Back to Journals » Infection and Drug Resistance » Volume 15
Phytochemical Investigation and Determination of Antibacterial Activity of Solvent Leave Extracts of Carissa spinarum
Authors Ayalew Tiruneh T, Ayalew Tiruneh G , Chekol Abebe E , Mengie Ayele T
Received 6 December 2021
Accepted for publication 15 February 2022
Published 4 March 2022 Volume 2022:15 Pages 807—819
DOI https://doi.org/10.2147/IDR.S352049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tizezew Ayalew Tiruneh,1 Gebrehiwot Ayalew Tiruneh,2 Endeshaw Chekol Abebe,3 Teklie Mengie Ayele4
1Department of Chemistry, College of Natural and Computational Science, University of Gondar, Gondar, Ethiopia; 2Department of Midwifery, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia; 3Department of Medical Biochemistry, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 4Department of Pharmacy, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Teklie Mengie Ayele, PO Box 272, Debre Tabor 6300, Ethiopia, Tel +251910111531, Email [email protected]
Background: Among many traditionally used medicinal plants, Carissa spinarum (Agam) is a well-known indigenous plant in Ethiopia. It is used in its raw form to treat different diseases in different parts of the country. Therefore, the aim of this study is to investigate extraction, isolation, and determination of the antibacterial properties of the solvent leaf extract of Carissa spinarum.
Methods: In this study, 800 g of powdered leaves of Carissa spinarum were macerated with 2500 mL of methanol and yielded 58 g (7.25%, w/w) of gummy material. The extract was then further partitioned by using ethyl acetate and chloroform. The extracts were subjected to phytochemical screening test. The antibacterial activity of the three solvent leaf extracts of Carissa spinarum were evaluated using disc diffusion method. The methanol extract was subjected to column chromatography silica gel (60– 200 mesh) by mixing methanol:petroleum ether (4:1). Then fractions were collected and investigated by TLC and finally identified using spectroscopy.
Results: The three extracts (methanol, ethyl acetate, and chloroform) of Carissa spinarum were presented to antimicrobial activity by disc diffusion method against four bacterial species using gentamycin and ampicillin discs as positive controls and DMSO as a negative control. All extracts had a relatively antibacterial effect with different extent zones of inhibition. However, the methanol extract showed superior antibacterial activity compared with DMSO and ethyl acetate and chloroform extracts. These could due to variation of the phytoconstituents. The most probable structure of the compound isolated was 5-(2’,3’,4’,6’-tetrahydroxy-5’-methoxycyclohexyloxy)-2,3,4 trihydroxypentanoic acid.
Conclusion: Data obtained from this study collectively indicated that the three solvent extracts of Carissa spinarum have a promising antimicrobial activity which supports the traditional claim of the plant for treatment of infection.
Keywords: antibacterial activity, Carissa spinarum, phytochemicals determination
Background
The role of plants and plant parts as sources of medicine is immense. Most of the drugs nowadays originate from plant sources. The use of plants for therapeutic purpose is probably the oldest phenomenon.1 Extraction and structural elucidation of numerous active secondary metabolites from these plants have been developed to high activity profile drugs. Plants would be the main sources for a variety of traditional and modern medicines. According to a report from the World Health Organization (WHO), around 80% of population in developing countries relies on traditional medicines. The largest proportion of these essential medicines are primarily related to herbal extracts or their active principles like, leaves, flowers, roots, fruits, and seeds.2 One plant species that is known for its medical use is Carissa spinarum (Agam).3
Traditional medicines are the leading foundation for biologically active remedies. There has been an increasing interest in medicinal plants as natural products in different parts of the world.4 The therapeutic significance of medicinal plants relies on the presence of pharmacologically active secondary metabolites and their effect in the human body. Phytochemicals are bioactive chemicals of plant origin. Many species have been recognized to have medicinal value and valuable effect for the well-being of humans, such as, infected wounds, which are a common public health concern.5
Carissa spinarum has a wide variety of applications in traditional medicine. Traditionally the leaf part of Carissa spinarum is used to treat external parasites and inflammation. Moreover, the various parts of Carissa spinarum are used to treat numerous ailments in Ethiopia. For instance, it is useful in treatment of rheumatism, purgative and snake repellent, wounds, and has emerged as a good source of the traditional medicine for the treatment of, arthritis, microbial infection, epilepsy, viral infection, cancer diseases, among others.6–9
In Ethiopia, there are numerous medicinal plants which are used simply in the raw form to treat different diseases. Among many traditionally used medicinal plants, Carissa spinarum (Agam) is one of the most well-known indigenous plant in Ethiopia in which the raw form is used to treat different diseases in different parts of the country.
Therefore, this study was mainly initiated by performing the process of crude extraction, phytochemical screening, antibacterial test and structural elucidation of the leaves of Carissa spinarum, which enables us to know the types of compounds found within this plant for use in the management of different ailments.
Materials and Methods
Apparatus and Chemicals
In this study we used the following apparatus: funnel, oven, pipettes (different sizes), TLC plate, water bath, UV lamp, beakers, electronic balance, conical flask, electronic grinder, and test tubes, capillary tube, Whatman No. 1 filter paper, glass rod, cotton, spatula, gas jar, tongs, pencil, ruler, scissors, aluminium foil, test tube ranker, glove, stand, column chromatography, round bottom flasks (different sizes), measuring cylinder, rotary evaporator (RE-200), petri dish, refrigerator, test tube holder, dropper, lamp, incubator, IR spectrometer, 1H-NMR spectrometer (400 MHz, Bruker), 13C-NMR spectrometer (100 MHz, Bruker) and DEPT-135 (100 MHz, Bruker). Further, chemicals used for this study include: solvents (petroleum ether, ethyl acetate, chloroform and methanol), sulphuric acid, hydrochloric acid, ferric chloride, magnesium, sodium hydroxide, sodium bicarbonate, copper sulphate, mercury chloride, potassium iodide, distilled water, glacial acetic acid, ammonia solution, acetic anhydride, phenolphthalein indicator, litmus paper, iodine crystal, silica gel (60–200 mesh), DMSO, Mueller–Hinton agar, denatured ethanol and standard tablets (ampicillin and gentamycin). All the instruments and apparatus were of analytical grade.
Collection and Authentication of Carissa spinarum
The healthy aerial parts of the leaves of Carissa spinarum were collected from Central North Gondar on January, 2021 (Figure 1). The collected plant material was authenticated by Dr Getnet Masresha a botanist from the Department of Biology at the University of Gondar and a voucher specimen TA/001/2021 was deposited in the University of Gondar herbarium. The plant material was splashed with distilled water to remove any dust material on it. The collected plant material was dried under shade immediately after collection. The shaded dried healthy leaves were powdered using a mechanical grinder in uniform powder size as shown in Figure 2. It was weighed and then the powdered sample was kept in a sealed container for extraction purposes to conduct this research.
 |
Figure 1 The leaves of Carissa spinarum. |
 |
Figure 2 Powdered leaves of Carissa spinarum. |
Extraction of Carissa spinarum
The air dried powdered leaves of Carissa spinarum (800 g) were macerated with methanol for 72 h, slightly shaking twice a day at room temperature. The mixture was filtrated with Whatman No. 1 filter paper. The methanol extract was concentrated by rota evaporator (RE-200) until it became completely dry and it yielded 58 g of black gummy material (7.25% by w/w).
The methanol extract was further partitioned by using lesser polarity (ethyl acetate and chloroform).
Phytochemical Screening
Preliminary phytochemical screening of secondary metabolites was carried out on the methanol, ethyl acetate, and chloroform extracts, according to the standard procedures described by many researchers.10
Test for Flavonoids
Two milliliters of each solvent leaf extract of Carissa spinarum solution was mixed with 1.5 mL of 50% methanol solution. Then the solution was heated and metal magnesium be added. Few drops of concentrated hydrochloric acid was added and the red color was observed for flavonoids.11
Test for Alkaloids
About 0.5 g of each solvent leaf extract of Carissa spinarum was dissolved in 2N HCl and it was filtered. The filtrates were tested with different reagents. Mayer's test: the filtrate was treated with Mayer's reagent, and then the appearance of creamish precipitate indicate the presence of alkaloids.12
Tannins Test
About 0.5 g of each solvent leaf extract of Carissa spinarum was mixed in 10 mL of distilled water in a test tube and then filtered. A few drops of 0.1% ferric chloride were added and observed for brownish green or blue black coloration.13
Test for Saponins
About 0.5 g of each solvent leaf extract of Carissa spinarum was diluted with 20 mL of distilled water in a test tube and boiled for 15 min. The formation of foam indicated the presence of saponins.14
Test for Steroids
Two milliliters of acetic anhydride was added to 2 mL each of solvent leaf extract of Carissa spinarum along with 2 mL concentration of sulphuric acid. The formation of blue or green that was detected for the presence of steroids.15
Test for Cardiac Glycosides
We dissolved 0.2 g of each solvent leaf extract of Carissa spinarum in 1 mL of glacial acetic acid containing and 1 drop of ferric chloride solution was added. This was then under layered with 1 mL of concentrated sulphuric acid. The formation of a brown ring obtained at the interface indicated the presence of cardiac glycosides.16
Test for Proteins
To 2 mL of the gram of each solvent leaf extract of Carissa spinarum solution 1 mL of 40% NaOH solution and 1 to 2 drops of 1% CuSO4 solution was added. Formation of violet color indicated the presence of peptide linkage of the molecule.17
Test for Anthraquinones
About 0.5 g of each solvent leaf extract of Carissa spinarum was taken in a dry test tube and 5 mL of chloroform was added and shaken for 5 min. The mixture was filtered and added 10% of ammonia solution. A pink violet or reddish color observed for the presence of anthraquinones.18
Partitioning of the Methanol Extract by Solvents
The methanol extract showed many spots when checked by TLC; as a result it was further macerated by ethyl acetate (400 mL) with occasional shaking for 48 h and then chloroform (200 mL) for 48 h for decreasing the number of components. The methanol extract (residue), chloroform extract and ethyl acetate extract were concentrated with a rotary evaporator and afforded 40 g, 7 g, and 4 g, respectively. The extracts were kept in an airtight container and stored in a deep freezer until the commencement of the experiment.
Antibacterial Activity Evaluation
Inoculums Preparation and Standardization
Antimicrobial susceptibility test implies the capability of an antimicrobial agent to inhibit bacteria and fungal growth. In this study the antimicrobial activities (specifically antibacterial) of ethyl acetate, chloroform, and methanol leaf extract of Carissa spinarum were done by disc diffusion method using gentamycin and ampicillin disc as standard antibiotic, two gram-positive bacteria (Staphylococcus aureus and Staphylococcus pneumonae) and two gram-negative bacteria (Escherichia coli and Klebsiella pneumonae). Bacteria cultures used in this study were obtained from department of Microbiology Laboratory, University of Gondar. The media was prepared by dissolving 38 g of Muller–Hinton agar (MHA) powder in 1000 mL of distilled water, heated, well shaken and allowed to boil. It was then put in autoclave at 1210°C for about 15 min to sterilize the media. Then the media was allowed to cool and poured in 12- plates and was put on a level surface. Finally the media was allowed to solidify, kept in an upright position in the incubator avoiding contamination from the hood.
Procedure for Performing the Disc Diffusion Test
After the preparation of the Muller–Hinton agar plates as per manufacturer instructions, the cultured bacteria were cleaned on the top of the pre-leveled media (100 μL) and allowed to dry for 10 min. A sterilized cork borer was used to bore holes on the plates and 100 mg/mL of each extracts (ethyl acetate, chloroform, and methanol) was introduced in the holes by using a gentamycin disc (10 μg/disc) and an ampicillin disc (10 μg/disc) as standard. The petri dishes were then nurtured at 370°C for 24 h. At the end of incubation period, the antibacterial activity of each sample was done in triplicates and manipulated by quantifying the diameters of the average inhibition zone by millimeter including the wells and discs as shown in Figures 3–5.
 |
Figure 3 Zone of inhibition shown by methanol fraction of leaves of Carissa spinarum. |
 |
Figure 4 Zone of inhibition shown by ethyl acetate fraction of leaves of Carissa spinarum. |
 |
Figure 5 Zone of inhibition shown by chloroform fraction of leaves of Carissa spinarum. |
Purification and Isolation of the Major Component
Out of the three extracts, the methanol extract showed better inhibition zone and it was subjected to column chromatography silica gel (60–200 mesh) by mixing the suitable solvents methanol:petroleum ether (4:1). A total of 24 fractions were collected and investigated by TLC. Fractions 6 to 20 showed similar Rf value and were mixed together. Then the solvent was removed completely. From these fractions brown gummy material was obtained. It was checked by TLC using different solvents and showed only a single spot. Finally it was sent to the Department of Chemistry, Addis Ababa University for spectroscopic analysis leveled as TA.
Results
Preliminary Examination
One drop of methanol solution of compound TA was put on blue litmus paper and changed the color to slightly red. Further one drop of phenolphthalein indicator was added to the compound and it became colorless with PH value of 4.8. Finally compound TA was found to be soluble in dilute NaOH as well as dilute NaHCO3 with formation of bubbles in the latter case. Therefore, the above tests indicate that compound TA contains the carboxylic group.
Phytochemical Screening Test Result
Phytochemical analysis of the solvent leaf extracts of Carissa spinarum revealed the presence of various secondary metabolites which are summarized in Table 1.
 |
Table 1 Phytochemical Screening Test of Different Solvent Leave Extracts of Carissa spinarum |
Antibacterial Activity of the Extracts of Ethyl Acetate, Chloroform, and Methanol Against Bacterial Strains
Evaluation of antibacterial activity of the crude extracts of ethyl acetate, chloroform and methanol of the leaves of Carissa spinarum were done by disc diffusion method using a gentamycin disc (10 μg/disc) and an ampicillin disc (10 μg/disc) as standard antibiotic; two gram-positive bacteria (S.aureus and S. pneumonae) and two gram-negative bacteria (E. coli and Klebsiella pneumonae) species in triplicate. The antibacterial activity of each sample was determined by measuring the average diameter of inhibition zone in mm including the wells and discs as shown in Table 2; Figures 3–5.
 |
Table 2 Antibacterial Activities of the Methanol, Chloroform, and Ethyl Acetate Leaf Extracts of Carissa spinarum Using Disc Diffusion Techniques |
Characterization of the Major Component of Methanol Leaves Extract of Carissa spinarum
The characterization of the major component of compound TA was done using spectroscopic methods such as, IR, 1H-NMR, 13C-NMR and DEPT-135 spectra.
IR Spectrum of Compound TA
The IR spectrum (Appendix 1 and 2) showed broad and strong bands at 3400 cm–1 and 3230 cm–1 indicating the presence of hydroxyl group (-OH). In addition to this the bands show the presence of hydrogen bonding (dimer) that partially obscure the C-H stretching bands which is a characteristic feature of carboxylic acids. The bands at 2884 cm–1 and 2830 cm–1 are assigned to C-H stretching; the bands at 1700 cm–1 to carbonyl group (C=O), the bands at 1235 cm–1 and 1055 cm–1 to C-O stretching and finally the bands at 1455 cm–1 and 1375 cm–1 for methylene and methyl group bending absorption respectively. The IR absorption of compound TA in comparison with estimated values is summarized in Table 3.
 |
Table 3 IR Spectrum of Compound TA |
1H-NMR Spectrum of Compound TA
In 1H-NMR spectrum of compound TA (appendix-2), the signals are not well resolved due to similar chemical shifts between two groups of protons, ie second-order spectrum. The –COOH proton absorption is not also observed, which could be off the chart to the left. Strong hydrogen bonding may also cause the peak to become broadened with very low intensity. In some cases the absorption may also disappear. For the characterization compound TA major signals are considered and others are omitted as impurities. Accordingly the signals at: 4.17 (C2H), 2.0 (C2OH), 3.7 (C3H), 2.0 (C3OH), 3.6 (C4H), 2.0 (C4OH), 3.62 and 3.4 (C5H2), 2.9 (C1ʹH), 3.6 (C2ʹH), 2.0 (C2ʹOH), 3.2 (C3ʹH), 2.0 (C3ʹOH), 3.6 (C4ʹH), 2.0 (C4ʹOH), 2.9 (C5ʹH), 3.80 (C6ʹH), 2.0 (C6ʹOH), and 3.2 (C1”H3) are assigned as described in Table 4.
 |
Table 4 1H-NMR Spectrum of Compound TA in Comparison with Estimated Value |
13C-NMR and DEPT-135 Spectra of Compound TA
The carbon-13 spectra of compound TA (Appendix 3 and 4) were not run properly since there is a difference in the number of carbon atoms of 13C-NMR (9 carbon atoms) and DEPT-135 (12 carbon atoms). In principle the numbers of carbon atoms in the DEPT-135 should have been less than that of 13C-NMR if quaternary carbon atoms are present. So, for the characterization of compound TA the carbon atoms from DEPT-135 spectrum are considered. In addition from many instances the peak intensity of carbonyl carbon is very weak and sometimes it may not appear unless enhanced. So from the DEPT-135 spectrum 12 carbon atoms are observed and out of these 9 are methine (CH), 1 methylene (CH2), 1 methyl (CH3) and 1 quaternary. Accordingly the peaks are assigned as follows, at: 174 (C-1), 74.11 (C-2), 72.65 (C-3), 70.39 (C-4), 71.08 (C-5), 81.51 (C-1’), 70.87 (C-2’), 72.48 (C-3’), 69.58 (C-4’), 82.74 (C-5’), 68.41 (C-6’) and 57.87 (C-1”). The 13C-NMR and DEPT-135 chemical shift of compound TA along with estimated values are summarized in Table 5. The spectroscopic data suggest that the most probable structure of compound TA can be: -5-(2’, 3’, 4’, 6’-tetrahydroxy-5’-methoxycyclohexyloxy)-2, 3, 4-trihydroxypentanoic acid as shown in Figure 6.
 |
Table 5 13C-NMR and DEPT-135 Spectra of Compound TA in Comparison with Estimated Value |
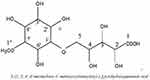 |
Figure 6 Proposed structure of compound TA. |
Discussion
Worldwide progression of resistance to the existing antimicrobials and adverse effects of those drugs used in the management of communicable ailments inspire the investigation of medicinal plants and plant derived phytochemicals. Several studies have been investigated about the antimicrobial activities of numerous medicinal plants. For instance, essential oils extracted from Ruta graveolens, Origanum vulgare and Myrtus communis showed antifungal activity against resistant strains of pityriasis versicolor.19–21 Essential oils isolated from lavender showed antibacterial activity against multidrug resistant strains of Pseudomonas aeruginosa.22 Moreover, extracts from medicinal plants showed activity against the tested organism. For instance, oils extracted from Zingiber officinale (ginger), Psidium guajava, Cyamopsis tetragonoloba (Gaur) L and Leucas cephalotes exhibited antiviral activity against the tested viral strains.23–27 Furthermore, phytochemicals extracted from Allium cepa exhibited antiparasitic activity against strains of Entamoeba gingivalis.28
Therefore, this study was executed to evaluate the phytochemicals and determine the antibacterial activity of leaf extracts of Carissa spinarum.
The methanol extract of Carissa spinarum showed higher zone inhibition against the tested bacterial species compared with the negative control (DMSO), ethyl acetate, and chloroform extract. This variation in growth inhibition between the methanol extract and the vehicle might be due to the existence of antibacterial secondary metabolites in the extract, which are in line with the previous reports on antimicrobial activity of medicinal plants.29 Moreover, the qualitative phytochemical screening test result of solvent extracts of Carissa spinarum revealed the presence of steroids, tannins, saponin, flavonoids, and terpenoids. These phytochemicals could be the reason for antimicrobial activity of the Carissa spinarum. The previous study by Singh and Verma revealed that phytochemical such as alkaloids had antibacterial activity against the tested bacterial species.30 The cardiac glycosides isolated from the methanol dried stems extract of Carissa spinarum revealed antimicrobial activity.31 Moreover, the methanol root extract of Carissa spinarum showed wound healing and antimicrobial activity as reported by Sanwal and Chaudhary.9 Furthermore flavonoids, tannins, saponins, and steroids were identified in methanol leaf extract of Carissa spinarum.32 Therefore, the antibacterial activity of the methanol leaf extract of Carissa spinarum could be due to a synergistic effect of these phytochemicals.33
The ethyl acetate and chloroform extracts of the 80% methanol extract of Carissa spinarum also exhibited antibacterial activity in all tested bacterial species with a different extent of zone of inhibition. The vehicle used for dissolving these extracts did not show any zone of inhibition in all tested bacterial species and was taken as a negative control. This shows the existence of antibacterial phytochemical in the extracts. An investigation reported by Clarence et al revealed that different extracts of Carissa spinarum had potential antibacterial activity against infections caused by pathogens such as S. aureus and E. coli,34 which is in line with our finding.
The zone of inhibition in all solvent extracts of Carissa spinarum was significantly smaller than the standard (gentamycin) against S. aureus and E. coli even at maximal concentration of the extracts. However, the inhibitions of against K. pneumoniae and S. pneumonae were comparable to gentamycin at a concentration of 100 mg/mL. Variation of the antibacterial activity among extracts and the standard might be the difference in the potential phytoconstituents essential for antibacterial activity.35
Ethyl acetate extract of Carissa spinarum showed larger zone of inhibition against E. coli, K. pneumonae, S. pneumonae. Besides, the presence of alkaloids, saponins, tannins, flavonoids, phenols, and terpenoids from the phytochemical test result support the broad antibacterial activity of ethyl acetate extract. Furthermore, the phytoconstituents found in ethyl acetate extract might relatively polar compared with the chloroform leaf extract of Carissa spinarum. Pooled evidence from different reports revealed that numerous phytoconstituents were isolated from solvent extracts of various parts of Carissa spinarum and have been studied for their antimicrobial activity.36
The chloroform extract displayed a somewhat lesser area zone of inhibition compared with the methanol and ethyl acetate leaf extracts of Carissa spinarum. This might be because of the lack of some potential phytoconstituents as indicated in our phytochemical screening test (Table 1).
From the bacterial strain tested, S. aureus exhibited smaller vulnerability to chloroform leaf extract of Carissa spinarum. This might be the inherent drug-resistant properties of the bacterial strain, which hinders the antibacterial activity of the phytoconstituents.37
In the present study, gram-positive bacteria (S. pneumonae and S. aureus) were more vulnerable to the all solvent leaf extracts of Carissa spinarum. The average zone of inhibitions of the all solvent leaf extracts were larger for gram-positives compared with gram-negatives at the same concentration. This variation might be due to the presence of special bacterial cell membrane structures in gram-negative strains such as lipolipopolysaccharide, which prevent the accumulation of phytoconstituents inside the bacteria cell.38
Largely, the presence of alkaloids in Carissa spinarum extracts of might possess antibacterial activity. The promising antibacterial action of alkaloids from medicinal plants might due to alteration of bacterial cell structures or inhibition of macromolecules such as bacterial enzymes and other target proteins.39
The current finding, as well as previous reports, revealed the presence of tannins in the extracts of Carissa spinarum.36 The antibacterial effect of this phytoconstituent could be due to their detrimental effect on bacterial cell membrane and by promoting uncoupling oxidative phosphorylation that might lead to death of the bacteria.39 The other phytoconstituents identified from the methanol and ethyl acetate leaf extract of Carissa spinarum are terpenoids. The possible antimicrobial activity of terpenoids could be related to its disruptive action on bacterial cell structures through causing them to malfunction.40,41
Flavonoids are also the other phytoconstituent from solvent extract of Carissa spinarum. The potential antibacterial action of flavonoids encompasses, formation of complex with bacterial cell walls and precipitation of bacterial cell machinery materials such as proteins and genes.42,43
Moreover, all leaf extracts of Carissa spinarum were rich in saponins. Previous reports revealed that saponins had multiple antibacterial effects. This effect might be probably due to membrane disruption and leakage of cell components.39,44
Furthermore, the most active leaf extract of Carissa spinarum (methanol extract) of was purified and isolated for characterization of the major component present in the extract. The isolated compound was trihydroxypentanoic acid. This isolated compound might contribute to the superior antibacterial activity of methanol leaf extract of the Carissa spinarum. This finding is in line with a study done by Ruchira et al in which many compounds were isolated and assessed for their numerous pharmacological activity such as antiherpetic, cytotoxic, antioxidant, and antibacterial effects.31 In addition, another report by Mudiganti and Anisha investigated the presence of several compounds that are isolated from the various solvent extract of Carissa spinarum plant through GC-MS analysis and their potential medicinal values are under investigation.32
In general, diverse speculations are set for antimicrobial mode of action of a herbal extract, depending on the phytoconstituent they comprise. The presence of the bioactive compound from a herbal extract leads to death of bacterial via several modes which include disruption of functional and structural proteins that are essential for the survival of the bacteria. Consequently, the overall antimicrobial effect of Carissa spinarum could be due to the existence of a higher quantity of phytoconstituent or due to synergistic action from different phytoconstituents.
In conclusion, findings from the current study collectively revealed that the methanol, ethyl acetate, and chloroform extracts of the leaves of Carissa spinarum have antibacterial activity against the tested bacterial species. All extracts of Carissa spinarum exhibited antibacterial action with a different spectrum of activity. The methanol fraction revealed the maximum antibacterial activity. The antibacterial activity of Carissa spinarum because of the existence of phytoconstituents (alkaloids, terpenoids, polyphenols, saponins, and flavonoids), can work either independently or synergistically. Furthermore, a compound was isolated and characterized with the help of IR, 1H-NMR, 13C-NMR and DEPT-135 spectroscopic techniques. The IR, 1H-NMR, 13C-NMR and DEPT-135 spectroscopic data in comparison with estimated value means that the most probable structure of isolated compound was 5-(2’, 3’, 4’, 6’ tetrahydroxy-5’-methoxycyclohexyloxy)-2,3,4-trihydroxypentanoic acid.
Abbreviations
13C-NMR, carbon-13 nuclear magnetic resonance; 1H-NMR, proton nuclear magnetic resonance; DEPT, distortionless enhancement by polarization transfer; DRC, Democratic Republic of Congo; IR, infrared spectroscopy; MHA, Mueller–Hinton agar; NMR, nuclear magnetic resonance; TA, trihydroxypentanoic acid; TLC, thin layer chromatography; TM, traditional medicine; TMPs, traditional medicinal practitioners; UoG, University of Gondar; WHO, World Health Organization; DMSO, dimethyl sulfoxide; PPM, parts per million; Rf, retention factor; MS, mass spectrometry; 2D-NMR, two-dimensional nuclear magnetic resonance.
Ethics Approval
Ethical clearance and permission was obtained from University of Gondar Research and Ethical Review Committee.
Acknowledgments
We are very grateful for University of Gondar for funding the study.
Author Contributions
All authors made a significant involvement to the work stated, that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
University of Gondar provided all the required resources for conducting this research project.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Huie CW. A review of modern sample preparation technique for the extraction and analysis of medicinal plants. Anal Bioanal Chem. 2002;373(1–2):23–30. doi:10.1007/s00216-002-1265-3
2. Abramov V. Traditional medicine. World Health Org. 1996;134:1–3.
3. Getaneh G. An ethnobotanical study of traditional use of medicinal plants and their conservation status in Mecha Wereda, west Gojjam zone of Amhara region, Ethiopia; 2011.
4. Saiprazanna B, Manobar B, Roja R, Prazanta K, Rajesheree P. Phytochemical investigation and study on antioxidant properties of Ocimum canum hydro alcohol leaf extracts. J drug deliv ther. 2012;2:122–128.
5. Odimegwu DC, Ibezim EC, Esimone CO, Nworu CS, Okoye FBC. Wound healing and antibacterial activities of the extract of Dissotis theifoila (Melastomataceae) stem formulated in a simple ointment base. J Med Plant Res. 2008;2:11–16.
6. Hegde K, Issac C, Joshi AB. Anarthric activity of Carissa spinarum root extract in Freund’s adjuvant induced polyarthritis in rats. Pharmacologyonline. 2010;2:713–718.
7. Hegde K, Satyanarayana D, Joshi AB. Evaluation of anticonvulsant activity of Carissa spinarum root extract. RGUHS J Pharm Sci. 2011;1(1):64–68.
8. Rao JM, Rao RJ, Kumar US, Reddy SV, Tiwari AK. Anoxidant and a new germacrane sesquiterpene from Carissa Spinarum. Nat Prod Res. 2004;19(8):763–769.
9. Sanwal R, Chaudhary AK. Wound healing and antimicrobial potenal of Carissa spinarum L. in albino mice. J Ethnopharmacol. 2011;135:792–796.
10. Beck NR, Namdeo KP. Preliminary phytochemical and pharmacognostical evaluation of Carissa spinarum leaves. Asian J Pharm Tech. 2013;3(1):30–33.
11. Vinod K, Vijay J, Ravinder S. Antibacterial activity of root and leaf extracts of Carissa spinarum against Methicillin-resistant Staphylococcus aureus (MRSA). Octa J Biosci. 2017;5(2):117–137.
12. Mamta S, Jyoti S. Phytochemical screening of Acorus calamus and Lantana camara. Int Res J Pharm. 2012;3(5):324–326.
13. Sindhu CG. Phytochemical screening of Calendula officinalis Linn leaf extract by TLC. Int J Ayurveda Res. 2010;1:131–134.
14. Doherty VF, Olaniran OO, Kanife UC. Antimicrobial activity of Aframomum melegueta (Allegator pepper). Int J Biol. 2010;2(2). doi:10.5539/ijb.v2n2p126
15. Ayoola GA, Lawore FM, Adelowotan T, et al. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). Afr J Microbiol Res. 2008;2:162–166.
16. Onotu CS, Jingfa YE, Benjamin JE, Kugu BA, Andrew T, Okoh KE. In vivo antitrypanosomal activity of ethanolic root extract of Carissa spinarum (Wild Karanda) in mice infected with trypanosoma brucei brucei Spp. IJES. 2013;2(11):124–128.
17. WC Evans. Trease and Evans Pharmacognosy.
18. Trease GE, Evans WC. Pharmacognosy. Vol. 69.
19. Donadu MG, Peralta-Ruiz Y, Usai D, et al. Colombian essential oil of ruta graveolens against nosocomial antifungal resistant candida strains. J Fungi. 2021;7(5):383. doi:10.3390/jof7050383
20. Aleksandra B, Matthew D, Donatella U, et al. Antifungal activity of Myrtus communis against Malassezia sp. isolated from the skin of patients with pityriasis versicolor. Infection. 2017. doi:10.1007/s15010-017-1102
21. Vittorio M, Elisabetta G, Giovanna R, et al. Clinical, cosmetic and investigational dermatology. 2020:233–239.
22. Matthew D, Donatella U, Antonio P, Tiziana P, Vittorio M, Maura F. Lavender essential oils against P. aeruginosa. J Infect Dev Ctries. 2018;12(1):009–014. doi:10.3855/jidc.9920
23. Samander K, Lalit D, Samander K, Jaya PY. Anti-dengue activity of super critical extract and isolated oleanolic acid of Leucas cephalotes using in vitro and in silico approach. BMC Complement Med Ther. 2021;21(1):227. doi:10.1186/s12906-021-03402-2
24. Kaushik S, Kaushik S, Sharma V, Yadav JP. Antiviral and therapeutic uses of medicinal plants and their derivatives against dengue viruses. Pharmacogn Rev. 2018;12(24):177–185. doi:10.4103/phrev.phrev_2_18
25. Sulochana K, Samander K, Ramesh K, Dar L, Jaya PY. In-vitro and in silico activity of Cyamopsis tetragonoloba (Gaur) L. supercritical extract against the dengue-2 virus. VirusDis. 2020;31(4):470–478. doi:10.1007/s13337-020-00624-9
26. Yashika S, Anubhuti K, Vikrant S, Divya D, Sulochana K, Jaya PY. In-vitro and in-silico evaluation of the anti-chikungunya potential of Psidium guajava leaf extract and their synthesized silver nanoparticles. VirusDis. 2021;32(2):260–265. doi:10.1007/s13337-021-00685-4
27. Sulochana K, Ginni J, Vaibhav K, Jaya PY, Samander K. Anti-viral activity of Zingiber officinale (Ginger) ingredients against the Chikungunya virus. VirusDis. 2020;31(3):270–276. doi:10.1007/s13337-020-00584-0
28. Ramesh K, Sumi N, Suman SK. Green synthesized Allium cepa nanoparticles with enhanced antiprotozoal activities for E gingivalis. Chem Biol Lett. 2020;7(4):247–250.
29. Murthy PN, Girma M, Ariaya H, Tsige GM. Antimicrobial and phytochemical screening of Justicia schimpriana. Ethiop Pharm J. 1993;11:47–53.
30. Singh S, Verma SSK. Antibacterial properties of alkaloid rich fractions obtained from various parts of Prosopis juliflora. Int J Pharmaceut Sci Res. 2011;2:114–120.
31. Ruchira W, Vimolmas L, Mekala G, Stephen NSG, Kittisak L. Bioactive Compounds from Carissa spinarum. Phytother Res. 2012;26(10):1496–1499. doi:10.1002/ptr.4607
32. Mudiganti RKR, Anisha G. Preliminary phytochemical and Gc Ms study of one medicinal plant carissaspinarum. Indo Am J pharm sci. 2018;8(03):414–421.
33. Akharaiyi FC. Antibacterial, phytochemical and antioxidant activities of Datura metel. Int J PharmTech Res. 2011;3(1):478–483.
34. Clarence R, Patrick N, Hamisi MM, Francis S. Individual and combined antibacterial activity of crude extracts from medicinal plants Carissa spinarum linn and Carica papaya linn. Eur J Med Plants. 2014;4(12):1513–1523. doi:10.9734/EJMP/2014/10599
35. Ehigbai IO, Faith EO, Ehimwenma SO. Quantitative phytochemical analysis and antimicrobial activities of fresh and dry ethanol extracts of Citrus sinensis (L.) Osbeck (sweet Orange) peels. Clin Phytoscience. 2020;6(1):46. doi:10.1186/s40816-020-00193-w
36. Gemechu B, Dagmawit A, Venkataramana K. A review of the medicinal and antimicrobial properties of Carissa spinarum L. Am J Biomed Res. 2020;8(2):54–58. doi:10.12691/ajbr-8-2-5
37. Harrient U, Nandita D. Mechanism of antibiotic resistance in Salmonella typhi. Int J Curr Microbiol Appl Sci. 2014;3(12):461–476.
38. Perlin DS, Shor E, Zhao Y. Update on antifungal drug resistance. Curr Clin Microbiol Rep. 2015;2(2):84–95. doi:10.1007/s40588-015-0015-1
39. Wink M. Modes of action of herbal medicines and plant secondary metabolites. Medicines. 2015;2(3):251–286. doi:10.3390/medicines2030251
40. Freiesleben SH, Jager AK. Correlation between plant secondary metabolites and their antifungal mechanisms. A review. Med Aromat Plant. 2014;3:2–6.
41. Brehm-Stecher BF, Johnson EA. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol and apritone. Antimicrob Agents Chemother. 2003;47:3357–3360. doi:10.1128/AAC.47.10.3357-3360.2003
42. Tsuchiya H, Sato M, Miyazaki T, et al. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. 1996;50(1):27–34. doi:10.1016/0378-8741(96)85514-0
43. Anandhi D, Srinivasan PT, Kumar JP, Jagatheesh S. DNA fragmentation induced by the glycosides and flavonoids from C.coriaria. Int J Curr Microbiol App Sci. 2014;3:666–673.
44. Netala VR, Ghosh SB, Bobbu P, Anitha D, Tartte V. Triterpenoid saponins: a review on biosynthesis, applications and mechanism of their action. Int J Pharm Pharm Sci. 2015;7:24–28.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
