Back to Journals » International Journal of General Medicine » Volume 14
Plasma Endogenous Sulfur Dioxide: A Novel Biomarker to Predict Acute Kidney Injury in Critically Ill Patients
Authors Jiang Y, Wang J, Zheng X , Du J
Received 21 March 2021
Accepted for publication 17 May 2021
Published 28 May 2021 Volume 2021:14 Pages 2127—2136
DOI https://doi.org/10.2147/IJGM.S312058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Yijia Jiang,1 Jingyi Wang,1 Xi Zheng,1 Jiantong Du2
1Department of Surgical Intensive Critical Unit, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Department of Ophthalmology, Peking University First Hospital, Beijing, People’s Republic of China
Correspondence: Jiantong Du
Department of Ophthalmology, Peking University First Hospital, No. 8 Xishiku Road, Xicheng District, Beijing, People’s Republic of China
Tel + 86-15010131615
Email [email protected]
Purpose: Sulfur dioxide (SO2) is a novel gaseous signaling molecule that plays an important role in inflammation, which contributes the pathogenesis of acute kidney injury (AKI). The aim of this study was to explore the predictive value of plasma SO2 for AKI in high-risk patients.
Patients and Methods: A prospective cohort of 167 patients who underwent major noncardiac surgery was enrolled in the study. Plasma SO2, urine neutrophil gelatinase-associated lipocalin (NGAL), tissue inhibitor of metalloproteinase-2 (TIMP-2), and insulin-like growth factor-binding protein 7 (IGFBP7) levels were detected immediately after the operation. The primary endpoint was new-onset AKI within 72 h after admission. The ability of biomarkers including SO2 and a clinical risk model to predict AKI was compared by receiver operator characteristic (ROC) curve analysis and decision curve analysis (DCA), additional contributions were evaluated by integrated discrimination improvement (IDI) and net reclassification improvement (NRI) analyses.
Results: A total of 61 (36.5%) patients developed AKI within 72 h of surgery. Compared to NGAL and [TIMP-2]·[IGFBP7], SO2 showed better predictive ability for new-onset AKI with an area under the ROC curve of 0.771 (95% confidence interval: 0.700– 0.832, p< 0.001). The improvement in predictive value by including SO2 in the clinical risk model was supported by NRI (0.28; P=0.04) and IDI (0.15; P< 0.001) analyses. The net benefit of the combination of SO2 and clinical variables was the max in DCA.
Conclusion: Plasma SO2 shows a useful value for predicting new-onset AKI, and improved AKI prediction based on clinical variables, which can guide the implementation of preventive measures for high-risk patients.
Keywords: gasotransmitter, AKI, predictive modelling, intensive care unit
Introduction
Acute kidney injury (AKI) is a major public health burden—occurring in more than half of critical ill patients and is associated with increased risk of both in-hospital mortality and long-term chronic kidney disease.1,2 Given the low efficacy of interventions for AKI, identification of high-risk patients and implementation of preventive strategies are critical to assure a good clinical outcome. Diagnosis of AKI is currently based on oliguria or/and elevated serum creatinine.3 However, the former is closely related to the patient’s overall fluid volume whereas the latter is a late marker that may not be detected even when glomerular filtration rate has decreased by half.4 Thus, these 2 functional markers are neither sensitive nor specific, which could delay diagnosis and treatment.5,6 Numerous studies have investigated potential biomarkers for identifying high-risk patients and facilitating the diagnosis of AKI.7 However, most biomarkers have disadvantages and limited utility in clinical practice.8,9
Sulfur dioxide (SO2), which was previously regarded as a toxic gas,10,11 is now recognized as a novel gasotransmitter.12 SO2 is endogenously produced by the metabolism of the sulfur-containing amino acid L-cysteine13 and plays important physiological and pathophysiological roles.14–16 SO2 levels are elevated in patients with acute pneumonia, chronic renal failure, and pediatric acute lymphoblastic leukemia with bacterial infection.17–19 We speculated that as an indicator of inflammation, SO2 may predict the development of AKI in high-risk patients. To test this hypothesis, in this study we used SO2 to develop an AKI prediction model and compared its performance with that of established markers of AKI in a prospective cohort of postoperative critically ill patients.
Materials and Methods
Study Setting and Population
The present study was carried out in a 20-bed surgical intensive care unit (ICU) of Beijing Chao-Yang Hospital from January 1, 2020 to October 31, 2020. The study was designed and conducted and is reported according to the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis statement.20 The study was approved by the human ethics committee of Beijing Chao-Yang hospital, Capital Medical University (Beijing, China) (no. 2020-ke-236). Informed consent from patients or their next of kin was obtained before consecutive patients were prospectively enrolled in the study. We included patients who underwent major noncardiac surgery and stayed in the ICU longer than 48 h. The exclusion criteria were age <18 years; development of AKI before ICU admission; insufficient data or blood samples; chronic kidney disease (CKD); operated by nephrectomy or kidney transplantation; and not transferred to the ICU immediately after the operation.
Definitions and Clinical Endpoints
The definition and classification of AKI were based on serum creatinine and urine output criteria proposed by Kidney Disease: Improving Global Outcomes.3 New-onset AKI was defined as AKI occurring within 72 h after the operation. CKD was defined according to National Kidney Foundation criteria as estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 for at least 3 months irrespective of the cause. GFR was estimated with the Cockcroft–Gault formula.21 If at least 5 values for serum creatinine were available, the median of all values available from 6 months to 6 days prior to enrollment was used as the baseline; otherwise, the lowest value in the 5 days prior to enrollment was used. If no pre-enrollment creatinine was available or the emergency patient’s serum creatinine was abnormal at the time of admission, baseline creatinine was estimated using the Modification of Diet in Renal Disease equation assuming that baseline eGFR was 75 mL/min per 1.73 m.22 Sepsis and septic shock were diagnosed according to the Sepsis 3.0 definition of the American College of Chest Physicians/Society of Critical Care Medicine.23
The primary endpoint was the development of AKI within 72 h after enrollment. Secondary endpoints for the purpose of characterizing the patient population included ICU and hospital mortality and length of ICU and hospital stays.
Data Collection
Demographic information and clinical data such as chronic illnesses, pre-ICU medications (including nephrotoxic drugs), surgical procedure, laboratory blood tests, duration of mechanical ventilation, and length of ICU and hospital stays were prospectively collected during the hospital stay and recorded in case report forms. Baseline serum creatinine, everyday creatinine, and hourly urine output on ICU admission and thereafter were measured and recorded. The severity of illness was estimated with Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment (SOFA) scores.
Sample and Laboratory Analysis
Paired blood and urine samples were collected immediately after the operation. After 30 min, the samples were centrifuged at 3000 rpm at 4°C for 10 min, and the supernatant (plasma) was stored at −80°C. SO2 concentration were analyzed by high-performance liquid chromatography analysis (HPLC) (Series number:USKH127476, produce year: 2020).15 A 100 µL volume of plasma was mixed with 70 µL of sodium borohydride and 10 µL of methyl 2-bromopropionate (both from Sigma-Aldrich, St. Louis, MO, USA), followed by incubation for 10 min at 42°C. Perchloric acid (40 µL) was added and the mixture was centrifuged at 12,400 rpm for 10 min at 23°C to remove precipitated proteins; 10 µL Tris-HCl (pH=3.0) was then added to the supernatant for neutralization before HPLC analysis. The mobile phases were a methanol: acetic acid: water buffer (5.00:0.25:94.75 by volume, pH=3.4) and pure methanol. All measurements were performed at an excitation wavelength of 392 nm and absorption wavelength of 479 nm. Quantification analysis was done by the standardization of sodium sulfite. According to previous research,15 we set up 7 sets of standard samples with different concentrations of sulfite, and measured their peak areas respectively using HPLC, and then obtained the standard curve (Figure S1). The R2 of the standard curve is 0.9986, which proves that the determination accuracy is excellent. Commercially available enzyme-linked immunosorbent assay kits were used to measure urine concentrations of neutrophil gelatinase-associated lipocalin (NGAL) (CSB-E09408h; Cusabio, Wuhan, China), tissue inhibitor of metalloproteinase-2 (TIMP-2) (DTM200; R&D Systems, Minneapolis, MN, USA), and insulin-like growth factor-binding protein 7 (IGFBP7) (ARG81498; arigo Biolaboratories, Shanghai, China). The assays were performed by technicians who were blinded to clinical data, and the supervising physicians were blinded to the biomarker test results.
Statistical Analysis
Continuous variables are presented as a median with 25th and 75th percentiles (interquartile range [IQR]), and categorical variables are presented as percentages. Continuous data were compared between groups with the Student’s t-test or Mann–Whitney U-test, and categorical variables were compared with the chi-squared test or Fisher’s exact test. Receiver operator characteristic (ROC) curve analysis was performed to assess the predictive value of biomarkers for AKI during follow-up. The cutoff point was the value with the highest sensitivity and specificity. Clinical parameters with p<0.10 in the univariate analyses were included in the multivariate logistic regression model. The calibration of the model was assessed with the Hosmer–Lemeshow test. Bootstrapping with repeated sampling was performed to confirm the stability of the model. We compared the predictive performance of the clinical risk model before and after adding different biomarkers by calculating the statistical significance of differences between area under the ROC curve (AUC) values24 which were defined as follows: 0.90–1.0, excellent; 0.80–0.89, good; 0.70–0.79, useful; 0.60–0.69, poor; and 0.50–0.59, not useful.25 Improvement in the predictive accuracy of the models was evaluated by calculating the relative integrated discrimination improvement (IDI) and net reclassification improvement (NRI).26 We also estimated the clinical utility and net benefit of the new predictive models by decision curve analysis (DCA),27 which identifies patients who are at risk of AKI based on the clinic prediction model with and without biomarkers. The x axis shows threshold values for AKI while the y axis represents the net benefit for the different threshold values of AKI; a higher net benefit is provided by prediction models that are farthest away from the slanted dashed gray line (assuming all adverse events) and horizontal black line (assuming no adverse event). For all analyses, statistical significance was taken as a 2-sided p value <0.05. Statistical analyses were performed using SPSS v25 (SPSS Inc, Chicago, IL, USA), MedCalc v.16.4.3 (MedCalc, Ostend, Belgium), and R 4.0.3 (R Project for Statistical Computing, Vienna, Austria).
Results
Subject Characteristics and Event Rates
We screened 282 subjects who were admitted to the ICU after major noncardiac surgery and typically had at least 1 recognized risk factor for AKI. A total of 115 patients were excluded for the following reasons: age <18 years (n=1); developed AKI before ICU admission (n=56); CKD (n=28); operated by nephrectomy or kidney transplantation (n=20); not transferred to the ICU immediately after the operation (n=8); and invalid or missing test results (n=2). Thus, 167 subjects were ultimately included in the prospective cohort; their demographic information is shown in Table 1.
 |
Table 1 Baseline Characteristics of Patients Stratified by New-Onset Acute Kidney Injury |
In total, 61 patients (36.5%) met the primary endpoint of new-onset AKI. Each AKI case was diagnosed based on elevated creatinine (34.4%), oliguria (41.0%), or both (24.6%). Of these cases, 57.4% (n=35) were stage I, 32.8% (n=20) were stage II, and 9.8% (n=6) were stage III. The median (IQR) length of stay in the ICU was 2 (2–4) days for patients with AKI and 6 (3–8) days for those without AKI (Table 2).
 |
Table 2 Outcomes Between Patients with and without New-Onset AKI |
Predictive Performance of SO2 Compared to Established AKI Biomarkers
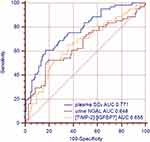
Plasma SO2 was higher in patients with new-onset AKI (Table 3) and increased with AKI severity (Figure 1). Plasma SO2, urine NGAL, and [TIMP-2]·[IGFBP7] were associated with the incidence of AKI in the univariate analysis (Table S1). Figure 2 shows the AUCs for plasma SO2 and established AKI biomarkers. Plasma SO2 had an AUC of 0.771 (95% confidence interval [CI]: 0.700–0.832, p<0.001) for predicting AKI, with an optimal cutoff value of 15.0 µmol/l. In contrast, NGAL and [TIMP-2]·[IGFBP7] showed lower performance in predicting AKI, with AUCs of 0.648 (95% CI: 0.570–0.720, p=0.001) and 0.655 (95% CI: 0.578–0.727, p<0.001), respectively. The statistical significance of the differences between the AUCs of SO2 and AKI biomarkers was confirmed with the DeLong method (p=0.045 for plasma SO2 and NGAL; p=0.036 for plasma SO2 and [TIMP-2]·[IGFBP7]).
 |
Table 3 Levels of Plasma SO2 and Urine Biomarkers in New-Onset AKI and Non-AKI Patients |
 |
Figure 1 Discrimination of plasma SO2 between non-AKI and AKI of different severities. ***Comparison between non-AKI and AKI of different severities (p<0.001). Abbreviations: AKI, acute kidney injury |
Predictive Superiority of Plasma SO2 Over Other Biomarkers in the AKI Risk Model
In the univariate analysis, hypertension, estimated blood loss, baseline creatinine, SOFA score, and use of vasopressors were associated with the occurrence of AKI. Of these variables, hypertension, estimated blood loss, and baseline creatinine were retained for the final model (Table S2). The calibration of the model was confirmed with the Hosmer–Lemeshow goodness-of-fit test (p>0.05). This clinical model predicted AKI with an AUC of 0.782 (95% CI: 0.712–0.843, p<0.001).
The inclusion of SO2 significantly improved the predictive ability of the clinical model for AKI, supported by NRI [0.282 (0.043–0.583), p=0.038] and IDI [0.145 (0.088–0.202), p<0.001] analyses and Delong’s test (p=0.013) (Table 4). In contrast, the model was not improved by including NGAL and [TIMP-2]·[IGFBP7]. In the multivariate logistic regression analysis, the risk of AKI based on the clinical–plasma SO2 risk prediction model was 1/(1 + e−z), z=0.005+1.171×plasma SO2+3.256×hypertension+1.001×estimated blood loss. The optimal cutoff probability was 0.395; patients with a probability higher than this value were at risk of developing AKI after major noncardiac surgery.
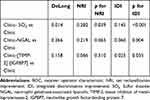 |
Table 4 Comparison of the ROC Curves, NRI and IDI of Combination vs Clinic Models in Predicting AKI |
Figure 3 shows the decision curves of 4 models (clinical, clinical–SO2, clinical–NGAL, and clinical–[TIMP-2]·[IGFBP7]) for predicting AKI. The clinical–SO2 model had the highest net benefit at 10%–60% of the probability threshold; that is, if a patient with a risk of AKI between 10% and 60% warranted further therapy (such as hemodynamic monitoring or preventive interventions), AKI screening using clinical–SO2 would have the most benefit for study participants after taking into account cost, adverse effects, and other negative factors in the screening tests. Although the net benefit of the 4 models tended to be similar with increasing probability threshold, they diverged significantly at low probability threshold.
Sensitivity Analysis
As SO2 is produced by neutrophils, the risk prediction analysis was repeated after excluding patients with white blood count <4*109/l (n=7). The predictive value of SO2 was enhanced when it was incorporated into the model with clinical variables (AUC=0.780 and 0.855, respectively; p<0.001).
Discussion
This is the first study to investigate the predictive utility of endogenous SO2 for AKI in critically ill patients. We found that plasma concentration of SO2 was significantly higher in patients who developed AKI after major noncardiac surgery than in those who did not, and was independently associated with the risk of AKI. Our results are novel because they show the plasma SO2 profile of critically ill patients and demonstrate its predictive capacity for AKI, which was superior to that of urine NGAL and [TIMP-2]·[IGFBP7] and enhanced that of a conventional clinical model, as supported by IDI and NRI analyses and DCA.
AKI is a common postoperative complication that is independently associated with poor prognosis in critically ill patients who have undergone surgery.28 However, early identification of high-risk patients can lead to initiation of preventive measures (eg, avoiding nephrotoxins, volume management, and individualized hemodynamic resuscitation)29,30 before renal damage occurs, which can reduce mortality and improve clinical outcomes. The predictive ability of plasma SO2 was compared to that of other AKI biomarkers. The most widely used biomarkers are TIMP-2 and IGFBP7, which are expressed in tubular cells in response to DNA damage and are markers for G1 arrest.31,32 Urine [TIMP-2]·[IGFBP7] showed excellent ability to predict AKI in the SAPPHIRE and TOPAZ trials;22,33 however, this has not been validated in different populations, which is important as AKI can be caused by different factors.9 NGAL, the most extensively studied AKI biomarker, is expressed in many tissues and the level in urine is elevated as early as 3 h after tubular injury.34–36 However, NGAL expression lacks specificity as a biomarker because it increases with age and infection and is higher in females.37 In the present study, both [TIMP-2]·[IGFBP7] and NGAL showed limit predictive value for AKI, possibly because in our cohort of postoperative patients, only a minority had sepsis; meanwhile, AKI-induced elevation in urinary TIMP-2/IGFBP7 level is associated with increased kidney filtration, which is more commonly associated with sepsis than with hemorrhage.38 Our multivariate logistic regression analysis suggested that estimated blood loss but not sepsis was a risk factor for new-onset AKI; thus, AKI in our cohort was mostly caused by ischemia, which is expected to affect TIMP-2 and GFBP7 levels. The upregulation of NGAL in AKI is positively correlated with the severity of kidney injury;34 however, most AKI patients in our cohort had only mild injury and therefore, the increase in NGAL expression was relatively insignificant. In our study, plasma SO2 showed better predictive performance than urine [TIMP-2]·[IGFBP7] and NGAL for the development of AKI in postoperative critically ill patients.
SO2 has recently been identified as a gasotransmitter in the cardiovascular system. Previously studies reported endogenous SO2 synthesis pathways in the heart, stomach, lung, kidney, spleen, liver, and retina in mammals that play an important role in systemic homeostasis12 SO2 was identified as a key regulator of inflammation in the pathogenesis of cardiovascular, ophthalmologic, and neurologic diseases39–41 that acts by attenuating tumor necrosis factor (TNF)-α-induced inhibitor of nuclear factor (NF)-κB (IκBα) phosphorylation and degradation and NF-κB p65 phosphorylation.42,43 Elevated levels of endogenous SO2 were detected in patients with acute early edge infection and postural tachycardia syndrome, suggesting that it is an early and sensitive indicator of inflammation.19,44 In the pathophysiology of AKI, inflammation induces endothelial and tubular cell injury;45 we therefore speculated that SO2 could be a biomarker of AKI, although the plasma level of SO2 in critical care patients and its relationship to AKI has not been previously reported. In the present study, we firstly revealed the existence of the plasma endogenous SO2 in critical ill patients and the elevation of SO2 demonstrated a good predictive performance in detecting new-onset AKI. Studies on AKI based on predictive analytics46–49 have used only conventional biomarkers or clinical variables, the novelty of our study is not only demonstrate a promising biomarker but also contribute to the pathophysiologic mechanisms of AKI.
Our study had some limitations. Firstly, it was a single-center study involving noncardiac postoperative patients, which limits the external validity of our findings. Secondly, we did not conduct a sample size analysis because of the absence of relevant data. Thirdly, we did not have a validation cohort and as the predictive model was evaluated in the same cohort as that used for model training, the possibility of overfitting cannot be excluded. Finally, we did not analyze time-dependent variables or include dynamic predictions of AKI after the operation, which should be addressed in future studies.
Conclusion
In summary, plasma SO2 level was significantly higher in patients who developed new-onset AKI within 72 h of ICU admission after major noncardiac surgery than in those who did not develop AKI. Additionally, plasma SO2 was a better predictor of AKI in our cohort than established AKI biomarkers such as urine [TIMP-2]·[IGFBP7] and NGAL. The predictive ability of SO2 was enhanced in combination with clinical variables. These findings provide a basis for further investigations on the role of SO2 in the pathogenesis of AKI in critically ill patients.
Abbreviations
SO2, Sulfur dioxide; AKI, acute kidney injury; NGAL, neutrophil gelatinase-associated lipocalin; TIMP-2, tissue inhibitor of metalloproteinases-2; IGFBP7, insulin-like growth factor-binding protein 7; ROC, receiver operator characteristic; DCA, decision curve analysis; IDI, integrated discrimination improvement; NRI, net reclassification improvement; AUC, area under the receiver operating characteristic curve; CI, confidence interval; CKD, chronic kidney disease; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes; eGFR, estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; CRF, case report forms; APACHE II, acute physiology and chronic health evaluation; SOFA, sequential organ failure assessment. HPLC, high-performance liquid chromatography analysis.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the human ethics committee of Beijing Chao-Yang hospital, Capital Medical University (Beijing, China). The chief ethics number was 2020-ke-236. Informed consent from patients or their next of kin was obtained before patients joined in the study. The study followed the ethical principles of the Declaration of Helsinki 1964.
Consent for Publication
The manuscript does not contain any individual person’s data in any form.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The work was supported by the National Natural Science Foundation of China (No. 81900872).
Disclosure
The authors declare that they have no competing interests.
References
1. Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI‑EPI study. Intensive Care Med. 2015;41:1411–1423. doi:10.1007/s00134-015-3934-7.
2. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–1964. doi:10.1016/S0140-6736(19)32563-2.
3. Palevsky PM, Liu KD, Brophy PD, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61:649–672. doi:10.1053/j.ajkd.2013.02.349.
4. Ronco C, Bellomo R, Kellum JA. Understanding renal functional reserve. Intensive Care Med. 2017;43:917–920. doi:10.1007/s00134-017-4691-6.
5. Lehner GF, Forni LG, Joannidis M. Oliguria and biomarkers of acute kidney injury: star struck lovers or strangers in the night? Nephron. 2016;134:183–190. doi:10.1159/000447979.
6. Sandrra K-G, Meersch M, Bell M. Biomarker-guided management of acute kidney injury. Curr Opin Crit Care. 2020;26:556–562. doi:10.1097/MCC.0000000000000777.
7. Koyner JL, Zarbock A, Basu RK, et al. The impact of biomarkers of acute kidney injury on individual patient care. Nephrol Dial Transplant. 2020;35:1295–1305. doi:10.1093/ndt/gfz188.
8. Ho J, Tangri N, Komenda P, et al. Urinary, plasma, and serum biomarkers’ utility for predicting acute kidney injury associated with cardiac surgery in adults: a meta-analysis. Am J Kidney Dis. 2015;66:993–1005. doi:10.1053/j.ajkd.2015.06.018.
9. Dewitte A, Joannès-Boyau O, Sidobre C, et al. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol. 2015;10:1900–1910. doi:10.2215/CJN.12651214.
10. Woerman AL, David M. Perinatal sulfur dioxide exposure alters brainstem parasympathetic control of heart rate. Cardiovasc Res. 2013;99:16–23. doi:10.1093/cvr/cvt057.
11. Min KB, Min JY, Cho SI, et al. The relationship between air pollutants and heart-rate variability among community residents in Korea. Inhal Toxicol. 2008;20:435–444. doi:10.1080/08958370801903834.
12. Huang Y, Tang C, Du JB, et al. Endogenous sulfur dioxide: a new member of gasotransmitter family in the cardiovascular system. Oxid Med Cell Longev. 2016;3:8961951. doi:10.1155/2016/8961951.
13. Singer TP, Kearney EB. Intermediary metabolism of L-cysteinesulfinic acid in animal tissues. Arch of Biochem Biophys. 1956;61:397–409. doi:10.1016/0003-9861(56)90363-0.
14. Ma HL, Huang XL, Liu Y, et al. Sulfur dioxide attenuates LPS-induced acute lung injury via enhancing polymorphonuclear neutrophil apoptosis. Acta Pharmacol Sin. 2012;33:983–990. doi:10.1038/aps.2012.70.
15. Du S, Jin H, Bu D, et al. Endogenously generated sulfur dioxide and its vasorelaxant effect in rats. Acta Pharmacol Sin. 2008;29:923–930. doi:10.1111/j.1745-7254.2008.00845.x.
16. Liang Y, Liu D, Ochs T, et al. Endogenous sulfur dioxide protects against isoproterenol—induced myocardial injury and increases myocardial antioxidant capacity in rats. Lab Invest. 2011;91:12–23. doi:10.1038/labinvest.2010.156.
17. Kajiyama H, Nojima Y, Mitsuhashi H, et al. Elevated levels of serum sulfite in patients with chronic renal failure. J Am Soc Nephrol. 2000;11:923–927.
18. Mitsuhashi H, Ikeuchi H, Yamashita S, et al. Increased levels of serum sulfite in patients with acute pneumonia. Shock. 2004;21:99–102. doi:10.1097/01.shk.0000105501.75189.85.
19. Wu W, Jia Y, Du S, et al. Changes of sulfur dioxide, nuclear factor-κB, and interleukin-8 levels in pediatric acute lymphoblastic leukemia with bacterial inflammation. Chin Med J. 2014;127:4110–4113.
20. Collins GS, Reitsma JB, Altman DG, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. 2015;350:g7594. doi:10.1136/bmj.g7594.
21. Bellomo R, Ronco C, Kellum JA, et al. Acute Dialysis Quality Initiative workgroup. Acute renal failure-definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:204–212. doi:10.1186/cc2872.
22. Kashani K, Al-Khafaji A, Ardiles T, et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit Care. 2013;17:R25. doi:10.1186/cc12503.
23. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–810. doi:10.1001/jama.2016.0287.
24. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845.
25. Glassford NJ, Schneider AG, Xu S, et al. The nature and discriminatory value of urinary neutrophil gelatinase associated lipocalin in critically ill patients at risk of acute kidney injury. Intensive Care Med. 2013;39:1714–1724. doi:10.1007/s00134-013-3040-7.
26. Pencina MJ, D’Agostino RBJ, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi:10.1002/sim.2929.
27. Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Med Decis Making. 2006;26:565–574. doi:10.1177/0272989X06295361.
28. Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, et al. Cost and mortality associated with postoperative acute kidney injury. Ann Surg. 2015;261:1207–1214. doi:10.1097/SLA.0000000000000732.
29. Ronco C. Acute kidney injury: from clinical to molecular diagnosis. Crit Care. 2016;20:201. doi:10.1186/s13054-016-1373-7.
30. Meersch M, Schmidt C, Hoffmeier A, et al. Prevention of cardiac surgery-associated AKI by implementing the KDIGO guidelines in high-risk patients identified by biomarkers: the PrevAKI randomized controlled trial. Intensive Care Med. 2017;43:1551–1561. doi:10.1007/s00134-016-4670-3.
31. Seo DW, Li H, Qu CK, et al. Shp-1 mediates the anti-prolferative activity of tissue inhibitor of metalloproteinase-2 in human microvascular endothelial cells. J Biol Chem. 2006;281:3711–3721. doi:10.1074/jbc.M509932200.
32. Zuo S, Liu C, Wang J. IGFBP-rP1 induces p21 expression through a p53-independent pathway, leading to cellular senscence of MCF-7 breast cancer cells. J Cancer Res Clin Oncol. 2012;138:1045–1055. doi:10.1007/s00432-012-1153-y.
33. Gunnerson KJ, Shaw AD, Chawla LS, et al. TIMP2•IGFBP7 biomarker panel accurately predicts acute kidney injury in high-risk surgical patients. J Trauma Acute Care Surg. 2016;80:243–249. doi:10.1097/TA.0000000000000912.
34. Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148:810–819. doi:10.7326/0003-4819-148-11-200806030-00003.
35. Cruz DN, Bagshaw SM, Maisel A, et al. Use of biomarkers to assess prognosis and guide management of patients with acute kidney injury. Contrib Nephrol. 2013;182:45–64. doi:10.1159/000349965.
36. Geus HRHD, Bakker J, Lesaffre EM, et al. Neutrophil gelatinase-associated lipocalin at ICU admission predicts for acute kidney injury in adult patients. Am J Respir Crit Care Med. 2011;183:907–914. doi:10.1164/rccm.200908-1214OC.
37. Srisawat N, Kellum JA. The role of biomarkers in acute kidney injury. Crit Care Clin. 2020;36:125–140. doi:10.1016/j.ccc.2019.08.010.
38. Johnson ACM, Zager RA. Mechanisms underlying increased TIMP2 and IGFBP7 urinary excretion in experimental AKI. J Am Soc Nephrol. 2018;29:2157–2167. doi:10.1681/ASN.2018030265.
39. Jin HF, Zhao M, Chen SY, et al. The role of sulfur dioxide in the regulation of mitochondrion-related cardiomyocyte apoptosis in rat with isopropylarterenol-induced myocardial injury. Int J Mol Sci. 2013;14:10465–10482. doi:10.3390/ijms140510465.
40. Du JT, Huang YQ, Li K, et al. Retina-derived endogenous sulfur dioxide might be a novel antiapoptotic factor. Biochem Biophys Res C Ommun. 2018;496:955–960. doi:10.1016/j.bbrc.2018.01.103.
41. Han Y, Yi W, Qin J, et al. Dose-dependent effect of sulfur dioxide on brain damage induced by recurrent febrile seizures in rats. Neurosci Lett. 2014;563:149–154.
42. Zhang H, Huang Y, Bu D, et al. Endogenous sulfur dioxide is a novel adipocyte-derived inflammatory inhibitor. Sci Rep. 2016;6:27026. doi:10.1016/j.neulet.2013.12.042.
43. Zhu Z, Zhang L, Chen Q, et al. Macrophage-derived sulfur dioxide is a novel inflammation regulator. Biochem Biophys Res Commun. 2020;524:916–920. doi:10.1016/j.bbrc.2020.02.013.
44. Li HX, Zheng XC, Chen SY, et al. Increased endogenous sulfur dioxide involved in the pathogenesis of postural tachycardia syndrome in children: a case-control study. Chin Med J (Engl). 2018;131:435–439. doi:10.4103/0366-6999.225051.
45. Kellum JA, Prowle JR. Paradigms of acute kidney injury in the intensive care setting. Nat Rev Nephrol. 2018;14:217–230. doi:10.1038/nrneph.2017.184.
46. Zhang ZH, Navarese EP, Zheng B, et al. Analytics with artificial intelligence to advance the treatment of acute respiratory distress syndrome. J Evid Based Med. 2020;13:301–302. doi:10.1111/jebm.12418.
47. Khan ZF, Alotaibi SR. Applications of artificial intelligence and big data analytics in m-health: a healthcare system perspective. J Healthc Eng. 2020;30:8894694. doi:10.1155/2020/8894694.
48. Zimmerman LP, Reyfman PA, Smith ADR, et al. Early prediction of acute kidney injury following ICU admission using a multivariate panel of physiological measurements. BMC Med Inform Decis Mak. 2019;19(Suppl 1):16. doi:10.1186/s12911-019-0733-z.
49. Zhang ZH, Ho KM, Hong Y. Machine learning for the prediction of volume responsiveness in patients with oliguric acute kidney injury in critical care. Crit Care. 2019;23:112. doi:10.1186/s13054-019-2411-z.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.