Back to Journals » Cancer Management and Research » Volume 13
Plasma Endoglin is Associated with Favorable Outcome for Pemetrexed-Based Therapy in Advanced Non-Small Cell Lung Cancer
Authors Li CH, Ko JL, Hsiao YP, Tsai MH, Lai YC, Hsin IL, Kang YT, Sheu GT , Lin WL, Wu MF
Received 12 September 2021
Accepted for publication 25 November 2021
Published 22 December 2021 Volume 2021:13 Pages 9305—9318
DOI https://doi.org/10.2147/CMAR.S338957
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Yong Teng
Che-Hsing Li,1,2,* Jiunn-Liang Ko,1,3,4,* Yu-Ping Hsiao,5,6 Ming-Hung Tsai,7 Yen-Chein Lai,8 I-Lun Hsin,3,4 Yu-Ting Kang,3,4 Gwo-Tarng Sheu,1,3,4 Wea-Lung Lin,6,9 Ming-Fang Wu1,4,6
1Divisions of Medical Oncology and Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, 402, Taiwan; 2Graduate Program in Immunology & Microbiology, Baylor College of Medicine, Houston, TX, 77030, USA; 3Institute of Medicine, Chung Shan Medical University, Taichung, 402, Taiwan; 4CSMU Lung Cancer Research Center, Chung Shan Medical University, Taichung, 402, Taiwan; 5Division of Dermatology, Chung Shan Medical University Hospital, Taichung, 402, Taiwan; 6School of Medicine, Chung Shan Medical University, Taichung, 402, Taiwan; 7Division of Hematology and Oncology, Department of Internal Medicine, China Medical University Hospital, Taichung, 40447, Taiwan; 8Department of Medical Laboratory and Biotechnology, Chung Shan Medical University, Taichung, 402, Taiwan; 9Department of Pathology, Chung Shan Medical University Hospital, Taichung, 402, Taiwan
*These authors contributed equally to this work
Correspondence: Ming-Fang Wu
Divisions of Medical Oncology and Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, 110, Sec. 1, Jianguo N. Road, Taichung, 40201, Taiwan
Tel +886-4-24739595#34711
Email [email protected]
Purpose: Pemetrexed-based chemotherapy (Pem-C) is the first-line chemotherapy for advanced non-squamous non-small cell lung cancer (NSCLC). However, limited tumor-associated proteins in blood are available to predict pemetrexed response and/or survival.
Patients and Methods: Plasma samples from three responders and three nonresponders with stage IIIB–IV NSCLC were collected prior to Pem-C and analyzed using Proteome ProfilerTM Human XL Oncology Array to detect 84 oncology-related proteins. The plasma concentrations of cathepsin S, endoglin (ENG), and matrix metalloproteinases 3 and 9 in 71 patients with advanced NSCLC treated with Pem-C were further measured using enzyme-linked immunosorbent assay based on the remarkable differences in the four proteins between responders and nonresponders in the array results.
Results: Pem-C responders had significantly higher ENG levels but not the other three markers than nonresponders (mean ENG level: 27.1 ± 7.4 vs 22.3 ± 6.9, p < 0.01). High ENG concentration was correlated with improved progression-free survival (hazard ratio [HR]: 0.52, 95% confidence interval [CI]: 0.31– 0.86, p < 0.01) and overall survival (HR: 0.55, 95% CI: 0.32– 0.94, p < 0.05) in patients treated with Pem-C, and the ENG level was an independent factor in our cohort (HR: 0.54, 95% CI: 0.33– 0.89, p < 0.05). ENG concentration in Pem-C responders also significantly increased at the time of best response (p < 0.05).
Conclusion: Cumulatively, this study reveals that ENG is correlated with Pem-C responsiveness in patients, which indicates the potential use of plasma ENG levels as a non-invasive biomarker for pemetrexed-based treatment in patients with non-squamous NSCLC.
Keywords: endoglin, pemetrexed-based therapy, prognostic factor, non-squamous non-small cell lung cancer, biomarker
Introduction
Lung cancer is the most common cause of cancer-related mortality in the world, and non-small cell lung cancer (NSCLC) accounts for around 85% of patients with lung cancer.1 In Taiwan, 5-year overall survival (OS) of patients with lung cancer is 15.9%, which indicates that lung cancer is a critical health issue.2 Given the progress of cancer treatment, the 2-year survival of advanced NSCLC had increased from around 13% for patients diagnosed in 2005–2006 to 20% in 2015–2016.3
More than 60% of NSCLC are non-squamous cell carcinoma. In the last 10 years, many new types of therapies have been developed for advanced non-squamous NSCLC, particularly lung adenocarcinoma. For example, the first-line treatment for patients with driver mutations is tyrosine kinase inhibitors,4 whereas the first-line therapy for patients with a high expression of programmed death-ligand 1 without driver mutation is immune check point inhibitors.5 However, many patients do not have driver mutations and do not respond to immunotherapy well. For these patients, the first-line treatment is pemetrexed and platinum-based chemotherapy.6
Pemetrexed is a folate antimetabolite that inhibits tumor cell replication. Pemetrexed targets multiple folate synthesis-related enzymes, including thymidylate synthase (TS) and dihydrofolate reductase (DHFR).7 Pemetrexed has been approved as the first-line chemotherapy alone or in combination with platinum for advanced non-squamous NSCLC.8 However, the best OS of pemetrexed-based chemotherapy (Pem-C) is still around 18 months,9 which reveals the necessity of clinical factors or biomarkers to predict potential pemetrexed responders.
Pemetrexed-based treatment prognostic biomarkers have been studied in many articles. Most studies showed that high TS expression instead of DHFR expression in NSCLC tumors predicts poor progression-free survival (PFS) in Pem-C.10,11 Two studies also demonstrated that TS gene polymorphisms predict the treatment outcome.12,13 Other studies also discussed the association between tumor driver mutations, such as epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement, as well as pemetrexed sensitivity.14,15 Our previous study also demonstrated that lipocalin-2 downregulation enhances pemetrexed sensitivity in lung cancer cell lines.16 However, detecting biomarkers non-invasively is more preferable in clinics to dynamically monitor patient response.
Many studies also used serum protein levels as a non-invasive approach for cancer prognosis. For instance, the expression levels of different members of the cysteine cathepsin protease family, also known as cathepsin, in tumor and circulation were used to predict therapeutic response and clinical prognosis.17 Different types of matrix metalloproteinases (MMPs) in tumor and peripheral blood were also studied as prognostic factors for various cancers and therapy outcome.18 Moreover, endoglin (ENG) is important to regulate the function of endothela and cancer angiogenesis.19 Currently, soluble ENG is also used to predict the outcome of aggressive prostate cancer.20
In this study, we analyzed the plasma samples collected from patients with advanced NSCLC prior to the first pemetrexed dose through the Proteome ProfilerTM Human XL Oncology Array and enzyme-linked immunosorbent assay (ELISA). We found that soluble ENG level is correlated with Pem-C response and survival. We aimed to develop a non-invasive strategy to detect ENG in blood as a Pem-C biomarker.
Methods
Patient Eligibility and Data Collection
Patients with pathologically or cytological confirmed NSCLC with pre-application for pemetrexed treatment in Chung Shan Medical University Hospital were initially screened from January 2012 to October 2018. Patients were excluded if they (1) were diagnosed with diseases other than stage IIIB–IV NSCLC; (2) had Eastern Cooperative Oncology Group performance status (ECOG PS) scale >2; (3) received pemetrexed treatment as neoadjuvant therapy; (4) were treated with EGFR inhibitors, bevacizumab, or other chemotherapy agents without combining with pemetrexed; (5) had cerebrovascular accident or infection within 1 month; and (6) lost contact during follow-up. The plasma samples of patients, as well as images, were collected at baseline (the baseline was defined as the time within one month before initial pemetrexed treatment) and every 9 ± 1 weeks of follow-up until disease progression. Patients were excluded for analysis if their blood samples were not collected at baseline. The clinical data of patients, including gender, age at the initial Pem-C treatment, smoking status, pathological type, tumor staging, EGFR mutation status, chemotherapy line, treatment courses, clinical images and outcomes were collected from medical records for analysis.
NSCLC diagnosis was confirmed by at least two pathologists in accordance with pathological examination through biopsy, surgical specimens or cytology of malignant pleural effusion, lymph node aspiration, and bronchial brushing. Tumor stage was determined using the 7th edition of the international lung cancer TNM staging system from the American Joint Committee on Cancer. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Chung Shan Medical University Hospital (CSMUH-IRB CS2-20146).
Treatment Response and Outcome Evaluation
Eligible patients were administered with 500 mg/m2 pemetrexed alone or in combination with 75 mg/m2 cisplatin or carboplatin at the area under the curve of 5–6 intravenously every 3 weeks. Pemetrexed was administered as the maintenance treatment after 4–6 cycles of platinum-containing treatment if without disease progression. Treatment dose was adjusted in accordance with toxicity.
Treatment response was evaluated every cycle (3 weeks) through chest radiography and every 9 ± 1 weeks through chest computed tomography together with whole-body bone scan or brain magnetic resonance imaging if with bone or brain metastasis, respectively. The Response Evaluation Criteria in Solid Tumor version 1.1 was applied to evaluate treatment response,21 which was categorized as complete remission (CR, total target lesions disappeared), partial response (PR), progressive disease (PD), or stable disease (SD).
Follow-up time is the time from the initial Pem-C until December 31, 2018. Pemetrexed responders are the patients who received Pem-C for more than 120 days without evidence of progression. PFS is the time from the date of the first pemetrexed dose to disease progression, treatment discontinuation, or death of any cause. OS is the time from the date of the first pemetrexed dose until death or the last follow-up.
Oncology-Related Soluble Protein Analysis
The plasma samples at baseline from three Pem-C-responsive (patient nos. 15, 17, and 20) and three Pem-C-nonresponsive (patient nos. 24, 27, and 34) patients were analyzed through the Proteome Profiler Human XL Oncology Array (R&D systems Inc., Minneapolis, MN, USA). The relative intensity of every spot was measured and compared with that of positive control spots.
ELISA
The plasma samples of 71 patients at baseline were analyzed using ELISA. Cathepsin S, ENG, MMP3, and MMP9 concentrations were detected using ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) in accordance with the manufacturer’s protocol.
Statistical Analysis
All quantitative data are presented as mean ± standard deviation. For the plasma concentrations of ENG, MMP3, and MMP9, the closest integer number of the median value was selected as the cut-off value to divide patients into two groups. For cathepsin S levels, the closest decile number of the median value was selected as the cut-off value.
All statistical analyses were performed using the Statistical Package for the Social Sciences software version 20.0 (SPSS, Inc., Chicago, IL, USA). All categorical clinical and biomarker variables were analyzed using the Pearson’s chi-squared test. Fisher's exact test was used if the expected frequency of cells in 2×2 contingency table was less than 5. Univariate and multivariate logistic regression analyses of patient characteristics were used to determine the predictive and independent factors of pemetrexed response. PFS and OS were plotted using the Kaplan–Meier method and compared using Log rank test. Multivariate analysis for PFS was also conducted through the Cox proportional hazard regression method. Generally, two-sided p < 0.05 was considered statistically significant.
Results
Patient Characteristics
One-hundred patients with NSCLC passed the pre-application of pemetrexed from January 2012 to October 2018. Eighty-six patients were enrolled into our study after the first-round of exclusion on the basis of the exclusion criteria. Patients who did not provide blood samples at baseline were also excluded in the second round. Finally, samples from 71 patients were analyzed in this study. The enrolment process is shown in Figure 1.
The clinical characteristics of the 71 pemetrexed-treated patients are demonstrated in Supplementary Table 1. The mean age at baseline was 60.5 years (range = 34–83 years). A total of 38 (54%) and 45 (63%) patients were female and nonsmokers, respectively. Sixty-nine patients (97%) were diagnosed with lung adenocarcinoma, one had squamous cell carcinoma, and one had poorly differentiated carcinoma. Sixty-seven patients (94%) were in stage IV, and four patients (6%) were in stage IIIB. Fifty-eight patients (82%) had N2–N3 lymph nodes, whereas 13 patients (18%) had N0–N1 lymph nodes.
Treatment-related factors are also recorded in Supplementary Table 1. Fifty-six patients (79%) had ECOG PS = 0–1 before the initial pemetrexed treatment, whereas 15 patients (21%) had ECOG PS = 2. Pemetrexed was chosen as first-, second-, and third-line chemotherapies in 54 (76%), 16 (23%) and 1 (1%) patients, respectively. Platinum was selected as the initial combination treatment in 59 patients (83%), whereas pemetrexed was received as monotherapy by 12 patients (17%).
In terms of treatment outcome, 18 patients (25%) achieved PR as the best response, whereas 25 patients (35%) had SD. The response of three patients could not be evaluated because of the rapid shifting to other treatments before the imaging study. The median PFS was 5.1 months (154 days; 95% confidence interval [CI] = 102–206 days), and the median OS was 17.8 months (533 days; 95% CI = 378–687 days).
Cathepsin S, ENG, MMP3, and MMP9 Were Potentially Associated with Pem-C Response
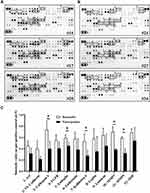
Plasma samples from three Pem-C responders and three nonresponders at baseline were analyzed using the Proteome Profiler Human XL Oncology Array to determine whether the plasma levels of some oncology-related proteins are correlated with the response to pemetrexed-based treatment. Figure 2A and B present the results from the responders and nonresponders, respectively. We observed whether the 12 markers in this panel had evident differences between the responder and nonresponder groups. Then, after the intensities of these 12 spot pairs were quantified and normalized through reference spots, we identified five markers with remarkable differences between responders and nonresponders, namely, cathepsin S, ENG, kallikrein 6, MMP3, and MMP9 (p < 0.05, Figure 2C). Most biomarker studies examined kallikrein-related peptidase family in tumor tissue or focused on their relationship with hormone-related tumors such as ovarian cancer and prostate cancer.22 Given that the levels of cathepsin S, ENG, MMP3 and MMP9 in blood serum were studied as prognostic factors in various types of cancer, we studied these four markers in the next research results.
High ENG Levels in Blood Were Correlated with Pem-C Responsiveness
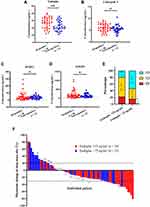
The plasma samples of 71 patients were analyzed using ELISA and divided into two groups in accordance with the patient’s Pem-C responsiveness to measure concentration of four markers at baseline in our cohort. While comparing these two groups, the ENG level of the responders (27.1 ± 7.4 ng/mL, n = 38) was significantly higher than that of nonresponders (22.3 ± 6.9 ng/mL, n = 33, p < 0.01; Figure 3A). Cathepsin S, MMP3, and MMP9 levels were not remarkably different between the two groups (Figure 3B–D). We further analyzed the correlation between ENG level and objective response. First, the ENG levels of all patients were divided into ENGhigh (n = 37) and ENGlow (n = 34) groups based on the cut-off point of 25 ng/mL. The cut-off point was chosen based on the closest integer number of the median value. The responses of three patients could not be assessed because of the lack of image records. Thus, the three patients were classified as nonresponders and excluded from the analyses of response rate. At around day 120, more patients in the ENGhigh group (n = 21, 58%) maintained SD than patients in the ENGlow group (n = 10, 31%), and less patients in the ENGhigh group (n = 7, 19%) had PD than patients in the ENGlow group (n = 17, 53%; Figure 3E). Maximum changes in the lung tumor and lymph node sizes of the other 68 patients, which were evaluated from the imaging studies of chest computed tomography during pemetrexed-based treatment, are demonstrated in Figure 3F. The partial response rate was 26.5% (n = 18) with 13 patients (72%) in the ENGhigh group and 5 patients (28%) in the ENGlow group. Twenty-five patients did not have any response after treatment and rapidly became PD. Among patients with PD, the number of patients with low ENG level at baseline (n = 18, 72%) was higher than that of patients with high ENG level at baseline (n = 7, 28%).
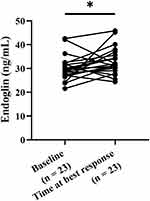
The plasma ENG concentrations of responders at baseline and the best response time were compared using paired t test to understand whether ENG levels would change during Pem-C treatment (Figure 4). ENG was significantly elevated after treatment (p < 0.05).
High ENG Levels Were Associated with Longer PFS and OS
The plasma concentration of the four markers were divided into the high- and low-level groups, and the relevance between marker level and survival were examined. The ENGhigh group was significantly correlated with longer PFS (median = 6.1 vs 2.8 months, hazard ratio [HR] = 0.52, 95% CI = 0.31–0.86, p < 0.01; Figure 5A). However, no significant difference in PFS between the high- and low-level groups was observed in the three other markers (Figure 5B–D). Consistent with the results of Pem-C response and PFS, the ENGhigh group also had significantly better OS than the ENGlow group (median = 19.2 vs 14.9 months, HR = 0.55, 95% CI = 0.32–0.94, p < 0.05; Figure 5E). Considering that some patients had endoglin levels near the median, the very high group (ENG > 30 ng/mL) and very low group (ENG < 20 ng/mL) were also compared. Very high ENG slightly was correlated with longer PFS and OS, but no significance was found (Supplementary Figure 1A and B). Given our findings on the relationship between ENG levels and the PFS of Pem-C, the following analyses were focused on ENG.
ENG Level in Blood Was an Independent Factor for Pem-C Response
We analyzed the correlation among all potentially associated clinical characteristics and ENG expression to understand whether other confounding clinical characteristics were present in patients with NSCLC. First, we divided patients into ENGhigh and ENGlow groups and analyzed the correlation between clinical factors and ENG expression through chi-squared test or Fisher-s exact test (Table 1). Clinical factors, including age before pemetrexed treatment, PS, and smoking, were significantly associated with ENG level (p < 0.05). We also examined whether clinical factors and ENG level were associated with Pem-C responsiveness (Table 1). A statistical association was observed between ENG level and Pem-C responsiveness only (p < 0.01).
 |
Table 1 Correlation of Pemetrexed-Based Therapy Responsiveness and Clinical, Pathological Factors, and Endoglin Level |
Second, we analyzed clinical characteristics through Cox proportional hazard regression models to understand whether the ENG level was an independent factor for Pem-C response (Table 2). In the univariate regression, the advanced lymph node stage (N2–N3; HR = 2.153, 95% CI = 1.088–4.260, p < 0.05) and low ENG level (HR = 1.994, 95% CI = 1.213–3.278, p < 0.01) were significantly correlated with shorter PFS. In the multivariate regression analysis, only ENG level was significantly associated with PFS (HR = 1.854, 95% CI = 1.125–3.054, p < 0.05), which indicates that ENG level might be an independent factor for Pem-C response.
 |
Table 2 Univariate and Multivariate Analysis of All Patients with Non-Small Cell Lung Cancer and Treated with Pemetrexed-Based Treatment |
ENG Level Was Not Associated with EGFR Mutation Status for Pem-C Responsiveness
Previous study demonstrated that patients with advanced lung adenocarcinoma harboring EGFR mutation are more responsive to Pem-C than EGFR wild type.15 Patients in our cohort were divided into EGFR wild type and mutation (excluding eight patients with unknown mutation status), and their ENG levels were compared to determine whether EGFR status was a confounding factor in our cohort (Supplementary Figure 2A). No significant differences in ENG were found between the EGFR wild type and mutation groups (wild type: 25.6 ± 7.5 ng/mL, n = 33 [including 3 ALK and 1 ROS1 translocation]; mutation: 25.7 ± 7.3 ng/mL, n = 30, p = 0.96). The PFS and OS of patients treated with Pem-C in the wild type and mutation group were also compared, and no significant difference was also observed (PFS: 5.6 vs 4.4 months, p = 0.21; OS: 33.2 vs 29.1 months, p = 0.81; Supplementary Figure 2B and C). Four patients in the EGFR wild type group had other driver mutations; therefore, we excluded these patients and still found no significant differences in the PFS and OS between the EGFR wild type and mutation groups (data not shown). As shown in Tables 1 and 2, EGFR mutation status was also not a confounding factor for Pem-C responsiveness based on the results of chi-square test and Cox regression models. These results indicated that ENG level is independent from EGFR mutation status.
Discussion
In the current research, we analyzed plasma samples collected from 71 patients with stage IIIB–IV NSCLC before undergoing Pem-C using human oncology array and ELISA. Pem-C responders had higher soluble ENG concentration, which was correlated with longer PFS and OS, and ENG level was increased at their best response time. ENG level was also an independent factor based on Cox regression models. Based on these findings, soluble ENG was associated with the tumor’s response to Pem-C and could be developed as a plasma biomarker for finding potential Pem-C responders.
ENG, also known as CD105, is a homodimer transmembrane glycoprotein, serves as the co-receptor of transforming growth factor-β (TGF-β). ENG is highly expressed in many types of normal human tissue. In endothelial cells, the long and short forms of ENG regulate the recruitment of TGF-β receptor 1 to induce Smad signaling, which results in the transcription of different endothelial cell proliferation genes.23 Many studies also showed that ENG is important for tumor angiogenesis. One study presented that ENG promotes the vascular endothelial growth factor receptor-mediated tip cell formation.24 Another study also demonstrated that targeting ENG by neutralizing antibody inhibits angiogenesis and metastasis in breast cancer in vitro and in vivo.25 Therefore, a monoclonal anti-ENG antibody, TRC105, was studied in cancer treatment clinical trials recently.26,27
Given that the angiogenesis effects of ENG are well-known, many studies also discuss the use of ENG as a prognostic factor in many cancer types. Most studies showed that high ENG expression in tumor predicts poor PFS and OS.28,29 In addition to the expression in tumor, other cell types expressing ENG in the tumor microenvironment are associated with prognosis. For example, ENG-expressing cancer-associated fibroblasts lead to colorectal cancer metastasis, and targeting ENG through TRC105 inhibits cancer-associated fibroblast-induced metastatic spread in a mouse model.30 Most studies view ENG as a poor prognostic factor, but one article showed that silencing ENG through methylation may induce endothelial–mesenchymal transition and increase the invasion of NSCLC cells.31 This previous study also revealed that in stage 1 patients, the ENG gene methylation predicts poor PFS, which indicates a positive prognostic value. However, few articles have discussed the relationship of ENG levels and cancer therapy.
In our cohort, patients with NSCLC who received Pem-C had a median PFS of 5.1 months, which is similar to the results of advanced non-squamous NSCLC in previous clinical trials.32,33 The median PFS in the ENGhigh group was 6.1 months. Most patients in this group completed 4–6 cycles of pemetrexed plus platinum combination therapy and received pemetrexed as maintenance; hence, the PFS was also comparable to the PARAMOUNT trial, which compared the use of pemetrexed to placebo in the maintenance phase.34
In our results, we found that high soluble ENG levels were associated with longer PFS after Pem-C treatment in advanced NSCLC, and ENG was an independent factor for Pem-C responsiveness. Most studies demonstrated that ENG promotes cancer progression, but soluble ENG has different functions. A previous study presented that soluble ENG reduces vascular endothelial growth factor-mediated vessel formation and mouse tumor burden, which indicates the countereffects of transmembrane and soluble ENG.35 A current clinical study also revealed that low soluble ENG levels in circulation are associated with high-grade prostate cancer.20 In addition, we also observed that the levels of soluble ENG were elevated when patients responded well to Pem-C. However, the ENG dynamic change in most non-responders could not be analyzed due to the short treatment period and the samples of some responders at the best response time were not enough for analysis. Therefore, more responders should be included in our future study to confirm the dynamic change in ENG during Pem-C. Despite the different cancer types, the aforementioned clinical study and our study confirmed the positive prognostic value of soluble ENG.
We also discovered that soluble ENG levels are not associated with EGFR mutation in our cohort. Common driver mutations such as EGFR in non-squamous NSCLC are known genetic factors for predicting Pem-C.15,36 However, in our cohort, we did not find differences in the ENG levels between EGFR wild type and mutation groups. We did not find any association between EGFR mutation and the PFS or OS of the pemetrexed-treated patients. Besides, ENG induces TGF-β signaling to regulate tumor angiogenesis, which is independent from EGFR-mediated cell proliferation pathways. Therefore, we speculated that soluble ENG level may be independent from driver mutations, at least EGFR, to affect Pem-C responsiveness. A large cohort study should be conducted to validate our results on ENG levels and tumor-harboring driver mutations.
Some limitations are present in this study. First, only patients with enough blood samples at baseline were included. All blood samples analyzed are from a small cohort. Other clinical factors may also affect Pem-C response, but we cannot evaluate all factors due to the small sample size. The small sample size may also explain why the mean values of ENG concentration in Pem-C responders and nonresponders are close even though statistically significant differences exist. However, we also performed subgroup analysis in the very-high- and very-low-ENG group and found similar correlations between ENG concentration and the PFS of patients treated with Pem-C. Second, we did not study the relevance of ENG expression; the main targets of pemetrexed (ie, TS and DHFR); and other uncommon driver mutations such as ALK and ROS1. Moreover, only three patients had ALK translocation and one had ROS1 translocation; therefore, we could not evaluate their relevance to ENG. Considering that TS and DHFR affect the nucleotide synthesis, DNA replication, and folate formation pathways, which are independent from TGF-β-related pathway, we still postulated that ENG level is an independent predictive factor. Nevertheless, our cohort still presents a remarkable association between ENG and Pem-C responsiveness.
Conclusions
In summary, high ENG concentration in the blood is correlated with favorable PFS and OS after Pem-C in patients with advanced non-squamous NSCLC, and ENG level is an independent factor for finding Pem-C responders. Currently, we are using lung cancer cell models to analyze how endogenous and exogenous ENG levels regulate pemetrexed-induced anti-tumor effects.
Abbreviations
NSCLC, non-small cell lung cancer; OS, overall survival; TS, thymidylate synthase; DHFR, dihydrofolate reductase; Pem-C, pemetrexed-based chemotherapy; PFS, progression-free survival; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; MMP, matrix metalloproteinase; ENG, endoglin; ELISA, enzyme-linked immunosorbent assay; ECOG PS, Eastern Cooperative Oncology Group performance status scale; CR, complete remission; PR, partial response; PD, progressive disease; SD, stable disease; CI, confidence interval; HR, hazard ratio; TGF-β, transforming growth factor-β.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Informed Consent
The use of clinical samples was approved by the institutional review board of Chung Shan Medical University Hospital (reference number: CSMUH-IRB CS2-20146). Informed consent was obtained from all subjects involved in the study.
Consent for Publication
All authors have agreed to the published version of the manuscript.
Author Contributions
Conceptualization, C.-H.L., J.-L.K., Y.-P.H., and M.-F.W.; Methodology, J.-L.K., M.-H.T., I.-L.H., Y.-T.K., and W.-L.L.; Validation, Y.-C.L.; Formal analysis, C.-H.L., J.-L.K., M.-H.T., I.-L.H., Y.-T.K., and M.-F.W.; Investigation, C.-H.L. and J.-L.K.; Resources, J.-L.K., Y.-P.H., and M.-F.W.; Data curation, C.-H.L. and J.-L.K.; Writing – original draft preparation, C.-H.L., Y.-C.L., I.-L.H., and M.-F.W.; Writing – review & editing, All authors; Visualization, Y.-C.L.; Supervision, J.-L.K. and M.-F.W.; Project administration, J.-L.K. and M.-F.W.; Funding acquisition, J.-L.K. and M.-F.W. All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
This work was supported by funding from the Ministry of Science and Technology, (Taipei, Taiwan) to Prof. Ko (MOST 108-2320-B-040 −015 -MY3) and Prof. Wu (MOST 110-2314-B-040 −029-) and from Chung Shan Medical University Hospital (Taichung, Taiwan) to Prof. Wu (CSH-2021-C-031).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94(8):1623–1640. doi:10.1016/j.mayocp.2019.01.013
2. Wang BY, Huang JY, Cheng CY, Lin CH, Ko J, Liaw YP. Lung cancer and prognosis in Taiwan: a population-based cancer registry. J Thorac Oncol. 2013;8(9):1128–1135. doi:10.1097/JTO.0b013e31829ceba4
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
4. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019;322(8):764–774. doi:10.1001/jama.2019.11058
5. Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
6. Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–1472. doi:10.6004/jnccn.2019.0059
7. Shih JY, Inoue A, Cheng R, Varea R, Kim SW. Does pemetrexed work in targetable, nonsquamous non-small-cell lung cancer? A narrative review. Cancers (Basel). 2020;12(9):Sep. doi:10.3390/cancers12092658
8. Treat J, Scagliotti GV, Peng G, Monberg MJ, Obasaju CK, Socinski MA. Comparison of pemetrexed plus cisplatin with other first-line doublets in advanced non-small cell lung cancer (NSCLC): a combined analysis of three Phase 3 trials. Lung Cancer. 2012;76(2):222–227. doi:10.1016/j.lungcan.2011.10.021
9. Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the Phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895–2902. doi:10.1200/JCO.2012.47.1102
10. Chen CY, Chang YL, Shih JY, et al. Thymidylate synthase and dihydrofolate reductase expression in non-small cell lung carcinoma: the association with treatment efficacy of pemetrexed. Lung Cancer. 2011;74(1):132–138. doi:10.1016/j.lungcan.2011.01.024
11. Takezawa K, Okamoto I, Okamoto W, et al. Thymidylate synthase as a determinant of pemetrexed sensitivity in non-small cell lung cancer. Br J Cancer. 2011;104(10):1594–1601. doi:10.1038/bjc.2011.129
12. Krawczyk P, Kucharczyk T, Kowalski DM, et al. Polymorphisms in TS, MTHFR and ERCC1 genes as predictive markers in first-line platinum and pemetrexed therapy in NSCLC patients. J Cancer Res Clin Oncol. 2014;140(12):2047–2057. doi:10.1007/s00432-014-1756-6
13. Li WJ, Jiang H, Fang XJ, et al. Polymorphisms in thymidylate synthase and reduced folate carrier (SLC19A1) genes predict survival outcome in advanced non-small cell lung cancer patients treated with pemetrexed-based chemotherapy. Oncol Lett. 2013;5(4):1165–1170. doi:10.3892/ol.2013.1175
14. Park S, Park TS, Choi CM, et al. Survival benefit of pemetrexed in lung adenocarcinoma patients with anaplastic lymphoma kinase gene rearrangements. Clin Lung Cancer. 2015;16(5):e83–9. doi:10.1016/j.cllc.2015.01.003
15. Wu SG, Yang CH, Yu CJ, et al. Good response to pemetrexed in patients of lung adenocarcinoma with epidermal growth factor receptor (EGFR) mutations. Lung Cancer. 2011;72(3):333–339. doi:10.1016/j.lungcan.2010.10.012
16. Wu MF, Hsiao YM, Huang CF, et al. Genetic determinants of pemetrexed responsiveness and nonresponsiveness in non-small cell lung cancer cells. J Thorac Oncol. 2010;5(8):1143–1151. doi:10.1097/JTO.0b013e3181e0b954
17. Olson OC, Joyce JA. Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer. 2015;15(12):712–729. doi:10.1038/nrc4027
18. Hadler-Olsen E, Winberg JO, Uhlin-Hansen L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013;34(4):2041–2051. doi:10.1007/s13277-013-0842-8
19. Rossi E, Bernabeu C, Smadja DM. Endoglin as an adhesion molecule in mature and progenitor endothelial cells: a function beyond TGF-beta. Front Med (Lausanne). 2019;6:10. doi:10.3389/fmed.2019.00010
20. Vidal AC, Duong F, Howard LE, et al. Soluble endoglin (sCD105) as a novel biomarker for detecting aggressive prostate cancer. Anticancer Res. 2020;40(3):1459–1462. doi:10.21873/anticanres.14088
21. Schwartz LH, Litiere S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–137. doi:10.1016/j.ejca.2016.03.081
22. Avgeris M, Mavridis K, Scorilas A. Kallikrein-related peptidases in prostate, breast, and ovarian cancers: from pathobiology to clinical relevance. Biol Chem. 2012;393(5):301–317. doi:10.1515/hsz-2011-0260
23. Schoonderwoerd MJA, Goumans MTH, Hawinkels L. Endoglin: beyond the endothelium. Biomolecules. 2020;10(2):Feb. doi:10.3390/biom10020289
24. Tian H, Huang JJ, Golzio C, et al. Endoglin interacts with VEGFR2 to promote angiogenesis. FASEB J. 2018;32(6):2934–2949. doi:10.1096/fj.201700867RR
25. Paauwe M, Heijkants RC, Oudt CH, et al. Endoglin targeting inhibits tumor angiogenesis and metastatic spread in breast cancer. Oncogene. 2016;35(31):4069–4079. doi:10.1038/onc.2015.509
26. Apolo AB, Karzai FH, Trepel JB, et al. A Phase II clinical trial of TRC105 (Anti-Endoglin Antibody) in adults with advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer. 2017;15(1):77–85. doi:10.1016/j.clgc.2016.05.010
27. Choueiri TK, Michaelson MD, Posadas EM, et al. An open label Phase Ib dose escalation study of TRC105 (Anti-Endoglin Antibody) with axitinib in patients with metastatic renal cell carcinoma. Oncologist. 2019;24(2):202–210. doi:10.1634/theoncologist.2018-0299
28. Davidson B, Stavnes HT, Forsund M, Berner A, Staff AC. CD105 (Endoglin) expression in breast carcinoma effusions is a marker of poor survival. Breast. 2010;19(6):493–498. doi:10.1016/j.breast.2010.05.013
29. Kauer J, Schwartz K, Tandler C, et al. CD105 (Endoglin) as negative prognostic factor in AML. Sci Rep. 2019;9(1):18337. doi:10.1038/s41598-019-54767-x
30. Paauwe M, Schoonderwoerd MJA, Helderman R, et al. Endoglin expression on cancer-associated fibroblasts regulates invasion and stimulates colorectal cancer metastasis. Clin Cancer Res. 2018;24(24):6331–6344. doi:10.1158/1078-0432.CCR-18-0329
31. O’Leary K, Shia A, Cavicchioli F, et al. Identification of Endoglin as an epigenetically regulated tumor-suppressor gene in lung cancer. Br J Cancer. 2015;113(6):970–978. doi:10.1038/bjc.2015.302
32. Okamoto I, Aoe K, Kato T, et al. Pemetrexed and carboplatin followed by pemetrexed maintenance therapy in chemo-naive patients with advanced nonsquamous non-small-cell lung cancer. Invest New Drugs. 2013;31(5):1275–1282. doi:10.1007/s10637-013-9941-z
33. Schuette WH, Groschel A, Sebastian M, et al. A randomized phase II study of pemetrexed in combination with cisplatin or carboplatin as first-line therapy for patients with locally advanced or metastatic non-small-cell lung cancer. Clin Lung Cancer. 2013;14(3):215–223. doi:10.1016/j.cllc.2012.10.001
34. Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247–255. doi:10.1016/S1470-2045(12)70063-3
35. Castonguay R, Werner ED, Matthews RG, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286(34):30034–30046. doi:10.1074/jbc.M111.260133
36. Jiang X, Yang B, Lu J, Zhan Z, Li K, Ren X. Pemetrexed-based chemotherapy in advanced lung adenocarcinoma patients with different EGFR genotypes. Tumor Biol. 2015;36(2):861–869. doi:10.1007/s13277-014-2692-4
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.