Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Plasmatic Levels and Response to Variable Doses of Monthly Aripiprazole and Three-Month Paliperidone in Patients with Severe Schizophrenia. Treatment Adherence, Effectiveness, Tolerability, and Safety
Authors Fernández-Miranda JJ , Díaz-Fernández S
Received 12 June 2023
Accepted for publication 26 August 2023
Published 5 October 2023 Volume 2023:19 Pages 2093—2103
DOI https://doi.org/10.2147/NDT.S425516
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Richard J Porter
Juan J Fernández-Miranda,1,2,* Silvia Díaz-Fernández1,2,*
1AGC de Salud Mental V, Hospital Universitario de Cabueñes, Servicio de Salud del Principado de Asturias (SESPA), Gijón, Spain; 2Instituto de Investigación Sanitaria del Principado de Asturias (ISPA), Oviedo, Spain
*These authors contributed equally to this work
Correspondence: Juan J Fernández-Miranda, AGC de Salud Mental V, Hospital Universitario de Cabueñes, Servicio de Salud del Principado de Asturias (SESPA), C/ Centro Especialidades Avelino González. Óran s/n, Gijón, Asturias, 33211, Spain, Email [email protected]; [email protected]
Introduction: There is a need when optimizing antipsychotic treatment to know the plasmatic levels (PLs) achieved with the different doses and their relationship with effectiveness and toxicity, especially in patients with poor clinical progress. This study investigates the dose-PL-response relationship of monthly aripiprazole (AOM) and three-month paliperidone (PP3M).
Methods: Observational, 52-week prospective study of patients with severe schizophrenia (CGI-S ≥ 5) treated with PP3M or AOM for at least one year before their inclusion in the study (N=68). Dose-PL relationship was determined. Subjects were included in standard-dose and high-dose (above labeled) and standard/therapeutic range-PLs and high-PLs (above range) groups. Treatment adherence, effectiveness (hospitalizations, severity), tolerability and safety were assessed. PLs and clinical response were evaluated.
Results: No clear linear relationship was found between doses and PLs. In a considerable number of cases, standard doses achieved PLs above the therapeutic range. A significant clinical improvement was related to high PLs, without less safety, tolerability, or treatment compliance being involved. Clinical severity decreased more frequently in patients who received high doses and reached high PLs. Hospital admissions decreased significantly in those patients with high PLs.
Conclusion: Taking into account the absence of a linear relationship between doses and PLs, the effectiveness in people with severe schizophrenia of AOM and PP3M depends on reaching high PLs, achieved with high doses, but also with standard doses in some cases, without leading to worse treatment tolerability, safety, or adherence.
Keywords: schizophrenia, long-acting injectable antipsychotic, dose, plasmatic levels, effectiveness, tolerability
Introduction
Antipsychotic medications (AP) are an effective maintenance treatment for schizophrenia1–4 and clearly reduce the risk of relapse and hospitalization.5–10 The lack of adherence is associated with relapse and (re)admission,10–13 and risk of suicide.14–16 It has been suggested that second-generation antipsychotics (SGAs) are more effective than first-generation antipsychotics and are also better tolerated and safer,3–7 which is why their use is already widespread.1,3,6,8,12,17–19 However, there is still debate about the use of oral or injectable long-acting (LAI) SGAs to improve adherence and treatment outcomes.18–24 LAI antipsychotics have shown to improve compliance10–13 and to reduce relapses and hospital admissions25–32 better than oral formulations,18–27 thus being recommended in recent years, especially in patients with poor adherence, with multiple relapses and hospitalizations, or with greater severity.33–39
The effectiveness of antipsychotics has been related to the achievement of optimal plasmatic levels (PLs) since it is known that low levels cause poor results in treatment.40–42 The quantification of PLs (“pharmacotherapeutic monitoring”) is considered to be a tool for improving decisions on the use and dosage of psychotropic drugs.43,44 However, there are still doubts regarding its usefulness in clinical practice, given the interindividual variability in the pharmacokinetics of APs and the dose-PL and dose-response (effectiveness and toxicity) relationships.40,41,44,45
A further topic of debate is the use of high doses of AP in complex, severe or resistant patients. Although this is not advised in clinical guidelines,46,47 there are some arguments to support the logic of high doses related to individual pharmacokinetic differences.48,49 In the treatment of schizophrenia, such doses are often prescribed due to the poor response to standard treatment50,51 and also because these pharmacokinetic differences imply low PL with standard doses in some patients, with resulting low effectiveness.48,49,52,53 Nevertheless, solid data on the relative risk of its adverse effects with high doses are still lacking.48–53
For these reasons, more appropriate treatment with AP would require knowledge of the PLs achieved with the different doses, their relationships with effectiveness and compliance, and their tolerability and safety. This is especially important in patients with poor clinical progress, or those considered severe or resistant. This study, therefore, investigates the dose-PL-response relationship of one-month aripiprazole (AOM) and three-month paliperidone (PP3M). The objectives are to show the relationship between the different doses administered in routine clinical practice (including high doses) and the PLs reached of AOM and PP3M in patients with severe schizophrenia, to find out which doses are needed to achieve therapeutic levels and to know which PL are associated with greater effectiveness and whether they imply lower tolerability, safety, or therapeutic adherence.
Method
An observational, prospective (52-week) study was conducted to follow up people with severe schizophrenia (defined as clinical global impression-severity, CGI-S ≥ 5), receiving treatment with stabilized doses of PP3M or AOM for at least one year prior the study (In “steady state”) (N=68). The subjects of the study were patients over 18 years of age receiving treatment in a program for people with severe mental illness (Asturian Mental Health-Spanish National Health System).
They were included in two groups: standard dose (PP3M ≤ 525 mg/3 months or AOM ≤ 400 mg/month) and high doses (above). The PLs were divided into therapeutic recommended range (TRR) or “standard” (PP3M: 20–60 ng/mL; AOM: 150–250 ng/mL) and high range (above). Of the 68 subjects, 22 were on PP3M standard doses, 21 on PP3M high doses, 12 on AOM standard doses, and 13 on AOM high doses. The greatest homogeneity across the groups was achieved by selecting patients from each one randomly.
Sociodemographic variables (gender, age), weight, smoking, and concomitant medications were collected. The PLs were determined, as well as their relationship with the doses administered. Adherence to treatment and its relationship with doses and PLs were evaluated. To assess effectiveness, CGI-S scale scores and hospital admissions records were used, including comparisons of both with the previous year; the relationship of effectiveness with doses administered and PL obtained was also studied. To assess tolerability, reported adverse effects, weight variation, blood count, general biochemistry, and prolactin were recorded, and its relationship with doses and PLs was also studied.
Blood samples were extracted after fasting and at the moment of lowest levels, immediately before the administration of the next dose of both PP3M and AOM. As stabilized patients after at least one year on the same dose, they were in a “steady state”. The quantification of aripiprazole and 9- hydroxyrisperidone in serum was performed by liquid chromatography/mass tandem. In the case of aripiprazole, the molecule is measured directly, and not its metabolite dehydroaripiprazole. Liquid chromatography (LC) is a widely used technique to separate compounds from a sample prior to analysis and is often combined with mass spectrometry. With LC, the separation of the sample components is based on the interactions of the compounds with the mobile and the stationary phases. After chromatographic separations, compounds are eluted from the column and then run into the mass spectrometer for analysis, allowing high sensitivity and specificity. To measure prolactin, a 30-minute rest was required prior to extraction.
The study was carried out in accordance with the ethical principles of the Declaration of Helsinki (World Medical Association). And it was approved by the Asturian Medical Research Ethics Committee (CEImPA Code 2021.604). All patients (or their legal representatives, if applicable) signed informed consent.
A descriptive analysis of patients’ sociodemographic and clinical variables at the beginning and at the end of the study was carried out. For the analysis between treatment groups, χ2 was used for qualitative and Student’s t-test for quantitative variables. The confidence interval was established at 95%. For data processing, the “R Development Core Team” program (v 3.4.1) MASS Package (7.3–45 version) was used.
Results
The sample is composed mainly of middle-aged men (all patients identified themselves as male/male or female/female), with smoking habit and prior antipsychotic treatment. There were no significant changes in weight or smoking through follow-up (Table 1).
 |
Table 1 Sociodemographic and Clinical Profile of the Sample |
The correlation between dose and PL was not strictly linear (Figures 1 and 2). In more than half of the subjects with standard doses, high PLs were reached (Table 2). This occurred mainly with PP3M. There were some patients with high doses who did not achieve levels above the standard, occurring more with AOM. In a few cases standard doses did not achieve therapeutic PLs. That is, the majority of patients studied had PLs above the therapeutic range, not only those with high doses.
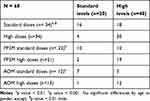 |
Table 2 Relationships Between Doses and Plasma Levels |
 |
Figure 1 Doses and plasmatic levels of one-month aripiprazole (AOM) and logarithmic trend line (The blue arrow indicates the maximum therapeutic level). |
 |
Figure 2 Doses and plasmatic levels of three-month paliperidone (PP3M) and logarithmic trend line (The blue arrow indicates the maximum therapeutic level). |
All subjects continued in treatment, regardless of type of AP, dose or PL. High doses or levels did not lead to treatment withdrawal. Clinical severity (CGI-S) decreased after one year of follow-up more frequently in patients with greater severity, but these were also patients with high doses. Hospital admissions decreased almost exclusively in those subjects with high PLs (Table 3).
 |
Table 3 Relationships Between Doses, Plasma Levels, Effectiveness and Toxicity |
More than a quarter of patients reported some adverse effect, but none was serious or involved a change in treatment. Moreover, such effects were not stronger with high doses or levels, except for parkinsonism with PP3M. The anticholinergic effects were related to the concomitant use of clozapine (Table 1). Notable changes in laboratory tests occurred in almost two thirds of the patients, although without severity (none were twice the upper limit of normality) or the need to change treatment. These changes were not related to the AP used or to the prescribed dose, but some were linked to high PLs. The most common change was elevation of lipids (two thirds of the subjects) and of prolactin (always in patients with PP3M, and significantly related to high PLs). Changes in blood count, glucose and renal function were found in almost one fifth of the patients (Table 1). Both the percentage of patients with adverse effects and with changes in laboratory results decreased during follow-up.
Concomitant medications (psychotropic drugs) were used by more than half of the patients. Benzodiazepines, other oral antipsychotics (mainly clozapine, especially in patients with AOM) and antidepressants were the most common. Their use decreased slightly during the study (especially benzodiazepines and antidepressants). Use was not related to clinical severity or to the AP used, nor to the doses or PLs (Table 1). Antiparkinsonian medications were used much more with PP3M, although without relation to dose or PL.
No significant differences were found in these results in relation to gender or age.
Discussion
Therapeutic Monitoring of Long-Acting Injectable Antipsychotics
SGA-LAIs have shown significantly greater adherence to treatment9–13,49,51 and a decrease in relapses, hospitalizations25–32,53,54 and suicide attempts14–16,54 compared to their oral counterparts.25,27,29,34,38,55,56 Knowledge of the PLs achieved with different doses of these APs, and their relationship with the progress of patients considered severe or resistant to treatment,19,40,41,48 is necessary for a better and individualized approach.17,19,35,39,55 In short, knowing the relationship between dose and PLs, and between PLs and effectiveness and tolerability, would enable optimization of treatment.41,43,45
As already mentioned, due to the lack of response in a significant number of patients, treatment with high doses of APs is often performed,40,47,48,50,51 although this is not generally recommended.5,8,46,47 However, efficacy/effectiveness studies rarely test high-dose regimens. The SGA doses needed to improve some severely ill patients may be significantly higher than standard.50–53 In addition, dose-PL-response variability could be related to pharmacokinetic (perhaps more than pharmacodynamic) differences.44,47–50,53
Therapeutic monitoring of AP to match treatment to each patient, and to improve response and reduce toxicity, has been called into question, given contradictory results53,57,58 and interindividual pharmacokinetic variability.48,49 It has also been reported that PLs vary due to factors such sex, age, weight or smoking, but without explaining the differences between patients.57–59 A greater correlation has been found between receptor occupancy and PLs than with prescribed dose.43–45 In any case, it seems clear that PLs are a better predictor of therapeutic effect than dose. Thus, by identifying the PLs associated with a greater response, the optimal dose with less toxicity could be determined for each patient.43,44,56,57
A study of SGA-LAI found an incidence of PL below the therapeutic reference range in more than half of the patients with recommended doses, so they could be underdosed.40,45 A meta-analysis of 20 antipsychotics showed that high doses may not provide greater efficacy but that, for some APs, high doses could be tried because their dose-PLs curves were not stable.44 Conversely, for APs with clearly increasing dose-response curves, high doses might be more effective.40,44,45 It is likely that the dose-response relationships in the most severe or treatment-resistant patients are different.44
With oral aripiprazole (ARI) no differences in efficacy were found according to doses.60 A study with AOM61 showed the effective dose to be 463 mg/4 weeks. Regarding LAI paliperidone, the effective dose found was 120–150 mg.62–64 Several studies found higher peak PLs after administration in the deltoid than in the gluteal.41,65 Although some studies showed a correlation between dose and PLs with both ARI and AOM,58,66,67 the evidence of a clear relationship between LAI PLs effectiveness and side effects is scarce,66,67 which It does not mean that it does not exist.68,69 In general, it is considered unlikely that high levels of SGA-LAI improve response, but also that they increase adverse effects.58,69
The dose/PL correlation in our study seems indicative (more linear) in the case of PP3M, but not so much in that of AOM. In any case, the findings of this investigation question the linear kinetics of these two drugs in this profile of patients since in a notable percentage of cases standard doses achieved higher plasmatic levels than the TRR. In fact, most of the patients studied had PLs above range, and not only those with high doses, which is a remarkable result. In short, it can be assumed that seriously ill patients required PLs above the TRR for their stabilization. It should be remembered that to support the use of high doses of APs, some arguments are based on possible individual pharmacokinetic differences.48,49
Given the above, the need for a more appropriate PLs TRR for severe/resistant patients can be posited, and related to the results of the patients in our study, in whom high PLs showed greater effectiveness.
Plasma Levels, Response and Toxicity
In this investigation, the clinical severity of the sample, measured with the CGI-S, decreased after one year of follow-up. This decrease was more frequent in those patients with greater severity, but who were also those who most frequently had high PLs. It is quite remarkable that hospitalizations (usual measure of effectiveness of antipsychotic treatment) decreased significantly in those patients with high PLs, and without affecting the good general therapeutic compliance. Therefore, it seems to point to the possible need for high PLs (over TRR) to achieve clinical stability in patients with severe schizophrenia, and also to the existence of pharmacokinetic factors that explain the non-linear relationship between doses and PLs achieved. Nevertheless, it is necessary to consider that treatment outcomes are not strictly PLs-dependent and that there is a difference between plasmatic and brain concentrations.
The fact that more than half of the patients were prescribed other psychoactive drugs is not surprising, since the use of antidepressants, mood stabilizers or benzodiazepines is common in this profile of patients. The concomitant use of oral antipsychotics (mainly clozapine) reflects clinical severity, but also raises the question of whether higher PLs of the LAI APs studied could make monotherapy possible. Nevertheless, concomitant medications prescribed in so many cases seemed not to have relevant interactions with both LAI-APs.
Although the likelihood and intensity of most AP side effects increase with dose, they are not always dose related.47,50,58–60 While some studies have found more extrapyramidal symptoms and elevated prolactin in high-dose SGA treatment, others have reported less parkinsonism and fewer dropouts with high doses.49–53 In general, there is no conclusive evidence in this regard, although several studies point to good tolerability and safety of high doses.48,49,55,69 Moreover, regarding the PLs/adverse effects relationship, most of the studies did not detect a clear association.59,60,70,71 In a study with ARI, patients with higher PLs scored lower on an akathisia scale.66
In our study, the good general tolerability is noteworthy. And although more than a quarter of patients reported some adverse effect, none was serious or involved changing treatment. The tolerability of high doses and high PLs was similar to that of standard doses and levels, and there was no treatment discontinuation with them. Side effects were more frequent only in the PP3M high-PLs group for parkinsonism. Laboratory tests results outside normal range were found in almost three quarter of the patients, although without severity (none twice the upper limit of normality) or need for treatment change. Lipid elevation occurred mainly in patients with PP3M, but regardless of dose or PL. Prolactin increase was found only with PP3M (which is not striking, given the known relationship of paliperidone with prolactin elevation and the non-incidence, or even decrease, with aripiprazole).69–71 This increase is related to high PP3M PLs. In short, there were no significant differences in terms of safety and tolerability between standard and high PLs of the two APs, which is important in order to try dose increases above the standards to achieve high PLs and this way reach better clinical outcomes without risk of intolerable side effects51,69,72 or less safety.72,73 On the other hand, the combination of LAI and oral APs (here, mainly clozapine) is not necessarily associated with more adverse events, and may even lead to fewer dropouts, which is partially confirmed in our research.19,40,41,48
In summary, no significant differences were observed between AOM and PP3M in terms of effectiveness and adherence, although a moderately higher tolerability of AOM was observed. Moreover, a certain greater need for high doses to achieve therapeutic PLs levels with AOM was found. These results raise the need to confirm the PLs achieved in routine practice, regardless of the prescribed doses, in patients with clinical severity and previous antipsychotic treatments. There is also a need to consider whether some patients, such as a considerable number of those studied, require PLs above the TRR (these TRR could be suitable for populations not so severely ill or without significant previous treatment failures), especially when tolerability and safety seem to be similar with standard rather than high PLs.
The relatively frequent use of LAI and oral AP combination could indicate a therapeutic hesitancy regarding the determination of a high dose of SGA LAI in a specific patient. Therapeutic monitoring could help to overcome this uncertainty.27,45,57,58 It has to be noted that the vast majority of patients have been previously treated with other antipsychotics without acceptable results, and a considerable number would be candidates for clozapine because they are “resistant”.49,50 (In fact, some of them combine clozapine with AOM or PP3M). The findings of this investigation point to the possibility of increasing LAI AP doses above marketed to achieve effective PLs (above the TRR), without a reduction in adherence, tolerability or safety, even as an alternative to clozapine treatment, with its known important adverse effects and supervision difficulties.
Limitations and Strengths of the Study
Some limitations of this research should be noted. This is a study with patients who were already being treated, so treatment was not assigned randomly, but based on clinical criteria. This has the risk that the study groups (patients with AOM or PP33M, and with standard or high doses) are not homogeneous in patient characteristics. An attempt was made to minimize this risk by selecting subjects in each of the four groups randomly.
We did not measure plasmatic levels of dehydroaripiprazole, although active moiety of aripiprazole could be important in clinical practice, but we think it is not relevant to this study’s results.
The CGI-S scale was used as a generally accepted measure of severity. This is a non-specific instrument and can therefore constitute a limitation. Moreover, no validated questionnaires were used for side-effects measurement.
All the patients were classified as severely ill, so the results cannot be generalized to patients who are not. Sample size was relatively small as it was conditioned precisely by this specific profile. Finally, the unmeasured factors related to the clinical decision to treat with one AP or another, and at standard or at high doses, are an inherent limitation of observational studies with this design.
Regarding strengths, the greatest is that it is the first study to measure the relationship of different doses with PLs of the most used SGA LAIs, and in the most severe patients with schizophrenia. In addition, the present research matches doses to PLs and both to treatment response (compliance, effectiveness, safety and tolerability). The number of high PP3M and AOM dose determinations is also remarkable, being the first research to measure their relationship with PLs achieved and the therapeutic response to them.
Conclusions
The search for the dose/PL relationship helps precision medicine in patients with schizophrenia, especially in the most severely ill or “resistant” to treatment, and those who are frequently prescribed high doses (above labeled) to achieve symptomatic stabilization. The knowledge of dose/PL/response relationships can guide routine practice and improve SGA AP use and dosage decisions.
This study describes the PLs achieved in patients with previous poor progress on different doses (including higher than approved) of the most recent SGA LAIs, and their relationship with effectiveness and toxicity. Results show no clear linear relationship between doses of AOM or PP3M and PLs. In addition, clinical improvements in these patients are related in many cases to PLs above the established therapeutic range, and without less safety, tolerability or therapeutic adherence.
The concomitant medications prescribed in many cases seemed not to have relevant interactions with both LAI-APs.
Finally, as this patient profile is a clozapine treatment candidate in many cases, these findings make it possible to consider AOM and PP3M, at PLs over TRR, as a possible alternative, more tolerable and safer, and with a greater probability of treatment compliance.
However, it is necessary to confirm these preliminary findings with studies with a larger number of subjects.
Ethics Statement
This study was approved by the Asturian Medical Research Ethics Committee (CEImPA Code 2021.604).
Acknowledgments
To Dr. F. Javier Cepeda-Piorno (Clinical Analysis Service, Cabueñes Universitary Hospital, Gijón. Asturian Health Service-SESPA) for his invaluable help in the antipsychotics plasmatic levels quantification and laboratory test measurement.
Funding
This research has received public funding from the Asturian Foundation for Biosanitary Research and Innovation (Fundación para la Investigación Biosanitaria del Principado de Asturias-FINBA). Code 2021-043-INTRAMURAL HC-FEMIJ.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barak Y, Aizenberg D. Clinical and psychosocial remission in schizophrenia: correlations with antipsychotic treatment. BMC Psychiatry. 2012;12(1):1. doi:10.1186/1471-244X-12-108
2. Kishi T, Ikuta T, Matsunaga S, Matsuda Y, Oya K, Iwata N. Comparative efficacy and safety of antipsychotics in the treatment of schizophrenia: a network meta-analysis in a Japanese population. Neuropsychiatr Dis Treat. 2017;13:1281–1302. doi:10.2147/NDT.S134340
3. Leucht S, Leucht C, Huhn M, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942. doi:10.1176/appi.ajp.2017.16121358
4. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149–160. doi:10.1002/wps.20516
5. Fabrazzo M, Cipolla S, Camerlengo A, Perris F, Catapano F. Second-generation antipsychotics’ effectiveness and tolerability: a review of real-world studies in patients with schizophrenia and related disorders. J Clin Med. 2022;11(15):4530. doi:10.3390/jcm11154530
6. Kearns B, Cooper K, Cantrell A, Thomas C. Schizophrenia treatment with second-generation antipsychotics: a multi-country comparison of the costs of cardiovascular and metabolic adverse events and weight gain. Neuropsychiatr Dis Treat. 2021;17:125–137. doi:10.2147/NDT.S282856
7. Ceraso A, Lin JJ, Schneider-Thoma J, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2020;2020(8). doi:10.1002/14651858.CD008016.pub3
8. Yasui-Furukori N, Kawamata Y, Sasaki T, et al. Prescribing trends for the same patients with schizophrenia over 20 years. Neuropsychiatr Dis Treat. 2023;19:921–928. doi:10.2147/NDT.S390482
9. Nagai N, Tani H, Yoshida K, et al. Drug attitude, insight, and Patient’s Knowledge about prescribed antipsychotics in schizophrenia: a Cross-Sectional Survey. Neuropsychiatr Dis Treat. 2020;16:781–787. doi:10.2147/NDT.S240377
10. Hui CLM, Poon VWY, Ko WT, et al. Risk factors for antipsychotic medication non-adherence behaviors and attitudes in adult-onset psychosis. Schizophr Res. 2016;174(1–3):144–149. doi:10.1016/j.schres.2016.03.026
11. Ljungdalh PMM. Non-adherence to pharmacological treatment in schizophrenia and schizophrenia spectrum disorders – an updated systematic literature review. Eur J Psychiatry. 2017;31(4):172–186. doi:10.1016/j.ejpsy.2017.08.001
12. Greene M, Yan T, Chang E, Hartry A, Touya M, Broder MS. Medication adherence and discontinuation of long-acting injectable versus oral antipsychotics in patients with schizophrenia or bipolar disorder. J Med Econ. 2018;21(2):127–134. doi:10.1080/13696998.2017.1379412
13. Díaz-Fernández S, López-Muñoz F, Fernández-Miranda JJ. Psychosocial and pharmacological approaches for improving treatment adherence and outcomes in people with severe schizophrenia: a 10-year follow-up. J Psychiatr Pract. 2021;27(6):417–426. doi:10.1097/PRA.0000000000000581
14. Pompili M, Orsolini L, Lamis DA, et al. Suicide prevention in schizophrenia: do Long-Acting Injectable Antipsychotics (LAIs) have a role? CNS Neurol Disord Drug Targets. 2017;16(4):454–462. doi:10.2174/1871527316666170223163629
15. Corigliano V, Comparelli A, Mancinelli I, et al. Long-acting injectable second-generation antipsychotics improve negative symptoms and suicidal ideation in recent diagnosed schizophrenia patients: a 1-year follow-up pilot study. Schizophr Res Treatment. 2018;2018:4834135. doi:10.1155/2018/4834135
16. Díaz-Fernández S, Frías-Ortiz DF, Fernández-Miranda JJ. Suicide attempts in people with schizophrenia before and after participating in an intensive case managed community program: a 20-year follow-up. Psychiatry Res. 2020;287(December 2018):112479. doi:10.1016/j.psychres.2019.112479
17. Fernández-Miranda JJ, Díaz-Fernández S, López-Muñoz F. Effectiveness of more personalized, case-managed, and multicomponent treatment for patients with severe schizophrenia compared to the standard treatment: a ten-year follow-up. J Pers Med. 2022;12(7). doi:10.3390/jpm12071101
18. López-Muñoz F, Tracy DK, Povedano-Montero FJ, et al. Trends in the scientific literature on atypical antipsychotic drugs in the United Kingdom: a bibliometric study. Ther Adv Psychopharmacol. 2019;9:2045125318820207. doi:10.1177/2045125318820207
19. Fernández-Miranda JJ, Díaz-Fernández S, López-Muñoz F. The use of second-generation antipsychotics in patients with severe schizophrenia in the real world: the role of the route of administration and dosage-a 5-year follow-up. Biomedicines. 2022;11(1). doi:10.3390/biomedicines11010042
20. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1–24. doi:10.4088/JCP.15032su1
21. Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA psychiatry. 2017;74(7):686–693. doi:10.1001/jamapsychiatry.2017.1322
22. Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208–224. doi:10.1002/wps.20632
23. Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387–404. doi:10.1016/S2215-0366(21)00039-0
24. Fernández-Miranda JJ, Díaz-Fernández S, López-Muñoz F. Oral versus long-acting injectable antipsychotic treatment for people with severe schizophrenia: a 5-year follow-up of effectiveness. J Nerv Ment Dis. 2021;209(5):330–335. doi:10.1097/NMD.0000000000001299
25. Lafeuille M-H, Laliberté-Auger F, Lefebvre P, Frois C, Fastenau J, Duh MS. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry. 2013;13(1):221. doi:10.1186/1471-244X-13-221
26. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192–213. doi:10.1093/schbul/sbs150
27. Ostuzzi G, Bertolini F, Tedeschi F, et al. Oral and long-acting antipsychotics for relapse prevention in schizophrenia-spectrum disorders: a network meta-analysis of 92 randomized trials including 22,645 participants. World Psychiatry. 2022;21(2):295–307. doi:10.1002/wps.20972
28. Taipale H, Mehtälä J, Tanskanen A, Tiihonen J. Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia-a nationwide study with 20-year follow-up. Schizophr Bull. 2018;44(6):1381–1387. doi:10.1093/schbul/sbx176
29. Munday J, Greene M, Chang E, Hartry A, Yan T, Broder MS. Early initiation of long-acting injectable antipsychotic treatment is associated with lower hospitalization rates and healthcare costs in patients with schizophrenia: real-world evidence from US claims data. Curr Med Res Opin. 2019;35(7):1231–1239. doi:10.1080/03007995.2019.1571295
30. Rubio JM, Schoretsanitis G, John M, et al. Psychosis relapse during treatment with long-acting injectable antipsychotics in individuals with schizophrenia-spectrum disorders: an individual participant data meta-analysis. Lancet Psychiatry. 2020;7(9):749–761. doi:10.1016/S2215-0366(20)30264-9
31. Kane JM, Schooler NR, Marcy P, et al. Effect of long-acting injectable antipsychotics vs usual care on time to first hospitalization in early-phase schizophrenia: a randomized clinical trial. JAMA psychiatry. 2020;77(12):1217–1224. doi:10.1001/jamapsychiatry.2020.2076
32. Díaz-Fernández S, Frías-Ortiz DF, Fernández-Miranda JJ. Mirror image study (10 years of follow-up and 10 of standard pre-treatment) of psychiatric hospitalizations of patients with severe schizophrenia treated in a community-based, case-managed programme. Rev Psiquiatr y Salud Ment. 2022;15(1):47–53. doi:10.1016/j.rpsmen.2022.01.002
33. Carpenter WT, Buchanan RW. Expanding therapy with long-acting antipsychotic medication in patients with schizophrenia. JAMA psychiatry. 2015;72(8):745–746. doi:10.1001/jamapsychiatry.2015.0485
34. Pilon D, Tandon N, Lafeuille M-H, et al. Treatment patterns, health care resource utilization, and spending in Medicaid beneficiaries initiating second-generation long-acting injectable agents versus oral atypical antipsychotics. Clin Ther. 2017;39(10):1972–1985.e2. doi:10.1016/j.clinthera.2017.08.008
35. Nasrallah HA. Triple advantages of injectable long acting second generation antipsychotics: relapse prevention, neuroprotection, and lower mortality. Schizophr Res. 2018;197(2018):69–70. doi:10.1016/j.schres.2018.02.004
36. Arango C, Baeza I, Bernardo M, et al. Long-acting injectable antipsychotics for the treatment of schizophrenia in Spain. Rev Psiquiatr Salud Ment. 2019;12(2):92–105. doi:10.1016/j.rpsm.2018.03.006
37. Park S-C, Choi MY, Choi J, et al. Comparative efficacy and safety of long-acting injectable and oral second-generation antipsychotics for the treatment of schizophrenia: a systematic review and meta-analysis. Clin Psychopharmacol Neurosci. 2018;16(4):361–375. doi:10.9758/cpn.2018.16.4.361
38. Fu AZ, Pesa JA, Lakey S, Benson C. Healthcare resource utilization and costs before and after long-acting injectable antipsychotic initiation in commercially insured young adults with schizophrenia. BMC Psychiatry. 2022;22(1):250. doi:10.1186/s12888-022-03895-2
39. Puspitasari IM, Sinuraya RK, Rahayu C, et al. Medication profile and treatment cost estimation among outpatients with schizophrenia, bipolar disorder, depression, and anxiety disorders in Indonesia. Neuropsychiatr Dis Treat. 2020;16:815–828. doi:10.2147/NDT.S240058
40. McCutcheon R, Beck K, D’Ambrosio E, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137(1):39–46. doi:10.1111/acps.12825
41. Schoretsanitis G, Kane JM, Correll CU, et al. Blood levels to optimize antipsychotic treatment in clinical practice. J Clin Psychiatry. 2020;81(3). doi:10.4088/JCP.19cs13169
42. Bogers JPAM, Hambarian G, Walburgh Schmidt N, Vermeulen JM, de Haan L. Risk factors for psychotic relapse after dose reduction or discontinuation of antipsychotics in patients with chronic schizophrenia. A meta-analysis of randomized controlled trials. Schizophr Bull. 2022. doi:10.1093/schbul/sbac138
43. Hiemke C, Bergemann N, Clement H, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1/2):9–62. doi:10.1055/s-0043-116492
44. Leucht S, Crippa A, Siafis S, Patel MX, Orsini N, Davis JM. Dose-response meta-analysis of antipsychotic drugs for acute schizophrenia. Am J Psychiatry. 2020;177(4):342–353. doi:10.1176/appi.ajp.2019.19010034
45. Hýža M, Šilhán P, Češková E, et al. Plasma levels of long-acting injectable antipsychotics in outpatient care: a retrospective analysis. Neuropsychiatr Dis Treat. 2021;17:1069–1075. doi:10.2147/NDT.S298050
46. CADTH. Systematic review of combination and high dose AAPs for schizophrenia. Vol 1; 2011. Available from: https://www.cadth.ca/sites/default/files/pdf/H0503_AAP_science-report_e.pdf.
47. Royal College of Psychiatrists. Consensus statement on high-dose antipsychotic medication. Royal College of Psychiatrists; 2014:1–53. Available from: http://www.rcpsych.ac.uk/files/pdfversion/cr138.pdf.
48. Sommer IE, Begemann MJH, Temmerman A, Leucht S. Pharmacological augmentation strategies for schizophrenia patients with insufficient response to clozapine: a quantitative literature review. Schizophr Bull. 2012;38(5):1003–1011. doi:10.1093/schbul/sbr004
49. Fernández-Miranda JJ, Díaz-Fernández S, López-Muñoz F. High doses of second-generation long-acting antipsychotics in the treatment of patients with severe resistant schizophrenia. A six-year mirror-image study. Psychiatry Clin Psychopharmacol. 2020;30(4):1. doi:10.5455/PCP.20201011042823
50. Meltzer HY, V. BW, Roy A, et al. A randomized, double-blind comparison of clozapine and high-dose olanzapine in treatment-resistant patients with schizophrenia. J Clin Psychiatry. 2008;69(2):274–285. doi:10.4088/JCP.v69n0214
51. Fernández-Miranda JJ, Díaz-Fernández S. Tolerability of effective high doses of paliperidone palmitate in patients with severe resistant schizophrenia. Int Clin Psychopharmacol. 2017;32(1):6–12. doi:10.1097/YIC.0000000000000151
52. Meltzer HYY, Lindenmayer J-P-P, Kwentus J, Share DBB, Johnson R, Jayathilake K. A six month randomized controlled trial of long acting injectable risperidone 50 and 100mg in treatment resistant schizophrenia. Schizophr Res. 2014;154(1–3):14–22. doi:10.1016/j.schres.2014.02.015
53. Fernández-Miranda JJ, Caramés-García V, Sánchez-García A. Effectiveness, good tolerability, and high compliance of doses of risperidone long-acting injectable higher than 75 mg in people with severe schizophrenia: a 3-year follow-up. J Clin Psychopharmacol. 2015;35(6):630–634. doi:10.1097/JCP.0000000000000400
54. Huang C-Y, Fang S-C, Shao Y-HJ. Comparison of long-acting injectable antipsychotics with oral antipsychotics and suicide and all-cause mortality in patients with newly diagnosed schizophrenia. JAMA Netw open. 2021;4(5):e218810. doi:10.1001/jamanetworkopen.2021.8810
55. Fernández-Miranda JJ, Díaz-Fernández S, De Berardis D, López-Muñoz F. Paliperidone Palmitate Every Three Months (PP3M) 2-year treatment compliance, effectiveness and satisfaction compared with Paliperidone Palmitate-Monthly (PP1M) in people with severe schizophrenia. J Clin Med. 2021;10(7):1408. doi:10.3390/jcm10071408
56. Lin D, Thompson-Leduc P, Ghelerter I, et al. Real-world evidence of the clinical and economic impact of long-acting injectable versus oral antipsychotics among patients with schizophrenia in the United States: a systematic review and meta-analysis. CNS Drugs. 2021;35(5):469–481. doi:10.1007/s40263-021-00815-y
57. Correll CU, Jain R, Meyer JM, et al. Relationship between the timing of relapse and plasma drug levels following discontinuation of cariprazine treatment in patients with schizophrenia: indirect comparison with other second-generation antipsychotics after treatment discontinuation. Neuropsychiatr Dis Treat. 2019;15:2537–2550. doi:10.2147/NDT.S210340
58. Hart XM, Eichentopf L, Lense X, et al. Therapeutic reference ranges for psychotropic drugs: a protocol for systematic reviews. Front Psychiatry. 2021;12:787043. doi:10.3389/fpsyt.2021.787043
59. Hoekstra S, Bartz-Johannessen C, Sinkeviciute I, et al. Sex differences in antipsychotic efficacy and side effects in schizophrenia spectrum disorder: results from the BeSt InTro study. NPJ Schizophr. 2021;7(1):39. doi:10.1038/s41537-021-00170-3
60. Auby P, Saha A, Ali M, Ingenito G, Wilber R, Bramer S. Safety and tolerability of aripiprazole at doses higher than 30 mg. Eur Neuropsychopharmacol. 2002;12:288. doi:10.1016/S0924-977X(02)80406-0
61. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085–1090. doi:10.4088/JCP.14m09741
62. Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072–2082. doi:10.1038/npp.2010.79
63. Pandina GJ, Lindenmayer J-P, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235–244. doi:10.1097/JCP.0b013e3181dd3103
64. Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol. 2010;25(5):247–256. doi:10.1097/YIC.0b013e32833948fa
65. Hard ML, Wehr A, von Moltke L, et al. Pharmacokinetics and safety of deltoid or gluteal injection of aripiprazole lauroxil NanoCrystal® Dispersion used for initiation of the long-acting antipsychotic aripiprazole lauroxil. Ther Adv Psychopharmacol. 2019;9:2045125319859964. doi:10.1177/2045125319859964
66. Hwang T-J, W-M L, Chan H-Y, et al. Fast versus slow strategy of switching patients with schizophrenia to aripiprazole from other antipsychotics. J Clin Psychopharmacol. 2015;35(6):635–644. doi:10.1097/JCP.0000000000000426
67. Lin S-K, Chen C-K, Liu Y-L. Aripiprazole and dehydroaripiprazole plasma concentrations and clinical responses in patients with schizophrenia. J Clin Psychopharmacol. 2011;31(6):758–762. doi:10.1097/JCP.0b013e3182356255
68. Citrome L. A review of aripiprazole in the treatment of patients with schizophrenia or bipolar I disorder. Neuropsychiatr Dis Treat. 2006;2(4):427–443. doi:10.2147/nedt.2006.2.4.427
69. Fernández-Miranda JJ, Díaz-Fernández S, López-Muñoz F. Adherence, tolerability and effective doses of aripiprazole once-monthly in the long-term treatment of patients with severe schizophrenia. Curr Pharm Des. 2021;27(39):4078–4085. doi:10.2174/1381612827666210701160013
70. Samara MT, Dold M, Gianatsi M, et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment-resistant schizophrenia. JAMA Psychiatry. 2016;73(3):199. doi:10.1001/jamapsychiatry.2015.2955
71. Misawa F, Kishimoto T, Hagi K, Kane JM, Correll CU. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2–3):220–230. doi:10.1016/j.schres.2016.07.018
72. Kishi T, Matsunaga S, Iwata N. Mortality risk associated with long-acting injectable antipsychotics: a systematic review and meta-analyses of randomized controlled trials. Schizophr Bull. 2016;42(6):1438–1445. doi:10.1093/schbul/sbw043
73. Correll CU, Solmi M, Croatto G, et al. Mortality in people with schizophrenia: a systematic review and meta-analysis of relative risk and aggravating or attenuating factors. World Psychiatry. 2022;21(2):248–271. doi:10.1002/wps.20994
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
