it is termed cancer-associated thrombosis (CAT).4,5 In CAT, vein thromboembolism (VTE) often develops.4 Various potential predictive biomarkers for VTE have been proposed,6–8 including the analysis of blood corpuscles.6 In addition, D-dimer, prothrombin1+2, or the density of sP-selectin might also predict the risk of VTE.8 Furthermore, the analysis of high-mobility group box-1 (HMGB1) or soluble endothelial protein C receptor (sEPCR) were reported to predict the risk of VTE development.9,10
Platelet-derived microparticles (PDMPs) are small circulating membrane fragments shed from the surface of platelets which have roles in normal hemostatic responses to vascular injury.11,12 Platelets shed PDMP during activation, storage, and apoptosis13 and it is thought that PDMPs contribute to thrombin generation and thrombus formation by generating tissue factors.11,14 Therefore, PDMPs that ultimately cause vascular complications might be used as a tool to assess thrombotic areas.15
Here, we evaluated the utility of PDMPs in cancer patients. We identified PDMPs, HMGB1 and sEPCR as useful prognosis indicators for CAT.
Patients and Methods
Patients
Cancer patients and healthy volunteers were recruited from Kansai Medical Hirakata Hospital and Kansai Medical University (Osaka, Japan) between September 2012 and March 2017. The study group included 50 normal controls and 232 cancer patients. Of the cancer patients, 24 (10.3%) had thrombotic complications within 6 months after their first examination (Table 1). The types of cancer included malignant lymphoma (ML; n = 53), multiple myeloma (MM; n = 56), and lung cancer (LC; n = 123) (Table 1). This study was conducted in accordance with the Declaration of Helsinki and was performed with approval from the Institutional Review Board of Kansai Medical University. Written informed consent was obtained from all participants.
 |
Table 1 Various Biomarker Levels in Cancer Patients |
Measuring HMGB1 and sEPCR
Patient blood samples were collected in plain or sodium citrate-containing tubes and left at room temperature for a minimum of 1 h. Serum and citrated plasma were isolated by centrifugation for 20 min at 1000 ×g at 4 °C. Serum was divided into aliquots and frozen at −30 °C until use. Recombinant products and standard solutions provided with commercial kits served as positive controls. HMGB1 was measured using the HMGB1 ELISA Kit II (Shino-test Corp., Kanagawa, Japan). Plasma sEPCR levels were measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems Inc., Minneapolis, MN, USA). All kits were used according to the manufacturers’ instructions. Normal ranges were as follows: HMGB1: 1.2–4.8 ng/mL and sEPCR: 30–150 ng/mL.
Measuring PDMPs
Blood samples were collected from a peripheral vein using a 21-gauge needle and placed into vacutainers containing ethylenediaminetetraacetic acid (NIPRO Co. Ltd., Osaka, Japan) to minimize platelet activation. The samples were handled as described in the manufacturer’s protocol. Briefly, the samples were gently mixed by inverting the tube once or twice, stored at room temperature for 2–3 h, and centrifuged at 8000 ×g for 5 min at room temperature. Immediately after centrifugation, 200 μL of the upper layer supernatant from the 2 mL samples was collected to avoid contamination with platelets. The collected samples were stored at −40°C until analysis. PDMP levels were measured twice and mean values were calculated. Furthermore, basic studies were carried out prior to this assessment using clinical specimens. An ELISA kit used for PDMP measurements was obtained from JIMRO Co. Ltd. (Tokyo, Japan).16,17 The kit included two monoclonal antibodies against glycoproteins CD42b and CD42a. One U/mL of PDMPs in the ELISA kit was defined as the amount of PDMPs obtained from 24,000 solubilized platelets/mL. The performance of the kit was acceptable as reproducible intra-assay (1.1–4.0%) and inter-assay (5.2–8.8%) coefficients of variation were obtained. Recombinant products and standard solutions provided with the commercial kit were used as positive controls, and the kit was used in accordance with the manufacturer’s instruction.
Specificity of PDMPs
In whole blood collected in citrate buffer, we observed that platelet activation occurred during centrifugation and resulted in a clear elevation of PDMPs compared with blood collected and anticoagulated with EDTA-ACD. We determined optimal centrifugation conditions needed to measure PDMP by changing gravitational acceleration and centrifugation time.17 The stabilizing values of PDMPs were obtained at a centrifugal acceleration of 6000 g and a centrifugation time of 3 min. Based on these data, we obtained standard conditions for centrifugal acceleration (8000 g) and time (5 min).17 PDMPs are considered ectosomes, which are ideal for estimating extracellular vesicle (EV) size.18 Ectosomes are EVs ranging in size from 10 to 1000 nm. PDMP and ectosomes are constitutively released from the surface of cells and their formation can be upregulated by cellular activation.18
Effect of HMGB1 on PDMPs in Normal Platelet-Rich Plasma
Platelet-rich plasma from healthy persons (n = 5) was treated with purified HMGB1 (R&D Systems, Minneapolis, MN, USA) at various concentrations (100–1200 ng/mL) for 60 min. After treatment, PDMPs were collected by the above mentioned method. PDMP levels were measured five times by ELISA, and mean volumes were calculated.
Statistics
Data are expressed as the mean ± standard deviation (SD). A receiver operating characteristics (ROC) curve analysis was used to estimate the value of each biomarker. Between-group comparisons were made using the Newman–Keuls test and Scheffe’s test. Comparison of the two variables was done by Pearson’s or Spearman correlation tests. The correlation between PDMP concentration and continuous variables was assessed using multivariate linear regression analysis. All statistical analyses were performed using StatFlex v7 software, with P-values < 0.05 considered statistically significant.
Results
Levels of Biomarkers in Cancer Patients
We confirmed that all biomarker values were distributed normally and we estimated the value of each biomarker using a ROC curve. Lung cancer was significantly more frequent in men than in healthy controls. The age of the cancer patients was higher than that of the healthy controls. The levels of PDMPs, HMGB1 and sEPCR were higher in cancer patients compared with controls (Table 1). In particular, these levels were significantly elevated in lung cancer patients compared with controls (p < 0.001; Table 1).
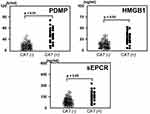
Analysis of Three Biomarkers in Relation to Thrombotic Complications
Next, we compared the concentrations of these three biomarkers (PDMPs, HMGB1 and sEPCR) in cancer patients stratified into groups based on CAT-positive or CAT-negative (Figure 1). Levels of PDMPs, HMGB1 and sEPCR were higher in CAT-positive patients compared with CAT-negative patients (Figure 1). In particular, PDMP levels in CAT-positive patients were significantly elevated compared with CAT-negative patients (p < 0.01; Figure 1).
Survival Analysis in Relation to the Three Biomarkers
The concentrations of all three biomarkers were higher in patients who died within 300 days of their first examination (Table 2). PDMP, HMGB1 and sEPCR levels showed a negative correlation with survival time; in particular, PDMP levels were significantly lower in patients who lived for more than 901 days after their first examination compared with the data in 0~300 days (p < 0.001; Table 2).
 |
Table 2 Survival Analysis Using Biomarkers |
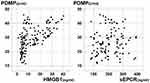
Correlation of PDMPs with Other Parameters
Figure 2 shows the correlation of PDMPs with HMGB1 or sEPCR in cancer patients (Pearson’s coefficient). PDMP levels were positively correlated with HMGB1 (correlation coefficient; r = 0.5933, p < 0.001). In contrast, PDMP levels were not significantly correlated with sEPCR (r = 1527, p = 0.1624).
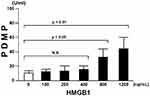
Effect of HMGB1 on PDMPs in Normal Platelet-Rich Plasma
We investigated whether PDMP secretion from platelets was enhanced by HMGB1. HMGB1 dose-dependently elevated PDMPs dose-dependently (HMGB1 concentrations of 800 and 1200 ng/mL) in vitro experiment using platelet-rich plasma from healthy persons (Figure 3).
Discussion
CAT causes significant morbidity and mortality in cancer patients.4,5 CAT is linked with a worse prognosis and thromboembolism is the second leading cause of death in cancer patients.19,20 Therefore, this study assessed the plasma concentrations of several biomarkers which may be related to CAT in cancer patients. We found that the concentrations of PDMP, sEPCR and HMGB1 were higher in cancer patients than in healthy controls. These results suggest that cancer patients likely have coagulation- and/or endothelial cell activation-related risk factors for coagulation abnormalities, resulting in the occurrence of CAT. The clinical significance of HMGB1 in cancer patients was previously reported; it was shown to be a potential prognostic factor for non-small cell lung cancer (NSCLC).9,21 Although Naumnik et al9 identified increased HMGB1 levels in advanced NSCLC patients undergoing chemotherapy, they concluded that HMGB1 concentrations did not influence survival times following NSCLC treatment because there was no significant difference in HMGB1 levels before and after chemotherapy. In contrast, Wang et al21 reported that HMGB1 was highly expressed in NSCLC and might be a valuable prognostic predictive marker for this disease. In the current study, HMGB1 levels were significantly different in cancer patients with or without CAT (p < 0.05).
We also found that sEPCR levels were significantly elevated in cancer patients with CAT compared with those without CAT (p < 0.05). Activated protein C, combined with its cofactor, protein S, acts as an anticoagulant, inactivating factor Va and factor VIIIa.22 EPCR, a transmembrane glycoprotein present on endothelial cells, enables protein C activation.23 EPCR is also found as a soluble form, sEPCR, which binds to activated protein C, in competition with cell-surface EPCR.24 Therefore, sEPCR is a biomarker of cancer-related hypercoagulability in human malignancies.10,25 In this study, 24 of 232 cancer patients with CAT exhibited significantly increased sEPCR levels. Additionally, sEPCR levels were associated with survival times similar to HMGB1. However, in this study, the strongest correlation between biomarker levels and survival times was PDMP, a platelet-related biomarker with procoagulant activity that contributes to thrombosis formation and atherosclerosis.11,12,26 PDMP levels have also been identified as a prognostic biomarker for cancer patients.27–29 Varon et al27 showed the effect of PDMP on cancer cell metastasis and their potential beneficial effect in an ischemic stroke model. Niki et al28 suggested that vascular complications associated with PDMP may contribute to a poor prognosis for NSCLC patients. Furthermore, Ma et al29 suggested that after chemotherapy platelets elevated PDMP-related procoagulant activity and might contribute to the hypercoagulative state of NSCLC. These previous studies support the results of the present study. Why does PDMP affect the prognosis of cancer patients? Liang et al30 demonstrated that platelet- secreted microRNA via PDMP promoted lung cancer cell invasion by targeting a tumor suppressor. In contrast, Michael et al31 reported that platelet-derived microRNA transfer in vivo to tumor cells in solid tumors via infiltrating PDMPs regulated tumor cell gene expression and modulated tumor progression. Thus, the role of PDMPs in cancer progression is controversial.
In the present study, we investigated the correlation of PDMPs with other parameters, and found that PDMP levels were positively correlated with HMGB1. In addition, HMGB1 induced the dose-dependent elevation of PDMPs (HMGB1 concentrations of 800 and 1200 ng/mL) in vitro using platelet-rich plasma from healthy persons. A previous study reported that HMGB1 induced the secretion of microparticles from various cells, especially Toll-like receptor 4 (TLR4).32,33 Therefore, one cause of PDMP production in cancer patients might be HMGB1 because platelets express TLR4.34 Unfortunately, this study has some limitations. First, the overall sample size is very small. Second, the samples were probably not representative of the entire cancer population experiencing CAT, because of a high percentage of patients with lung cancer. Patients in the GI cancer (e.g. stomach, pancreas) group are more prone to having CAT, thus fewer cases were observed. Finally, we did not completely define the relationship between PDMP and sEPCR in this study. Therefore, it remains unknown whether the high PDMP levels in cancer patients are directly linked to sEPCR levels. Confirmation of these observations in future prospective studies will be necessary.
Conclusion
Our findings have two potential implications. First, we showed that the combined increase in PDMP and HMGB1 levels was related to CAT in cancer patients. Second, we described how coagulatory dysfunction may result from increased levels of these biomarkers and contribute to a poor prognosis in cancer patients. This study had some limitations. We were unable to determine whether any relationship exists between PDMP and sEPCR. Additionally, we did not investigate how different therapeutic strategies affect the utility of the identified prognostic markers. Further confirmation of our observations in prospective studies is necessary.
Acknowledgments
This study was supported in part by a grant from the Advanced Medical Care from the Ministry of Health and Welfare of Japan, and a grant (13670760 to S.N.) from the Ministry of Education, Science and Culture of Japan. We thank Edanz Group for editing a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722. doi:10.1001/jama.293.6.715
2. Khorana AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(29):4839–4847. doi:10.1200/JCO.2009.22.3271
3. Monie DD, DeLoughery EP. Oathogenesis of thrombosis: cellular and pharmacogenetic contributions. Cardiovasc Diagn Ther. 2017;7(Suppl 3):S291–S298. doi:10.21037/cdt.2017.09.11
4. Elyamany G, Alzahrani AM, Bukhary E. Cancer-associated thrombosis: an overview. Clin Med Insig Oncol. 2014;8:129–137.
5. Li A, Garcia DA, Lyman GH, et al. Direct oral anticoagulant (DOAC) versus low-molecular-weight heparin (LMWH) for treatment of cancer associated thrombosis (CAT): a systematic review and meta-analysis. Thromb Res. 2019;173:158–163. doi:10.1016/j.thromres.2018.02.144
6. Simanek R, Vormittag R, Ay C, et al. High platelet count associated with venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). J Thromb Haemost. 2010;8:114–120. doi:10.1111/jth.2009.8.issue-1
7. Mackman N. New insights into the mechanisms of venous thrombosis. J Clin Invest. 2012;122(7):2331–2336. doi:10.1172/JCI60229
8. Ferroni P, Martini F, Portarena I, et al. Novel high-sensitive D-dimer determination predicts chemotherapy-associated venous thrombo- embolism in intermediate risk lung cancer patients. Clin Lung Cancer. 2012;13(6):482–487. doi:10.1016/j.cllc.2012.03.005
9. Naumnik W, Nilklińska W, Ossolińska M, et al. Serum levels of HMGB1, survivin, and VEGF in patients with advanced non-small cell lung cancer during chemotherapy. Folia Histochem Cytobiol. 2009;47(4):703–709. doi:10.2478/v10042-009-0024-0
10. Ducros E, Mirshahi SS, Faussat AM, et al. Soluble endothelial protein C receptor (sEPCR) is likely a biomarker of cancer-associated hypercoagulability in human hematologic malignancies. Cancer Med. 2012;1(2):261–267. doi:10.1002/cam4.11
11. Nomura S, Ozaki Y, Ikeda Y. Function and role of microparticles in various clinical settings. Thromb Res. 2008;123(1):8–23. doi:10.1016/j.thromres.2008.06.006
12. Nomura S, Shimizu M. Clinical significance of procoagulant microparticles. J Intensive Care. 2015;3(1):2–11. doi:10.1186/s40560-014-0066-z
13. Piccin A, Murphy WG, Smith OP. Circulating microparticles: pathophysiology and clinical implications. Blood Rev. 2007;21(3):157–171. doi:10.1016/j.blre.2006.09.001
14. Miyazaki Y, Nomura S, Miyake T, et al. High shear stress can initiate both platelet aggregation and shedding of procoagulant containing microparticles. Blood. 1996;88(9):3456–3464. doi:10.1182/blood.V88.9.3456.bloodjournal8893456
15. Geng XY, Xiao N, Han Y. et al. Platelet microparticles: a tool to predict infarction area in rats. J Invest Surg;2019. 1–6. doi:10.1080/08941939.2019.1606369
16. Osumi K, Ozeki Y, Saito S, et al. Development and assessment of enzyme immunoassay for platelet-derived microparticles. Thromb Haemost. 2001;85(2):326–330. doi:10.1055/s-0037-1615688
17. Nomura S, Shouzu A, Taomoto K, et al. Assessment of an ELISA kit for platelet-derived microparticles by joint research at many institutes in Japan. J Atherscler Thromb. 2009;16(6):878–887. doi:10.5551/jat.2642
18. Nomura S. Extracellular vesicles and blood diseases. Int J Hematol. 2017;105(4):392–405. doi:10.1007/s12185-017-2180-x
19. Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. doi:10.1182/blood-2013-04-460121
20. Campello E, Henderson MW, Noubouossie DF, et al. Contact system activation and cancer: new insight in the pathophysiology of cancer-associated thrombosis. Thromb Haemost. 2018;118(2):251–265. doi:10.1160/TH17-08-0596
21. Wang H, Li Y, Yu W, et al. Expression of the receptor for advanced glycation end-products and frequency of polymorphism in lung cancer. Oncol Lett. 2015;10(1):51–60. doi:10.3892/ol.2015.3200
22. Castellino FJ, Ploplis VA. The protein C pathway and pathologic processes. J Thromb Haemost. 2009;7(Suppl 1):140–145. doi:10.1111/jth.2009.7.issue-s1
23. Griffin JH, Fernández JA, Gale AJ, et al. Activated protein C. J Thromb Haemost. 2007;5(Suppl 1):73–80. doi:10.1111/j.1538-7836.2007.02491.x
24. Fukudome K, Kurosawa S, Stearns-Kurosawa DJ, et al. The endothelial cell protein C receptor. Cell surface expression and direct ligand binding by the soluble receptor. J Biol Chem. 1996;271(29):17491–17498. doi:10.1074/jbc.271.29.17491
25. Althawadi H, Alfarsi H, Besbes S, et al. Activated protein C upregulates ovarian cancer cell migration and promotes unclottability of the cancer cell microenvironment. Oncol Lep. 2015;34(2):603–609.
26. Nomura S. Microparticle and atherothrombotic diseases. J Atheroscler Thromb. 2016;23(1):1–9. doi:10.5551/jat.32326
27. Varon D, Hayon Y, Dashevsky O, et al. Involvement of platelet derived microparticles in tumor metastasis and tissue regeneration. Thromb Res. 2012;130(Suppl 1):S98–S99. doi:10.1016/j.thromres.2012.08.289
28. Niki M, Yokoi T, Kurata T, et al. New prognostic biomarkers and therapeutic effect of bevacizumab for patients with non-small-cell lung cancer. Lung Cancer (Auckl). 2017;8:91–99. doi:10.2147/LCTT.S138887
29. Ma R, Bi Y, Kou J, et al. Enhanced procoagulant activity of platelets after chemotherapy in non-small cell lung cancer. Cancer Biol Ther. 2017;18(8):627–634. doi:10.1080/15384047.2017.1345387
30. Liang H, Yan X, Pan Y, et al. MicroRNA-223 delivered by platelet-derived microparticles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3. Mol Cancer. 2015;14:58. doi:10.1186/s12943-015-0327-z
31. Michael JV, Wurtzel JGT, Mao GF, et al. Platelet microparticles infiltrating solid tumors transfer miRNAs that suppress tumor growth. Blood. 2017;130(5):567–580. doi:10.1182/blood-2016-11-751099
32. Saenz R, Futalan D, Leutenez L, et al. TLR4-dependent activation of dendritic cells by an HMGB1-derived peptide adjuvant. J Transl Med. 2014;12:211. doi:10.1186/1479-5876-12-211
33. Chen Q, Bel JJ, Liu C, et al. HMGB1 induces secretion of matrix vesicles by macrophages to enhance ectopic mineralization. PLoS One. 2016;11(5):e0156686. doi:10.1371/journal.pone.0156686
34. Alarcon M. Genaration of platelet-derived microparticles through the activation of the toll-like receptor 4. Heliyon. 2019;5(4):e01486. doi:10.1016/j.heliyon.2019.e01486
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.