Back to Journals » International Journal of Nanomedicine » Volume 18
Plant-Derived Vesicle-Like Nanoparticles: Clinical Application Exploration and Challenges
Authors He W, Zheng S, Zhang H , Gao B, Jin J, Zhang M , He Q
Received 3 July 2023
Accepted for publication 23 September 2023
Published 6 October 2023 Volume 2023:18 Pages 5671—5683
DOI https://doi.org/10.2147/IJN.S428647
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Wenfang He,1,* Siqiang Zheng,1,* Hua Zhang,2 Bowen Gao,3 Juan Jin,1 Mingzhen Zhang,3,4 Qiang He1
1Department of Nephrology, the First Affiliated Hospital of Zhejiang Chinese Medical University (Zhejiang Provincial Hospital of Traditional Chinese Medicine), Hangzhou, Zhejiang, 310000, People’s Republic of China; 2Hangzhou Medical College, Hangzhou, Zhejiang, People’s Republic of China; 3School of Basic Medical Sciences, Xi’an Key Laboratory of Immune Related Diseases, Xi’an Jiaotong University, Xi’an, Shaanxi, 710061, People’s Republic of China; 4Key Laboratory of Environment and Genes Related to Diseases, Xi’an Jiaotong University, Ministry of Education, Xi’an, Shaanxi, 710061, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qiang He; Mingzhen Zhang, Email [email protected]; [email protected]
Abstract: The utilization of plant-derived vesicle-like nanoparticles (PDVLNs) has shown effectiveness in the prevention/treatment of inflammatory-mediated diseases, malignancies, and immune-related diseases, such as acute liver injury, allergic asthma, gastric cancer and so on. This highlights the promising potential of PDVLNs as biotherapeutics. Furthermore, it should be noted that PDVLNs possess the ability to function as both natural and engineered drug carriers, making them an appealing option. This review aims to present the appropriate extraction methods of PDVLNs, summarize the applications of PDVLNs in different diseases, and provide an outlook on the prospects of PDVLNs. At the same time, the authors also express their discussion on the current limitations of PDVLNs.
Keywords: plant-derived vesicle-like nanoparticles, extraction methods, anti-inflammatory, immune regulation, antitumor
Introduction
Extracellular vesicles (EVs) are membranous structures located outside of cellular boundaries, exhibiting diameters ranging from 40–1000 nm.1 Furthermore, these substances are sourced from organic entities and cellular structures, encompassing plants, animals, and microorganisms.2 EVs were found to participate in the horizontal transfer of bioactive cargos, including proteins, nucleic acids (DNA, mRNA, and microRNA), as well as other molecules that include carbohydrates, lipids, and secondary metabolites.3–6 The investigation of EVs has resulted in an increase in scientific research in the discipline of zoological biology, and their capacity to facilitate intercellular communication and regulate pathological mechanisms has been progressively explicated.7 In contrast, the identification of plant-derived vesicle-like nanoparticles (PDVLNs) did not occur until 2009. Subsequently, research on PDVLNs has garnered significant interest and attention from numerous scientists and researchers.8
For PDVLNs, it is also called exosomes, EVs, as well as nanoparticles.9,10 In this article, the authors use a uniform definition of “PDVLNs” to refer to them.11 PDVLNs have been extensively studied as a means of pathogen defence, as plant cells have been observed to secrete PDVLNs through various mechanisms in response to pathogenic infection.12–14 Several studies have revealed several ways of PDVLNs generation and summarized into the following three pathways: exocyst-positive organelle (EXPO), exocyst-positive organelle (MVBs), the most frequently documented PDVLN production route, and Vacuole pathways.15–17
PDVLNs share similar characteristics as mammalian cell-derived exosomes, such as size (a particle size between 10 nm–1 μm), morphology (a spherical, oval or cup-shaped morphology), and Zeta potential (negative surface charge), as well as serving as tools for maintaining cellular homeostasis and intercellular communications, and all dependent on plant species and extraction procedure.1,18,19 Moreover, PDVLNs exhibit a significant degree of heterogeneity in origins and physical features and can be distinguished by the primary methodologies employed for the evaluation of mammalian EVs biochemical constituents, including Western blot (WB) and enzyme-linked immunosorbent assay (ELISA).20,21
Unlike mammals, they are primarily derived from edible plants (medicinal plants and fruits), contain natural active ingredients, and are easily accessible on a large scale.22 Studies have demonstrated that dietary nanoparticles are capable of penetrating mammalian cells and modulating their corresponding functions.23 Furthermore, PDVLNs do not carry zoonotic or human pathogens, seem safer, and can avoid causing unnecessary ethical problems. In addition, it can be used as nanocarriers in its original form and transformed form further to make an effort in disease diagnosis and treatment.9,22,24 However, it remains a huge challenge for researchers to get PDVLNs from specific plants, as current methods cannot be uniformly applied to any plant.25 Prior research has primarily concentrated on investigating the potent biological properties of the subject in vitro and animal models. However, it has been impeded by various constraints and remains unfeasible for clinical implementation.
Therefore, this review aims to present the appropriate extraction methods of PDVLNs, summarize the applications of PDVLNs in different diseases, and provide an outlook on the prospects of PDVLNs.
Preparation and Obligation of the PDVLNs
Medical and edible plants are closely associated with human life. More and more attention has been focused on the role of disease treatment and health management of PDVLNs. Compared to the mammalian cell-derived exosomes commonly used in current research and clinical use, it has a more readily available way of obtaining ingredients. However, it remains challenging to obtain PDVLNs industrially and specifically.
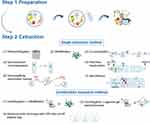
ISEV-2018 recommended that the purity and specialty of an EV preparation should depend on the experimental question and EV end-use.26 The updating of MISEV published in 2021 considered that ISEV-2018 effectively improves EV research’s quality and extraction methods.27 The critical aspect of PDVLNs is the maintenance of uniformity and control over product quality.28 As a result, the primary focus for the clinical application of PDVLNs is centered on the industrialization and efficient extraction of these substances. The following section provides further details on the commonly used techniques for isolating PDVLNs (Figure 1).
Pre-Processing and Collection of the Bulk Solution
First, prior to the isolation process, try to keep the state of raw materials consistent. Subsequently, the raw materials we selected need to be cleaned repeatedly. Then, juice the selected material. The procedure above has the potential to impact both the constitution and functionality of the product. In this step, the speed of juicing needs to be carefully selected according to the materials.
A Single Extraction Method
Ultracentrifugation
Ultracentrifugation is regarded as the most reliable methodology for EV isolation. Varying-size particles would be separated according to different sedimentation rates caused by centrifugal force. In actual application, a standardized procedure cannot suit different plants. Therefore, the centrifugation conditions and times must be established based on the sample.29 This low-cost method presents a reduced contamination risk, making it a viable option for processing large samples.30
Moreover, density gradient ultracentrifugation is always employed to help differential centrifugation achieve further purification. A density gradient can be created by layering liquids with a lower density in a centrifuge tube.31 Although gradient mediums, including sucrose and commercialized ones, are commonly used, this method is not recommended for industrial extraction because of the inevitable impurities.
Ultrafiltration
Ultrafiltration is also a common-used strategy in PDVLN extraction. The fluid pressure follows to cause smaller molecules (PDVLNs) to migrate through a polymeric membrane and block the passage of remaining macromolecules.32 Two primary modes of membrane filtration for ultrafiltration are direct flow filtration (DFF) and constant flow filtration (TFF). DFF makes efforts by increasing pressure and may not increase separation due to the restrictive layer it forms.33 In contrast, the TFF mode facilitates the passage of components through the membrane holes. Moreover, the TFF mode separation velocity and efficacy made it capable of processing large samples.34,35
Efficiency and commercial availability are the main advantages of ultrafiltration. However, attention should still be paid to the complicated technology of ultrafiltration membranes.
Co-Precipitation
Unlike physical methods, co-precipitation depends on a special co-precipitator to combine and thus decrease the solubilities of plant-derived vesicles.36 EVs derived from ginger through PEG precipitation demonstrate biochemical properties almost identical to those obtained through the ultracentrifugation method.37 Imperfectly, the presence of residual co-precipitants may impede the execution of additional investigations. Currently, specific commercially available kits for EV precipitation have gained widespread usage. However, the limited level of purity and high costs associated with these preparations impose limitations on their practicality.
Size-Exclusion Chromatography (SEC)
SEC is a chromatographic technique employed to separate samples based on the size of their molecules. Isolation using SEC was revealed to produce EVs that exhibit higher intact biophysical features.38 Mol et al verified that EVs obtained from SEC have higher functionality than those obtained from ultracentrifugation.39 The uniformity of size and elevated purity of EVs were demonstrated through their separation via SEC and subsequent examination under an electron microscope. However, this approach still presents particular challenges. It requires specific equipment and costs a lot of time.
Field Flow Fractionation
The field flow fractionation methodology utilizes a force field that is applied perpendicularly to the direction of the sample flow to achieve separation based on size and molecular weight variations. The absence of a stationary phase, reduced system pressure, and minimal or no shear influence render this method less disruptive to samples. The use of asymmetric flow field flow fractionation technology (AF4) has been effective in separating various EVs originating from B16F10 cells. This technology has the potential to advance the production of superior molecular diagnostics and therapeutics based on exosomes and exomeres.40,41 This approach can become the preferred method for accurate isolation and functional analysis of PEVs in the future.
Immunoaffinity Enrichment Method
The process depends on the interaction between antibodies and distinct proteins on the PDVLN membrane. However, certain drawbacks include limited antibody availability, low yield, and small volume. The marker proteins of PDVLN remain uncertain and deficient in commercially available antibodies. Its high cost may limit its applicability to research studies involving a limited sample size, thereby hindering its potential therapeutic utilization.
Microfluidics
The process of exosome isolation involves the utilization of microfluidic devices that operate on conventional principles such as immunity, size, and density. Additionally, novel sorting mechanisms, including acoustic, electrophoretic, and electromagnetic manipulations, nanowire-based traps (NTs), nano-sized deterministic lateral displacement (nano-DLD), and viscoelastic flow, are employed in the isolation process.31,42,43 Microfluidic systems offer a variety of advantages, including simplified operations, rapid processing, reduced sample intake volume, increased sensitivity, and enhanced functional module integration.
Combination Extraction Method
Any single method appears to have shortcomings in some way. Recent studies demonstrate that a suitable combination extraction method can improve yield and specificity.
Cong et al developed an optimal methodology that creatively integrates the benefits of differential centrifugation and ultrafiltration on Houttuyniae herba.28 The resultant products produced through the combination approach exhibit equivalent purity levels to those obtained via ultracentrifugation and demonstrate complete morphology and favourable dispersity. The combined method exhibits promise as a viable alternative due to its good cost-efficiency and suitability for scale-up.
Woith et al44 employed a combination of differential centrifugation and agarose gel electrophoresis to obtain purified EVs from Nicotiana tabacum L., Vinca minor L., and Viscum album L. Furthermore, this approach exhibits specific attributes: 1) it represents a straightforward substitute technique to visualize simultaneously with purification, and 2) this methodology may prove less complex than density gradient centrifugation or SEC.
Yang et al45 employed an electrophoretic technique with a 300 kDa cut-off dialysis bag, called ELD, to isolate PDEVs from the lemon. The resulting intact vesicles were verified to have a size and quantity compared to those obtained through the standard ultracentrifugation method, as confirmed by nanoparticle tracking analysis and transmission electron microscopy. The isolation method was highly efficient and could be executed in a standard biological laboratory without requiring specialized equipment.
The Role of PDVLNs in Diseases
The Role of PDVLNs in Anti-Inflammatory Disease
The academic community is currently highly interested in the potential of natural compounds as anti-inflammatory agents due to the undesirable and harmful side effects associated with current therapies.46 PDVLNs show their powerful anti-inflammatory capacity in different ways (Figure 2). J.Y. You et al47 successfully isolated high-purity cabbage-derived vesicle-like nanoparticles (CDVLNs) and Rabex-derived vesicle-like nanoparticles (RDVLNs). It was shown that they only enhanced cell proliferation and did not harm human cells after different concentrations of EVs were added to HaCaT, HDF, and RAW264.7 cells. Notably, the pro-inflammatory transcripts, IL-6/1β and COX-2, were significantly attenuated in LPS-induced cells with increasing levels of CDVLN. Meanwhile, IL-6/1β showed the same trend in the RDVLNs added group. On pro-inflammatory protein expression, COX-2 was significantly reduced in LPS-induced cells with increasing CDVLN levels. According to these findings, it is possible that Rabex may govern post-transcriptional events that occur throughout COX-2 expression in LPS-treated cells. In general, it was observed that Cabex and Rabex exhibited distinct anti-inflammatory properties and effectively inhibited the generation of pro-inflammatory molecules in cellular systems.
The NLRP3 inflammasome is a multimeric cytosolic protein complex that assembles in response to cellular perturbations. The presence of inflammatory cytokines plays a role in the emergence of systemic low-grade inflammation. Additionally, abnormal activation of NLRP3 can cause the establishment of a persistent inflammatory condition within the body, influencing the development of diseases related to inflammation.48 The study in the obesity model revealed that garlic chive(GC)-DVLNs exhibited a dose-dependent inhibition of all downstream events associated with activation of the NLRP3 inflammasome, including Casp1 autocleavage and IL-18/1β secretion. Interestingly, GC-DVLNs can also be found to reduce acute inflammatory responses and alleviate liver injury in GalN/LPS-challenged mice. It might make efforts by suppressing the NLRP3 inflammasome, thereby diminishing mature IL-1β, ultimately leading to a decrease in macrophage infiltration and detection of inflammatory gene expression in liver tissue.49
In line with the results of GC-DVLNs, honey (H)-DVLNs in bone marrow-derived macrophages (BMDMs) exhibited a dose-dependent inhibition of all the downstream events of NLRP3 inflammasome activation, including Casp1 autocleavage product Casp1 p10 generation, IL-1β/18 secretion, and pyroptotic cell death. The administration of nanoparticles to mice during in vitro experimentation reduced inflammation and liver damage in cases of experimentally induced acute liver injury. miR-4057 in H-DVLNs was observed to exhibit inhibitory effects on NLRP3 inflammasome. Vesicle-like nanoparticles derived from natural products have been identified as potential agents for treating diseases due to their anti-inflammatory properties.
Some fruit-DVLNs also show enormous potential in anti-inflammatory. Raimondo et al50 found that pretreatment with lemon (L)-DVLNs suppressed gene and protein expression of pro-inflammatory cytokines, including IL- 6/1-β and TNF- α. Furthermore, the authors showed that LEVs had anti-inflammatory properties in both vitro and in vivo by obstructing the ERK1/2-NF-κB cascade. NF-κB is considered to be a vital regulator of the inflammatory pathway. Therefore, modulation of the NF- κB pathway could offer advantages in regulating inflammatory stimuli.
The Role of PDVLNs in Immune Regulation
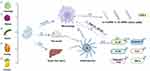
PDVLNs still have many potentials and unknowns waiting to be discovered by scholars in the field of immune regulation.51 The following part lists the role of immune regulation of PDVLNs in the current study (Figure 3). Dendritic cells (DCs) are recognized as proficient APCs and are integral in initiating and regulating innate and adaptive immune responses.52 Han et al53 first applied Petasites japonicus (PJ)-DVLNs to induce DC maturation through overexpressing surface molecules (CD80/86 and MHC-I/II), producing Th1-polarizing cytokines (TNF-α and IL-12p70), and achieving antigen-presenting capability via MAPK and NF-κB pathways. It may promote the initiation of Th1 polarization and CD8+ T cell activation in addition to being able to initiate anticancer immunity.
 |
Figure 3 Summary of immune regulation effects and mechanisms of different PDVLNs. ① refers to Petasites japonicus, ② refers to Celery, ③ refers to Aster yomena Callus. Created with BioRender.com. |
However, increased stimulation of DC maturation will cause an excessive immune response, resulting in inevitable cytokine secretion, normal cell stimulation, cell apoptosis induction, immune cell secondary stimulation by dead cells, and ultimately leading to acute or chronic inflammatory disorders.54 Aster yomena Callus (AYC)-DVLNs have been identified as potential contributors to the reduction of excessive T cell responses in cases of Allergic Asthma. This is achieved by inhibiting DC maturation and infiltration.55 Furthermore, scientists identified the primary metabolites in AYC-DVLN comprising a range of omega-3/6/7 and omega-fatty acids and their derivatives, which were thought to inhibit DC maturation and contribute to macrophage activation.56 Apart from AYC-DVLNs, the regulation effect of Celery- derived vesicle-like nanoparticles (C- DVLNs) on T cells has also aroused the interest of scientific researchers.57 The inhibitory impact of C- DVLNs was observed by inducing an immune response in T cells and PBMC cells, which are critical players in the immune response.
These findings indicated that the materials above have the potential as substitutes for therapeutic agents derived from plants. Additionally, these systems have the potential to serve as an alternative technology that can address a range of issues.
The Role of PDVLNs in Anti-Tumor
PDVLNs have recently received significant attention due to their established therapeutic properties, particularly their anti-cancerous activities, which are attributed to bioactive metabolites and other endogenous molecules (Figure 4). Kim et al58 identified VLNs from four cytotoxic effect plants and verified that VLNs from D. morbifera and P. densiflora sap exhibited significant cytotoxic influences on various neoplasms. The authors of the study have shown that the administration of DM-DVLNs and PD-DVLNs in combination is superior to the use of DM-DVLNs alone. This combined treatment resulted in a synergistic enhancement of cytotoxic effects, which was observed through the inhibition of growth and induction of apoptosis.
VLNs of cannabis were found to arrest the G0/G1 phase in the cell cycle and effectively trigger cell death through the activation of mitochondrial-dependent apoptosis signalling pathways in two HCC cell lines.59 The study revealed that Bitter melon (BM)-DVLNs have the potential to arrest the S-phase in the cell cycle, leading to apoptosis in OSCC cells. Furthermore, when combined with 5-fluorouracil (5-FU), BM-DVLNs were found to enhance OSCC apoptosis by increasing ROS generation. These findings offer a novel approach to overcoming cancer resistance.60 Furthermore, L-DVLNs were found to cause the arrest of the S phase in the cell cycle of gastric cancer cells and trigger cell apoptosis by the mediation of ROS generation.45 VLNs derived from garlic can decrease the anti-apoptotic Bcl-2 expression levels and increase pro-apoptotic Cas3 genes, including Bax, P53, and Cas9, causing S phase cell cycle arrest in A498/A549 cells.61 In addition to vasculogenesis, it decreased VEGF secretion in A498/A549 cells.
Boccia et al62 found that HR-derived VLNs from S. dominica exhibited significant and selective pro-apoptotic effects on pancreatic and mammary cancer cells. A study on D. morbifera sap-derived extracellular vesicles (D-DVLNs) exhibits an inhibitory impact on cancer-associated fibroblasts (CAFs) in a dose-dependent manner. CAFs are recognized as crucial mediators of cancer metastasis.63 VLNs derived from Kaempferia parviflora (KP) were also found to be internalized by human gastric cells, exhibiting cytotoxicity against AGS cells in a dose-dependent manner.64 Ginseng (G)-DVLNs have an anti-tumor effect in another way. It was shown to achieve macrophage polarization that relies mainly on TLR4 and MyD88 signalling.65 Surprisingly, VLNs from Artemisia annua (ADVLNs) were found can inhibit tumor growth and boost anti-tumor immunity in lung cancer. It depends on the mitochondrial DNA (mtDNA), contained in ADVLNs, which was found can serve to induce the cGAS-STING pathway driving the shift of pro-tumor macrophages to anti-tumor phenotype. Further, ADNVs were found can greatly improve the efficacy of PD-L1 inhibitor.66
The Role of PDVLNs in Skin Diseases
A variety of natural compounds, specifically PDVLNs, have been synthesized for achieving skin regeneration. Moreover, VLNs obtained from multiple distinct cells were found to have anti-inflammatory influences and enhance cell proliferation and angiogenesis, both of which are crucial in the latter phases of wound healing.67 It indicates that VLNs represent a valuable biomaterial for skin regeneration. Kim et al68 found that AS-DVLNs can be a natural biomaterial for chronic skin wound healing by attenuating pro-inflammatory cytokine expression, promoting dermal fibroblast proliferation and migration, and promoting angiogenesis. Grapefruit (G)-DVLNs play a similar role in wound healing as AS-DVLNs.69 It achieves wound healing through increased cellular viability and migration, coupled with a decrease in intracellular ROS production in HaCaT cells.
Furthermore, treatment with G-DVLN resulted in an enhancement of the tube formation potential of treated HUVEC cells. In addition, PDVLNs have the potential to function as an anti-melanogenic agent, helping to advance natural cosmetics.70 Cucumber (C) DWLNs exhibited a significant improvement in the dermal penetration efficiency of a lipophilic AI surrogate, resulting in a 200% increase. This finding highlights the potential of C-DVLNs as a viable option for dermal drug delivery.71
The Role of PDVLNs in Other Diseases
Due to the considerable potential of PDVLNs, many researchers have conducted research on their relation to heart diseases, hoping to apply the results in clinics. Cui et al72 found the protective effects of Momordica.charantia (MC) -DVLNs in cardiomyocytes against radiation-induced injury have been observed to involve the mitigation of DNA damage and mitochondrial dysfunction. The efficacy of citraVesTM, a recently patented plant-based product that contains Citrus limon (L.)-sourced VLN, has been demonstrated in healthy individuals concerning two critical determinants of cardiometabolic risks, namely waist circumference and LDL-C.73
Similarly, in terms of metabolism, orange juice (O)-DVLNs protected both the intestine and the liver from fat overload associated with a high-fat, high-sucrose diet.20 More interestingly, watermelon (W)-DVLNs can regulate placental function avoiding fetal growth restriction by actively being taken up by human intestinal cells (Caco-2).74 And Lemon-DVLNs were found can regulate homeostasis and restore subcellular function by suppressing the CaOx-induced endoplasmic reticulum stress response of tubule cells, thus indirectly inhibiting stone formation.75
PDVLNs have also attracted the attention of neurologists. Drynariae Rhizoma root (DRR) DVLNs were identified using proteomics and bioinformatics, and the result showed that DDVLNs could have applications in the treatment of neurological diseases and identified the possible molecular basis.76
Discussion
Some studies show that monitoring exosome biogenesis can predict the occurrence and intensity of immunological response in the asthmatic niche and the duality of animal-derived exosomes in disease treatment has led people to turn their attention to natural plant-derived exosomes.77,78 Recently, related research on natural products has been in full swing, especially that of PDVLNs. Research on PDVLNs in medicine focuses mainly on the following two aspects: 1) how to gain high-quality PDVLNs; and 2) explore the underlying mechanism and expand the application of PDVLNs.
Some effective extraction methods for PDVLNs and their current basic or clinical exploration and applications are summarized in this review. The enhancement and update of the isolation method for PDVLNs undoubtedly enhance the quantity and quality of PDVLNs. However, there are many updated methods to obtain relatively pure and effective PDVLNs; it remains difficult for researchers to choose a suitable method. Due to the various characteristics, the part of the plant chosen for gaining VLNs is different for each plant, which leads to the specificity of a suitable method, as well as some other factors. For example, plants, whether wet or dry, might all offer vesicles to varying degrees.76 To some extent, this discovery has dramatically solved the problem that its use is restricted by origin, traffic, seasonal, and regional factors. This advantage further increases the possibility of its clinical application. Thus, researchers must consider all factors and choose a suitable extraction method.
This review reveals that PDVLNs can make numerous efforts to treat human diseases. First, itself, the lipids, proteins, and nucleic acids present in them have the potential to improve our understanding of their physiological functions and contribute to the advancement of human disease research.79–81 Second, although they possess exceptional intrinsic properties, natural vectors are intentionally altered to confer multiple functionalities, including increased permeability, loading capacity, and specificity. Utilizing structural alteration and vector transformation, PDVLNs are widely used as superior vehicles for conveying contrast agents, chemotherapy drugs, nucleic acids, and genes to specific locations for the treatment of recalcitrant diseases.82,83 At the same time, based on the drug resistance of current therapies and the multi-target characteristics of diseases, plant-derived vesicles can be used as therapeutic agents and Multidrug co-delivery vectors for some diseases, which can make up for certain disadvantages in these two aspects.28 Combined with nanozymes, artificial intelligence, and other fields, it may be able to explore its scope of application.84,85 For example, developing a new artificial intelligence system that, combined with engineered PDVLNs, can be used in diagnosing and treating diseases will be a significant breakthrough for the application of PDVLNs. Lastly, PDVLNs are derived from agricultural products.86 Undoubtedly, PDVLNs are more economical due to more scalable sources.
How far PDVLNs from the clinical application? At least these problems should be resolved. According to steps the authors tend to divide it into three steps: production, modification, and application. First, in production, the authors think the critical point is unifying the industry standard, ensuring isolation, purification, physicochemical characterization, and quality control. As mentioned above, the research in this area still needs further exploration. Second, there have been more studies on the modification of exosomes derived from animal cells but few studies on plants. The prospect is believed to be infinite, but it also takes a long time to explore and verify. Finally, in the application, oral, injection, or inhalation administration is determined by further study. Furthermore, the dose, safety, and efficacy of the drug still need a large number of preclinical studies to provide evidence and support.
There is still a long way to go between the research and clinical application of plant vesicles. However, from relevant clinical trials of mesenchymal stem cell-derived exosomes, we have reason to believe that the clinical application of plant-derived exosomes is full of promise.85,87 The clinical benefits of biotherapeutics have gained prominence, leading to a growing market share and industry recognition. As such, we maintain a positive outlook on this trend. The above is believed to significantly influence the degree of consciousness and trends in healthcare practices for humans.
Totally, we introduce in detail the characteristics of plant exosome vesicles, their extraction methods, and their current research and applications in diseases such as inflammation, immune regulation, tumours, and other diseases. However, due to the relative lack of current research, more potentials and applications of PDVLNS have not yet been explored, we cannot fully summarize the role of PDVLNS and this article can only summarize and discuss the current research.
Abbreviations
PDVLNS, Plant-derived Vesicle-like Nanoparticles; EVs, Extracellular vesicles; EXPO, exocyst-positive organelle; MVBs, exocyst-positive organelle; WB, Western blot; ELISA, enzyme-linked immunosorbent assay; DFF, direct flow filtration; TFF, constant flow filtration; SEC, Size-exclusion chromatography; nano-DLD, nano-sized deterministic lateral displacement.
Acknowledgments
Thanks to Biorender for giving the drawing support. Thanks to Home for Researchers for the language polish.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (Grant No. LHDMZ22H050001); the Construction of Key Projects by Zhejiang Provincial Ministry (Project No.WKJ-ZJ-2302); The Zhejiang Province Chinese Medicine Modernization Program (Project No. 2020ZX001); The Key Project of Scientific Research Foundation of Chinese Medicine (2022ZZ002); The “Pioneer” and “Leading Goose” R&D Program of Zhejiang (2022C03118; 2023C03075); The Key project of Basic Scientific Research Operating Funds of Hangzhou Medical College (KYZD202002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grange C, Bussolati B. Extracellular vesicles in kidney disease. Nat Rev Nephrol. 2022;18(8):499–513. doi:10.1038/s41581-022-00586-9
2. Van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–228. doi:10.1038/nrm.2017.125
3. Pathan M, Fonseka P, Chitti SV, et al. Vesiclepedia 2019: a compendium of RNA proteins lipids and metabolites in extracellular vesicles. Nucleic Acids Res. 2019;47(D1):D516–D519. doi:10.1093/nar/gky1029
4. Kalluri R, LeBleu VS. The biology function and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
5. Yue B, Yang H, Wang J, et al. Exosome biogenesis secretion and function of exosomal miRNAs in skeletal muscle myogenesis. Cell Prolif. 2020;53(7):e12857. doi:10.1111/cpr.12857
6. Shaban SA, Rezaie J, Nejati V. Exosomes derived from senescent endothelial cells contain distinct pro-angiogenic miRNAs and proteins. Cardiovasc Toxicol. 2022;22(6):592–601. doi:10.1007/s12012-022-09740-y
7. Mahbubfam S, Rezaie J, Nejati V. Crosstalk between exosomes signaling pathway and autophagy flux in senescent human endothelial cells. Tissue Cell. 2022;76:101803. doi:10.1016/j.tice.2022.101803
8. Pinedo M, de la Canal L, de Marcos Lousa C. A call for Rigor and standardization in plant extracellular vesicle research. J Extracel Vesicl. 2021;10(6):e12048. doi:10.1002/jev2.12048
9. Karamanidou T, Tsouknidas A. Plant-Derived Extracellular Vesicles as Therapeutic Nanocarriers. Internat J Mol Sci. 2021;23(1):191. doi:10.3390/ijms23010191
10. Cui Y, Gao J, He Y, Jiang L. Plant extracellular vesicles. Protoplasma. 2020;257(1):3–12. doi:10.1007/s00709-019-01435-6
11. Feng J, Xiu Q, Huang Y, Troyer Z, Li B, Zheng L. Plant derived vesicle-like nanoparticles as promising biotherapeutic tools: present and future. Adv Mater. 2023;35:e2207826. doi:10.1002/adma.202207826
12. Bayat F, Afshar A, Baghban N. Algal cells-derived extracellular vesicles: a review with special emphasis on their antimicrobial effects. Front Microbiol. 2021;12:785716. doi:10.3389/fmicb.2021.785716
13. De Palma M, Ambrosone A, Leone A, et al. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9:12.
14. Castillo-González C, Zhang X. The trojan horse of the plant kingdom. Cell Host Microbe. 2018;24(1):1–3. doi:10.1016/j.chom.2018.06.015
15. He B, Cai Q, Qiao L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nature Plants. 2021;7(3):342–352. doi:10.1038/s41477-021-00863-8
16. Movahed N, Cabanillas DG, Wan J, Vali H, Laliberte JF, Zheng H. Turnip mosaic virus components are released into the extracellular space by vesicles in infected leaves. Plant Physiology. 2019;180(3):1375–1388. doi:10.1104/pp.19.00381
17. Cui Y, Cao W, He Y, et al. A whole-cell electron tomography model of vacuole biogenesis in Arabidopsis root cells. Nature Plants. 2019;5(1):95–105. doi:10.1038/s41477-018-0328-1
18. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi:10.3390/biom11010087
19. Thongboonkerd V. Roles for exosome in various kidney diseases and disorders. Front Pharmacol. 2019;10:1655. doi:10.3389/fphar.2019.01655
20. Berger E, Colosetti P, Jalabert A, et al. Use of nanovesicles from orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Molecul Therap. 2020;18:880–892. doi:10.1016/j.omtm.2020.08.009
21. Xu XH, Yuan TJ, Dad HA, et al. Plant exosomes as novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells in vitro and in vivo. Nano Letters. 2021;21(19):8151–8159. doi:10.1021/acs.nanolett.1c02530
22. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Molecul Therap. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
23. Zhou LK, Zhou Z, Jiang XM, et al. Absorbed plant MIR2911 in honeysuckle decoction inhibits SARS-CoV-2 replication and accelerates the negative conversion of infected patients. Cell Discov. 2020;6(1):54. doi:10.1038/s41421-020-00197-3
24. Fang Z, Liu K. Plant-derived extracellular vesicles as oral drug delivery carriers. J Control Rel. 2022;350:389–400. doi:10.1016/j.jconrel.2022.08.046
25. Jackson KK, Mata C, Marcus RK. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta. 2023;252:123779. doi:10.1016/j.talanta.2022.123779
26. Thery C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracel Vesicl. 2018;7(1):1535750. doi:10.1080/20013078.2018.1535750
27. Witwer KW, Goberdhan DC, O’Driscoll L, et al. Updating MISEV: evolving the minimal requirements for studies of extracellular vesicles. J Extracell Vesicl. 2021;10(14):e12182. doi:10.1002/jev2.12182
28. Cong M, Tan S, Li S, et al. Technology insight: plant-derived vesicles-How far from the clinical biotherapeutics and therapeutic drug carriers? Advan Drug Deliv Rev. 2022;182:114108. doi:10.1016/j.addr.2021.114108
29. Rutter BD, Innes RW. Growing pains: addressing the pitfalls of plant extracellular vesicle research. New Phytolog. 2020;228(5):1505–1510. doi:10.1111/nph.16725
30. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118(4):1917–1950. doi:10.1021/acs.chemrev.7b00534
31. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
32. Yang D, Zhang W, Zhang H, et al. Progress opportunity and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. doi:10.7150/thno.41580
33. Jia Y, Yu L, Ma T, et al. Small extracellular vesicles isolation and separation: current techniques pending questions and clinical applications. Theranostics. 2022;12(15):6548–6575. doi:10.7150/thno.74305
34. Priglinger E, Strasser J, Buchroithner B, et al. Label-free characterization of an extracellular vesicle-based therapeutic. J Extracell Vesicl. 2021;10(12):e12156. doi:10.1002/jev2.12156
35. Staubach S, Bauer FN, Tertel T, et al. Scaled preparation of extracellular vesicles from conditioned media. Advan Drug Deliv Rev. 2021;177:113940. doi:10.1016/j.addr.2021.113940
36. Rider MA, Hurwitz SN, Meckes DG. ExtraPEG: a polyethylene glycol-based method for enrichment of extracellular vesicles. Sci Rep. 2016;6:23978. doi:10.1038/srep23978
37. Kalarikkal SP, Prasad D, Kasiappan R, Chaudhari SR, Sundaram GM. A cost-effective polyethylene glycol-based method for the isolation of functional edible nanoparticles from ginger rhizomes. Sci Rep. 2020;10(1):4456. doi:10.1038/s41598-020-61358-8
38. Nordin JZ, Lee Y, Vader P, et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11(4):879–883. doi:10.1016/j.nano.2015.01.003
39. Mol EA, Goumans MJ, Doevendans PA, Sluijter JPG, Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. 2017;13(6):2061–2065. doi:10.1016/j.nano.2017.03.011
40. Zhang H, Freitas D, Kim HS, et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20(3):332–343. doi:10.1038/s41556-018-0040-4
41. Zhang H, Lyden D. Asymmetric-flow field-flow fractionation technology for exomere and small extracellular vesicle separation and characterization. Nat Protoc. 2019;14(4):1027–1053. doi:10.1038/s41596-019-0126-x
42. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation characterization biological function and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi:10.3390/cells8040307
43. Momen-Heravi F, Balaj L, Alian S, et al. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. doi:10.3389/fphys.2012.00162
44. Woith E, Melzig MF. Extracellular vesicles from fresh and dried plants-simultaneous purification and visualization using gel electrophoresis. Inter J Molecul Sci. 2019;20(2):357. doi:10.3390/ijms20020357
45. Yang M, Liu X, Luo Q, Xu L, Chen F. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18(1):100. doi:10.1186/s12951-020-00656-9
46. Arulselvan P, Fard MT, Tan WS, et al. Role of antioxidants and natural products in inflammation. Oxid Med Cell Longev. 2016;2016:5276130. doi:10.1155/2016/5276130
47. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioactive Materials. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
48. Sharma BR, Kanneganti TD. NLRP3 inflammasome in cancer and metabolic diseases. Nat Immunol. 2021;22(5):550–559.
49. Liu B, Li X, Yu H, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11(19):9311–9330. doi:10.7150/thno.60265
50. Raimondo S, Urzì O, Meraviglia S, et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J Cellul Molecul Med. 2022;26(15):4195–4209. doi:10.1111/jcmm.17404
51. Yi Q, Xu Z, Thakur A, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. doi:10.1016/j.phrs.2023.106733
52. Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20(1):7–24. doi:10.1038/s41577-019-0210-z
53. Han JM, Song HY, Lim ST, Kim KI, Seo HS, Byun EB. Immunostimulatory potential of extracellular vesicles isolated from an edible plant petasites japonicus via the induction of murine dendritic cell maturation. Inter J Molecul Sci. 2021;22(19):10634. doi:10.3390/ijms221910634
54. Wu DD, Li T, Ji XY. Dendritic cells in sepsis: pathological alterations and therapeutic implications. J Immunol Res. 2017;2017:3591248. doi:10.1155/2017/3591248
55. Kim WS, Ha JH, Jeong SH, et al. Immunological effects of aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells. 2022;11(18):2805. doi:10.3390/cells11182805
56. Ishida T, Nishiumi S, Tanahashi T, et al. Linoleoyl ethanolamide reduces lipopolysaccharide-induced inflammation in macrophages and ameliorates 2,4-dinitrofluorobenzene-induced contact dermatitis in mice. Europ J Pharmacol. 2013;699(1–3):6–13. doi:10.1016/j.ejphar.2012.11.030
57. Taşlı PN. Usage of celery root exosome as an immune suppressant; Lipidomic characterization of Apium graveolens originated exosomes and its suppressive effect on PMA/ionomycin mediated CD4(+) T lymphocyte activation. J Food Biochem. 2022;46:e14393. doi:10.1111/jfbc.14393
58. Kim K, Yoo HJ, Jung JH, et al. Cytotoxic effects of plant sap-derived extracellular vesicles on various tumor cell types. J Funct Biomat. 2020;11(2):22. doi:10.3390/jfb11020022
59. Tajik T, Baghaei K, Moghadam VE, Farrokhi N, Salami SA. Extracellular vesicles of cannabis with high CBD content induce anticancer signaling in human hepatocellular carcinoma. Biomed Pharmacot. 2022;152:113209. doi:10.1016/j.biopha.2022.113209
60. Yang M, Luo Q, Chen X, Chen F. Bitter melon derived extracellular vesicles enhance the therapeutic effects and reduce the drug resistance of 5-fluorouracil on oral squamous cell carcinoma. J Nanobiotechnol. 2021;19(1):259. doi:10.1186/s12951-021-00995-1
61. Özkan İ, Koçak P, Yıldırım M, et al. Garlic (Allium sativum)-derived SEVs inhibit cancer cell proliferation and induce caspase mediated apoptosis. Sci Rep. 2021;11(1):14773. doi:10.1038/s41598-021-93876-4
62. Boccia E, Alfieri M, Belvedere R, et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Communicat Bio. 2022;5(1):848. doi:10.1038/s42003-022-03781-3
63. Kim K, Jung JH, Yoo HJ, et al. Anti-metastatic effects of plant sap-derived extracellular vesicles in a 3D microfluidic cancer metastasis model. J Funct Biomat. 2020;11(3):49. doi:10.3390/jfb11030049
64. Nemidkanam V, Chaichanawongsaroj N. Characterizing Kaempferia parviflora extracellular vesicles a nanomedicine candidate. PLoS One. 2022;17(1):e0262884. doi:10.1371/journal.pone.0262884
65. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. J Immunot Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
66. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21(1):78. doi:10.1186/s12951-023-01835-0
67. Casado-Diaz A, Quesada-Gomez JM, Dorado G. Extracellular vesicles derived from mesenchymal stem cells (MSC) in regenerative medicine: applications in skin wound healing. Front Bioeng Biotechnol. 2020;8:146. doi:10.3389/fbioe.2020.00146
68. Kim M, Park JH. Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;14(9):1905. doi:10.3390/pharmaceutics14091905
69. Savcı Y, Kırbaş OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Function. 2021;12(11):5144–5156. doi:10.1039/D0FO02953J
70. Lee R, Ko HJ, Kim K, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracel Vesicl. 2020;9(1):1703480. doi:10.1080/20013078.2019.1703480
71. Abraham AM, Wiemann S, Ambreen G, et al. Cucumber-derived exosome-like vesicles and plantcrystals for improved dermal drug delivery. Pharmaceutics. 2022;14(3):476. doi:10.3390/pharmaceutics14030476
72. Cui WW, Ye C, Wang KX, et al. Momordica. charantia-derived extracellular vesicles-like nanovesicles protect cardiomyocytes against radiation injury via attenuating DNA damage and mitochondria dysfunction. Front Cardiovascul Med. 2022;9:864188. doi:10.3389/fcvm.2022.864188
73. Raimondo S, Nikolic D, Conigliaro A, et al. Preliminary results of citraVes™ effects on low density lipoprotein cholesterol and waist circumference in healthy subjects after 12 weeks: a pilot open-label study. Metabolites. 2021;11(5):276. doi:10.3390/metabo11050276
74. Timms K, Holder B, Day A, McLaughlin J, Forbes KA, Westwood M. Watermelon-derived extracellular vesicles influence human ex vivo placental cell behavior by altering intestinal secretions. Molr Nut Food Res. 2022;66(19):e2200013. doi:10.1002/mnfr.202200013
75. Zhang L, Li S, Cong M, et al. Lemon-derived extracellular vesicle-like nanoparticles block the progression of kidney stones by antagonizing endoplasmic reticulum stress in renal tubular cells. Nano Letters. 2023;23(4):1555–1563. doi:10.1021/acs.nanolett.2c05099
76. Cao Y, Zhao Q, Liu F, et al. Drug value of drynariae rhizoma root-derived extracellular vesicles for neurodegenerative diseases based on proteomics and bioinformatics. Plant Signal Behav. 2022;17(1):2129290. doi:10.1080/15592324.2022.2129290
77. Almohammai A, Rahbarghazi R, Keyhanmanesh R, Rezaie J, Ahmadi M. Asthmatic condition induced the activity of exosome secretory pathway in rat pulmonary tissues. J Inflamm. 2021;18(1):14. doi:10.1186/s12950-021-00275-7
78. Hassanpour M, Rezaie J, Darabi M, Hiradfar A, Rahbarghazi R, Nouri M. Autophagy modulation altered differentiation capacity of CD146(+) cells toward endothelial cells pericytes and cardiomyocytes. Stem Cell Res Therapy. 2020;11(1):139. doi:10.1186/s13287-020-01656-0
79. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi:10.1016/j.ajps.2021.05.006
80. Teng Y, Ren Y, Sayed M, et al. Plant-derived exosomal MicroRNAs shape the gut microbiota. Cell Host Microbe. 2018;24(5):637–652 e8. doi:10.1016/j.chom.2018.10.001
81. Hedayat M, Ahmadi M, Shoaran M, Rezaie J. Therapeutic application of mesenchymal stem cells derived exosomes in neurodegenerative diseases: a focus on non-coding RNAs cargo drug delivery potential perspective. Life Sci. 2023;320:121566. doi:10.1016/j.lfs.2023.121566
82. Ding H, Cai Y, Gao L, et al. Exosome-like nanozyme vesicles for H(2)O(2)-responsive catalytic photoacoustic imaging of xenograft nasopharyngeal carcinoma. Nano Letters. 2019;19(1):203–209. doi:10.1021/acs.nanolett.8b03709
83. de Jong OG, Kooijmans SAA, Murphy DE, et al. Drug delivery with extracellular vesicles: from imagination to innovation. Acc Chem Res. 2019;52(7):1761–1770. doi:10.1021/acs.accounts.9b00109
84. Zhang X, Zhu X, Li Y, Hai X, Bi S. A colorimetric and photothermal dual-mode biosensing platform based on nanozyme-functionalized flower-like DNA structures for tumor-derived exosome detection. Talanta. 2023;258:124456. doi:10.1016/j.talanta.2023.124456
85. Rezaie J, Nejati V, Mahmoodi M, Ahmadi M. Mesenchymal stem cells derived extracellular vesicles: a promising nanomedicine for drug delivery system. Biochem Pharmacol. 2022;203:115167. doi:10.1016/j.bcp.2022.115167
86. Wang Y, Wei Y, Liao H, et al. Plant exosome-like nanoparticles as biological shuttles for transdermal drug delivery. Bioengineering. 2023;10:1.
87. Ahmadi M, Mahmoodi M, Shoaran M, Nazari-Khanamiri F, Rezaie J. Harnessing normal and engineered mesenchymal stem cells derived exosomes for cancer therapy: opportunity and challenges. Inter J Mol Sci. 2022;23(22):13974. doi:10.3390/ijms232213974
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.