Back to Journals » Breast Cancer: Targets and Therapy » Volume 11
Plasma Level Of miR-21 And miR-451 In Primary And Recurrent Breast Cancer Patients
Authors Motamedi M , Hashemzadeh Chaleshtori M, Ghasemi S, Mokarian F
Received 23 July 2019
Accepted for publication 3 October 2019
Published 25 October 2019 Volume 2019:11 Pages 293—301
DOI https://doi.org/10.2147/BCTT.S224333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Pranela Rameshwar
Maryam Motamedi,1 Morteza Hashemzadeh Chaleshtori,1 Sorayya Ghasemi,1 Fariborz Mokarian2
1Cellular and Molecular Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran; 2Department of Hematology and Oncology, Faculty of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
Correspondence: Sorayya Ghasemi
Cellular and Molecular Research Center, Basic Health Sciences Institute, Shahrekord University of Medical Sciences, Shahrekord, Iran
Tel/fax +98 38 33331471
Email [email protected]
Purpose: MiR-21 and miR-451 are closely associated with tumor initiation, drug resistance, and recurrence of breast cancer (BC). This study was conducted to evaluate the possible value of the plasma level of miR-21 and miR-451 as potential biomarkers for the detection of primary and recurrent BC.
Patients and methods: In this descriptive–analytical study, the plasma level of miR-21 and miR-451 was measured in 23 primary BC patients, 24 recurrent (local/distant metastasis) BC patients, and 24 aged-match women as healthy controls using quantitative reverse transcription-polymerase chain reaction (qRT-PCR). Finally, data were analyzed using SPSS software, and the area under the receiver operating characteristic (ROC) curve of miRNAs was measured.
Results: The plasma level of miR-21 was significantly increased in both groups of primary (P<0.001) and recurrent (P<0.001) BC patients in comparison with healthy women. However, the plasma level of miR-451 was not significantly changed in primary (P=0.065) and recurrent (P=0.06) BC patients than healthy controls. The elevation of both miR-21 and miR-451 plasma level was not significantly changed in recurrent patients compared with non-recurrent (primary) patients (P=0.481, and P=1, respectively). Based on the ROC analyses, the areas under the curves (AUC) for miR-21 in discriminating primary BC and recurrent BC patients from healthy controls were 0.828 (95% CI: 0.712 to 0.944) and 0.865 (95% CI: 0.756 to 0.974), respectively.
Conclusion: These data indicating that plasma miR-21 may be useful as a biomarker for the detection of both primary and recurrent BC. However, plasma miR-451 lacks enough sensitivity in the detection of primary and recurrent BC, and more studies are needed in this area.
Keywords: miRNA-21, miRNA-451, breast cancer, biomarker
Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer death among females worldwide.1 Most BC-related deaths are because of its recurrence and distant metastasis, which could be prevented through early diagnosis of this cancer.2 In some cases, current diagnostic tools lack sufficient specificity and sensitivity and are unable to distinguish aggressive from non-aggressive tumors. For example, mammography, the gold standard diagnostic tool of BC, has a false positivity of 8-10%, and in young women with dense breast tissue, it does not exhibit satisfactory sensitivity and specificity.3,4 Tumor markers such as carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) are promising in BC management, but because of their low sensitivity, they are not useful in early diagnosis of BC.5–7
Several studies have shown that microRNAs (miRNAs), which are ∼22 nucleotides in length, participated in almost all of the cancers, acting either as tumor suppressors or oncogenes.8 Circulating miRNAs, including serum and plasma miRNAs, not only can be easily accessed and measured but also can effectively distinguish cancer patients from healthy individuals.9,10 Among them, miRNA-21 (miR-21) and miR-451 have drawn more attention and interest because of their important roles in the initiation, drug resistance, and development of several cancers, including BC.11–13 The miR-21 gene is located in the FRA17B, which is a fragile site that frequently found to be amplified in different types of cancers as well as BC.14 The increasing number of researches indicate that miR-21 acts as an oncomiR through the repression of tumor suppressor genes such as Phosphatase and tensin homolog (PTEN), tropomyosin 1 alpha (TMP1), and Programmed Cell Death 4 (PDCD4) and incorporates in drug resistance of tumors.12 The role of miR-21 in tumorigenesis after treatment, i.e., recurrence of breast cancer, is unclear. It is also unclear whether the alteration of its expression can also be used as a promising plasma biomarker of breast cancer recurrence.
The human miR-451 gene, located at 17q11.2, has been shown to be widely dysregulated in cancers and plays roles in tumor initiation and progression.15 In fact, there are dual reports about miR-451. While some studies have shown that miR-451 is reduced in cancers and acts as a tumor suppressor, some suggest an increase in its expression level in cancer patients than in healthy subjects.16,17 Therefore, more studies are needed to further clarify the subject. No study has yet been conducted to investigate the relationship between miR-451 and its potential use as a plasma biomarker for recurrence of breast cancer.
In this study, we explored the potential value of plasma miR-21 and miR-451 in discriminating primary and recurrent BC patients from healthy controls. Furthermore, we investigated the differences in plasma level of miR-21 and miR-451 between the primary and recurrent BC patients, which have not been identified to our knowledge.
Materials And Methods
Bioinformatics Study
In addition to the studies and articles, two databases, dbDEMC 2.0 and miRwayDB, were reviewed for miR-21 and miR-451.
Study Population
Following written informed consent and questionnaire, whole blood samples and essential data were collected from 23 women with primary invasive ductal carcinoma (IDC) and/or ductal carcinoma in situ (DCIS) BC, and 24 women with local recurrence or distant metastasis of BC from Parsian Hospital (ShahreKord, Iran) and Seyed Al-Shohada Hospital (Isfahan, Iran) between 2015 and 2017. The control group included 24 healthy aged-match women with no current or previous malignancy, based on the oncologist confirmation and negative familial history of BC. Blood samples from primary BC patients, whose breast tissues showed the tumor for the first time and were in lower stages (I or II), were collected before chemotherapy. Recurrent BC patients were those with advanced-stage (IV)/or distant metastatic BC or had shown the tumor for the second time in their follow-up.
Samples Processing and miRNA Extraction
Up to 4 mL of whole blood from each participant was collected in K2EDTA plasma tubes (Sunphoria Co., Ltd VP3082, Taiwan). Plasma was isolated within 2 h after blood collection, as follows: EDTA plasma was isolated by centrifugation (Hettich Mikro 200R, Germany) of whole blood at 2700 rpm for 7 mins at room temperature. Plasma supernatant was recovered, divided into aliquots, and immediately stored at −70°C.
For qRT-PCR analysis, miRNA was extracted from 250 µL of plasma using miRCURYTM RNA Isolation Kits–Biofluids (Cat No. 300112, Exiqon, Denmark) according to the manufacturer’s instructions.
The Efficiency Of Primers And Quantification By Real-Time PCR
MicroRNA analysis was performed by SYBR green qRT-PCR assay. In brief, miRNA was extracted from plasma. A total of 4.5 µL of isolated total miRNA was polyadenylated with the addition of 2 µL poly(A) polymerase buffer, 1 µL adenosine-triphosphate, and 0.5 µL poly(A) enzyme. Five microliters of poly(A)-miRNA was reverse transcribed to cDNA with the addition of 2 µL 5X first strand buffer, 1 µL deoxynucleoside-5´-triphosphate, 0.5 µL reverse transcription enzyme, and 0.5 µL of each cDNA synthesis primers and was incubated at 44°C for 1 hr. The polyadenylation and reverse transcription were performed using MiR-Amp kit (Cat No. 00101005, Parsgenome, Iran).
The qRT-PCR reaction was performed with the manufacturer-provided miRNA-specific reverse and forward primers (Cat No. 00101007, Parsgenome, Iran) and SYBR® Premix Ex Taq™ II (Tli RNase H Plus) (Takara Bio, Kyoto, Japan) in Rotor-gene 6000® real-time PCR system (Qiagen, Hilden, Germany). U6 small nuclear RNA (U6 snRNA) was used as a reference gene. The total volume of each reaction was 10 µL containing 5 µL of SYBR Green master mix, 1 µL of cDNA, which was diluted 15-fold with RNase free water, and 0.4 µL of miRNA-specific primers. The process steps were 1 initial incubation cycle at 95°C for 5 mins, followed by 40 amplification cycles of 95°C, 5 s, 63.5°C, 20 s, and 72°C, 25 s. The run was performed in duplicates for all samples. To estimate the relative expression level of miR-21 and miR-451, the difference in the threshold cycle (Ct) values was assessed between the target and the endogenous control (U6 snRNA). For more accuracy, standard curves were generated for each of the miR-21, miR-451, and U6 snRNA primers using a cDNA serial dilution of a healthy control sample, which was the same for all primers.
The efficiency of primer from standard curve = 10 (−1/slope) – 1
The fold changes in miR-21 and miR-451 expression for each primary and recurrent BC samples were calculated relative to the average expression in normal control samples. Fold changes were calculated based on the Ct values using the M. W. Pfaffl formula.18
Fold change = (E target gene) ∆Ct target gene/(E reference gene) ∆Ct reference gene
∆Ct target gene = Ct GOI healthy control – Ct GOI patient sample
∆Ct reference gene = Ct ref healthy control – Ct ref patient sample
E= efficiency of primer
Statistical Analysis
The expression level of miR-451 and miR-21 was compared between the groups using the Kruskal–Wallis test followed by Dunn test. Receiver operating characteristics (ROC) curves were established to assess the feasibility of using plasma levels of miRNAs as diagnostic tools for differentiating BC patients from controls. All P-values were 2-tailed and results less than 0.05 were considered statistically significant. All statistical analyses were performed by SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Evaluation of miRNA expression changes was repeated three times for each sample.
Results
Bioinformatics Results
Regarding the data obtained from the dbDEMC 2.0 database, miR-21 was selected (http://www.picb.ac.cn/dbDEMC/search.php). Despite numerous articles about miR-451 on the BC, it has not been included in this database for this cancer. In the miRwayDB database, miR-451 only in MCF-7 has been reported (http://www.mirway.iitkgp.ac.in/search_results). So it looked like that miR-451 is interesting for our study.
Patient Characteristics
A total of 71 participants including 23 primary BC patients, 24 local recurrent/or distant metastatic BC patients, and 24 normal control subjects were recruited. Details on patient’s specimens, including age, stage, lymph node metastasis, specimen characteristics (such as hemolysis determined by visual inspection), etc. are summarized in Table 1. There were no significant differences in age between primary BC patients (52.5 ± 10.1 years), locally recurrent/or metastatic BC patients (49.6 ± 10 years), and controls (45.6 ± 14.3 years) (P = 0.138, Fisher’s exact test).
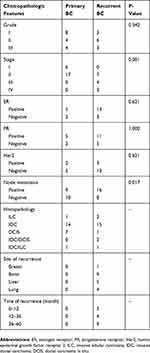 |
Table 1 Clinicopathological Characteristics Of BC Patients |
The Efficiency Of Primers
The linearity of the qRT-PCR between the logarithmic values of the miRNAs and the Ct values was confirmed for miR-21, miR-451, and U6 snRNA (R2 = 0.986, R2 = 0.995 and R2 = 0.996, respectively). The efficiency of miR-21, miR-451, and U6 snRNA primers were calculated as 1.095, 2.02, and 2.06, respectively.
Relative Expression Of Plasma miRNAs
Our data showed that plasma level of miR-21 was significantly elevated in both primary BC patients (2.71 folds, P<0.001) and recurrent BC patients (6.2 folds, P<0.001) than those in controls. However, the plasma level of miR-451 was not significant between primary and recurrent patients compared to healthy individuals (P=0.065 and P=0.06, respectively) (Figure 1). As a side analysis, the elevated expression levels of both miR-21 and miR-451 were not significant between recurrent patients compared with primary patients (P=0.481 and P=1, respectively). Median expression levels of miRNAs in primary BC, recurrent BC patients, and healthy individuals are presented in Table 2.
 |
Table 2 The Plasma Expression Level Of miR-21 And miR-451 |
ROC curve analyses revealed that miR-21 was valuable in distinguishing both primary BC and recurrent BC from normal controls with AUCs of 0.828 (95% CI: 0.712 to 0.944) and 0.865 (95% CI: 0.756 to 0.974), respectively (Figure 2). At the optimal cutoff value of 2.3257 with the maximal value of sensitivity + specificity-1 for miR-21 in discriminating primary BC group from healthy controls, the sensitivity and specificity were 60.86% and 95.83%, respectively. While at the cutoff value of 2.0445 for miR-21 in discriminating recurrent BC group from healthy controls, the sensitivity and specificity were 83.33% and 79.16%, respectively.
Discussion
With respect for searching effective and non-invasive biomarkers for early BC detection, management, and monitoring its relapse during or after the course of treatment to prolong the survival of BC patients, many studies have focused on circulating miRNAs.19,20 In the present study, the levels of two microRNAs (miR-21 and miR-451) were quantified in the plasma samples of non-recurrent (primary) and recurrent BC patients.
Previous researches have shown that blood-based miRNAs are resistant to RNase degradation, and also their expression levels are stable and reproducible during the time and freezing/thaw period.21 Due to easy access and non-invasiveness of the blood collection process, circulating miRNAs (as plasma miRNAs) have important roles in the diagnosis and follow-up of cancers.22
Consistent with other studies in gastric cancer,23 lung cancer,24 colorectal cancer,25 and BC,26 our data also showed that plasma expression level of miR-21 was significantly higher in both primary and recurrent BC patients than in healthy subjects. In order to validate the potential role of plasma miR-21 discriminating primary (early stage) BC patients and recurrent BC patients from healthy individuals, more accuracy in predicting recurrent patients (AUC=0.865) than primary patients (AUC=0.828) was demonstrated. The increased plasma level of miR-21 in the recurrent group (6.2 times) was higher than the primary group (2.71 times), which was in line with other reports. Yan et al (2008) showed that high expression level of miR-21 in BC tissues was significantly correlated with advanced clinical stages, poor prognosis, and lymph node metastases of patients.27 Wang et al (2015) demonstrated that the expression level of miR-21 distinguishes BC patients from healthy individuals and distant metastatic BC patients from those with locoregional disease.28
Different mechanisms have been proposed for miR-21 upregulation including gene amplification, transcriptional activation, and post-transcriptional regulation.29 It has been shown that there are several enhancer sequences upstream of miR-21 promoter, which are recognized by different transcriptional factors such as activation protein 1 (AP-1; composed of Jun and Fos family), signal transducer and activator of transcription 3 (STAT3), serum-response factor (SRF), and nuclear factor-kappa B (NF-κB). The elevated activity of these factors through aberrant signaling pathways in cancers could induce miR-21 upregulation. Moreover, miR-21 regulation could be affected by post-transcriptional events via the transforming growth factor β (TGFβ)/bone morphogenetic protein (BMP) signaling that promotes the maturation of miR-21 by the DROSHA complex29,30 (Figure 3).
At present, the great immense of miRNAs potentials has been revealed in the development of novel targeting therapies. Chemotherapy, radiotherapy, and antiendocrine therapy are important components of therapeutic strategies for cancers. However, the resistance of tumors to these methods is frequently seen in various cancers which results in cancer recurrence. Accumulating pieces of evidence have shown that some miRNAs incorporate in therapy response regulation. MiR-21 is implicated in chemotherapy response due to the enhancement of resistance of cancer cells to anti-cancer drugs through the inhibition of PTEN and PDCD430,31 or by upregulation of multidrug resistance type 1 (MDR1), which contribute to the multi-drug resistance and recurrence of cancers.32 Furthermore, it also has been demonstrated that miR-21 involves radiotherapy desensitization.12
Interestingly, some miRNAs play dual roles in different cancers. They may act as an oncogene or a tumor suppressor. For example, miR-451 plays roles as a tumor suppressor and decreases in tumor tissues compared with normal adjacent tissues in a variety of cancers.33,34 However, some studies have indicated that miR-451 may show opposite expression pattern in tumor tissues compared to bio-fluids such as plasma or serum.35,36 The function of miR-451 in each cancer depends mainly on the tissue type and specific targets.37 For example, one study showed an increase in the expression of MDR1 in the presence of miR-451,38 while in another study, MDR1 expression decreased in the presence of miR-451.39 However, many studies suggest that miR-451 is a valuable miR in the initiation, progression, and tumor relapse of some cancers. In the present study, the elevation of plasma miR-451 in patient groups versus healthy participants was not significant, which is due to the ineffective role of this miR in BC. However, this finding may also be due to the small sample size, different samples, sample collection techniques, and the normalizing method. Furthermore, miRNAs can be regulated through different pathways. In our observations, metastasis to lymph nodes between primary and recurrent BC is significantly different. The results of this difference had a meaningful relationship within the level expressions of miR-451 and miR-21.40 The decrease of expression of miR-451 and increase of miR-21 expression in plasma of BC patients is related to lymph node metastasis. It is said that the release of some miRNAs from tumor cells into the circulation can be selective or circulating miRNAs are secreted through unknown mechanisms from healthy tissues.17 But, our laboratory test showed that plasma miR-451 level was not different in BC, and therefore, despite the contradictory results regarding this miR in BC, miR-451 does not seem to serve as a biomarker in the plasma of BC primary or recurrent patients.
Conclusion
In summary, plasma miR-21 may be a potential biomarker for the detection of primary BC as well as those patients who show cancer for the second time in their follow-up course. On the other hand, miR-451 does not seem to be a marker for this cancer. Future investigations using larger sample sizes are needed to evaluate the role of plasma miR-21 or other plasma miRs associated with primary and recurrent BC detection. In addition, discriminating different groups of patients is needed to develop new prognostic biomarkers.
Abbreviations
BC, breast cancer; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; ROC, receiver operating characteristic; AUC, areas under the curves; CEA, carcinoembryonic antigen; CA 15-3, carbohydrate antigen 15–3; miRNAs, microRNAs; PTEN, phosphatase and tensin homolog; TMP1, tropomyosin 1 alpha; PDCD4, Programmed Cell Death 4; IDC, invasive ductal carcinoma; DCIS, ductal carcinoma in situ; AP-1, activation protein 1; STAT3, signal transducer and activator of transcription 3; SRF, serum-response factor; NF-κB, nuclear factor-kappa B; TGFβ, transforming growth factor β; BMP, bone morphogenetic protein; MDR1, multidrug resistance type 1.
Ethics Approval And Consent To Participate
The study procedure was conducted in accordance with principles for human experimentation as defined in the Declaration of Helsinki and was approved by the Shahrekord University of Medical Sciences Research Ethics Committee (Ethical permit no. IR.SKUMS.REC.1394.178), Iran. The written informed consent was provided from all patients related to this study.
Acknowledgments
We would like to acknowledge the Foundation for funding this project. This study was supported by Cellular and Molecular Research Center of Shahrekord University of Medical Sciences, Shahrekord, Iran (Grant No. 2581). We would also like to acknowledge the patients and the healthy controls who participated in this study and Dr. Amiri for his assistance in sample collection.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Mabe NW, Fox DB, Lupo R, et al. Epigenetic silencing of tumor suppressor Par-4 promotes chemoresistance in recurrent breast cancer. J Clin Invest. 2018;128(10):4413–4428. doi:10.1172/JCI99481
3. Asaga S, Kuo C, Nguyen T, et al. Direct serum assay for microRNA-21 concentrations in early and advanced breast cancer. Clin Chem. 2011;57(1):84–91. doi:10.1373/clinchem.2010.151845
4. Taplin S, Abraham L, Barlow WE, et al. Mammography facility characteristics associated with interpretive accuracy of screening mammography. J Nat Cancer Inst. 2008;100(12):876–887. doi:10.1093/jnci/djn172
5. Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi:10.1200/JCO.2007.14.2364
6. Uehara M, Kinoshita T, Hojo T, et al. Long-term prognostic study of carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA 15-3) in breast cancer. Int J Clin Oncol. 2008;13(5):447. doi:10.1007/s10147-008-0773-3
7. Duffy MJ, Evoy D, McDermott EW. CA 15-3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta. 2010;411(23–24):1869–1874. doi:10.1016/j.cca.2010.08.039
8. Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nature Rev Cancer. 2015;15(6):321. doi:10.1038/nrc3932
9. Wang N, Wang L, Yang Y, Gong L, Xiao B, Liu X. A serum exosomal microRNA panel as a potential biomarker test for gastric cancer. Biochem Biophys Res Commun. 2017;493(3):1322–1328. doi:10.1016/j.bbrc.2017.10.003
10. Zhou X, Wen W, Zhu J, et al. A six-microRNA signature in plasma was identified as a potential biomarker in diagnosis of esophageal squamous cell carcinoma. Oncotarget. 2017;8(21):34468.
11. van Schooneveld E, Wildiers H, Vergote I, Vermeulen PB, Dirix LY, Van Laere SJ. Dysregulation of microRNAs in breast cancer and their potential role as prognostic and predictive biomarkers in patient management. Breast Cancer Res. 2015;17(1):21. doi:10.1186/s13058-015-0526-y
12. Xue J, Niu J, Wu J, Wu Z-H. MicroRNAs in cancer therapeutic response: friend and foe. World J Clin Oncol. 2014;5(4):730–743. doi:10.5306/wjco.v5.i4.730
13. Yan L-X, Liu Y-H, Xiang J-W, et al. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 2016;48(2):471–484. doi:10.3892/ijo.2015.3287
14. Li J, Zhang Y, Zhang W, et al. Genetic heterogeneity of breast cancer metastasis may be related to miR-21 regulation of TIMP-3 in translation. Int J Surg Oncol. 2013;2013(1):1–7. doi:10.1155/2013/875078
15. Pan X, Wang R, Wang ZX. The potential role of miR-451 in cancer diagnosis, prognosis, and therapy. Mol Cancer Ther. 2013;12(7):1153–1162. doi:10.1158/1535-7163.MCT-12-0802
16. Su Z, Zhao J, Rong Z, et al. MiR-451, a potential prognostic biomarker and tumor suppressor for gastric cancer. Int J Clin Exp Pathol. 2015;8(8):9154.
17. Konishi H, Ichikawa D, Komatsu S, et al. Detection of gastric cancer-associated microRNAs on microRNA microarray comparing pre-and post-operative plasma. Brit J Cancer. 2012;106(4):740–747. doi:10.1038/bjc.2011.588
18. Pfaffl MW. Quantification strategies in real-time PCR: the real-time PCR encyclopedia A–Z of quantitative PCR. Inte Univer Line La Jolla. 2004;1(1):89–113.
19. Bahrami A, Aledavood A, Anvari K, et al. The prognostic and therapeutic application of microRNAs in breast cancer: tissue and circulating microRNAs. J Cell Physiol. 2018;233(2):774–786. doi:10.1002/jcp.25813
20. Hamam R, Hamam D, Alsaleh KA, et al. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 2017;8(9):3045. doi:10.1038/cddis.2017.518
21. Glinge C, Clauss S, Boddum K, et al. Stability of circulating blood-based microRNAs–pre-analytic methodological considerations. PLoS One. 2017;12(2):0167969. doi:10.1371/journal.pone.0167969
22. Si H, Sun X, Chen Y, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139(2):223–229. doi:10.1007/s00432-012-1315-y
23. Li B-S, Zhao Y-L, Guo G, et al. Plasma microRNAs, miR-223, miR-21 and miR-218, as novel potential biomarkers for gastric cancer detection. PLoS One. 2012;7(7):41629. doi:10.1371/journal.pone.0041629
24. Zhang H, Mao F, Shen T, et al. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncology Lett. 2017;13(2):669–676. doi:10.3892/ol.2016.5462
25. Tsukamoto M, Iinuma H, Yagi T, Matsuda K, Hashiguchi Y. Circulating exosomal microRNA-21 as a biomarker in each tumor stage of colorectal cancer. Oncology. 2017;92(6):360–370. doi:10.1159/000463387
26. Han J-G, Jiang Y-D, Zhang C-H, et al. A novel panel of serum miR-21/miR-155/miR-365 as a potential diagnostic biomarker for breast cancer. Ann Surg Treat Res. 2017;92(2):55–66. doi:10.4174/astr.2017.92.2.55
27. Yan L-X, Huang X-F, Shao Q, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14(11):2348–2360. doi:10.1261/rna.1034808
28. Wang G, Wang L, Sun S, Wu J, Wang Q. Quantitative measurement of serum microRNA-21 expression in relation to breast cancer metastasis in Chinese females. Ann Lab Med. 2015;35(2):226–232. doi:10.3343/alm.2015.35.2.226
29. Kumarswamy R, Volkmann I, Thum T. Regulation and function of of miR-21 in health and disease. RNA Biol. 2011;8(5):706–713. doi:10.4161/rna.8.5.16154
30. Wang Z-X, Lu -B-B, Wang H, Cheng Z-X, Yin Y-M. MicroRNA-21 modulates chemosensitivity of breast cancer cells to doxorubicin by targeting PTEN. Arch Med Res. 2011;42(4):281–290. doi:10.1016/j.arcmed.2011.06.008
31. Gong C, Yao Y, Wang Y, et al. Up-regulation of miR-21 mediates resistance to trastuzumab therapy for breast cancer. J Biol Chem. 2011;286(21):19127–19137. doi:10.1074/jbc.M110.216887
32. Xie Z, Cao L, Zhang J. miR-21 modulates paclitaxel sensitivity and hypoxia-inducible factor-1α expression in human ovarian cancer cells. Oncol Lett. 2013;6(3):795–800. doi:10.3892/ol.2013.1432
33. Wang G, Yao L, Yang T, et al. MiR-451 suppresses the growth, migration, and invasion of prostate cancer cells by targeting macrophage migration inhibitory factor. Transl Cancer Res. 2019;8(1):647–654. doi:10.21037/tcr.2019.03.28
34. Mamoori A, Wahab R, Vider J, et al. The tumour suppressor effects and regulation of cancer stem cells by macrophage migration inhibitory factor targeted miR-451 in colon cancer. Gene. 2019;697(2):165–174. doi:10.1016/j.gene.2019.02.046
35. Chan M, Liaw CS, Ji SM, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–4487. doi:10.1158/1078-0432.CCR-12-3401
36. Cuk K, Zucknick M, Heil J, et al. Circulating microRNAs in plasma as early detection markers for breast cancer. Int J Cancer. 2013;132(7):1602–1612. doi:10.1002/ijc.27799
37. Wang Z, Zhang H, Zhang P, et al. Upregulation of miR-2861 and miR-451 expression in papillary thyroid carcinoma with lymph node metastasis. Med Oncol. 2013;30(2):577. doi:10.1007/s12032-013-0577-9
38. Zhu H, Wu H, Liu X, et al. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76(5):582–588. doi:10.1016/j.bcp.2008.06.007
39. Kovalchuk O, Filkowski J, Meservy J, et al. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7(7):2152–2159. doi:10.1158/1535-7163.MCT-08-0021
40. Motamedi M, Hashemzadeh Chaleshtori M, Ghasemi S, et al. The association of mir-451 and mir-21 in plasma with lymph node metastases in breast cancer. J Babol Univ Med Sci. 2018;20(4):12–16.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.