Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 18
Physical Activity, Depression and Quality of Life in COPD – Results from the CLARA II Study
Authors Horner A , Olschewski H , Hartl S, Valipour A, Funk GC , Studnicka M , Merkle M, Kaiser B, Wallner EM , Brecht S, Lamprecht B
Received 14 August 2023
Accepted for publication 1 November 2023
Published 29 November 2023 Volume 2023:18 Pages 2755—2767
DOI https://doi.org/10.2147/COPD.S435278
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Min Zhang
Andreas Horner,1 Horst Olschewski,2 Sylvia Hartl,3 Arschang Valipour,4 Georg-Christian Funk,5 Michael Studnicka,6 Monika Merkle,7 Bernhard Kaiser,1 Eva Maria Wallner,8 Stephan Brecht,8 Bernd Lamprecht1
1Johannes Kepler University Linz, Kepler University Hospital, Department of Pulmonology, Linz, Austria; 2Division of Pulmonology, Department of Internal Medicine, Medical University of Graz, and Ludwig Boltzmann Institute for Lung Vascular Research, Graz, Austria; 3Department of Respiratory and Critical Care Medicine, Klinik Penzing and Sigmund Freud University, Medical School, Vienna, Austria; 4Karl-Landsteiner-Institute for Lung Research and Pulmonary Oncology, Klinik Floridsdorf, Vienna, Austria; 5Department of Internal and Respiratory Medicine, Klinik Ottakring, Vienna, Austria; 6Department of Pulmonary Medicine, Paracelsus Medical University, Salzburg, Austria; 7Specialist Office for Pulmonology Dr. Merkle, Vienna, Austria; 8A. Menarini Pharma GmbH, Vienna, Austria
Correspondence: Andreas Horner, Johannes Kepler University Linz, Kepler University Hospital, Department of Pulmonology, Altenberger Strasse, 69, 4040 Linz and Krankenhausstrasse 9, 4020 Linz, Austria, Email [email protected]
Background: Symptoms of depression, pain and limitations in physical activity may affect quality of life in COPD patients independent from their respiratory burden. We aimed to analyze the associations of these factors in outpatients with COPD in Austria in a stable phase of disease.
Methods: We conducted a national, cross-sectional study among patients with COPD. For depression, the Patient Health Questionnaire-9 (PHQ-9) and for respiratory symptoms the St. George’s Respiratory Questionnaire for COPD patients (SGRQ-C) were used along with 10-point scales for physical activity and pain.
Results: After exclusion of 211 patients due to non-obstructive spirometry or missing data, 630 patients (62.5% men; mean age 66.8 ± 8.6 (SD) years; mean FEV1%pred. 54.3 ± 16.5 (SD)) were analyzed. Of these, 47% reported one or more exacerbations in the previous year, 10.4% with hospitalization. A negative depression score was found in 54% and a score suggesting severe depression (PHQ-9 score ≥ 15) in 4.7%. In a multivariate linear regression model, self-reported pain, dyspnea, and number of exacerbations were predictors for higher PHQ-9-scores. A negative pain score was found in 43.8%, and a score suggesting severe pain in 2.9% (8– 10 points of 10-point scale). Patients reporting severe pain were more often female, had more exacerbations, and reported more respiratory and depressive symptoms, a lower quality of life, and less physical activity. About 46% of patients rated their physical activity as severely impaired. These patients were significantly older, had more exacerbations, concomitant heart disease, a higher pain and depression score, and a lower quality of life (SGRQ-C – total score and all subscores).
Conclusions: In Austria, nearly half of stable COPD outpatients reported symptoms of depression, which were associated with lower levels of self-reported physical activity, more pain, and respiratory symptoms. The associations were particularly strong for depression with SGRQ-C.
Keywords: chronic obstructive pulmonary disease, depression, PHQ-9, pain, quality of life, St. George’s Respiratory Questionnaire, physical activity
Introduction
COPD (chronic obstructive pulmonary disease) is an obstructive airway disease, characterized by chronic respiratory symptoms, as dyspnea, cough, excess sputum production, due to airway abnormalities that cause persistent airflow obstruction.1 Diagnosis of COPD is confirmed by the presence of not completely reversible obstructive airflow limitation as assessed by spirometry. Apart from preventive measures, especially smoking cessation, treatment of COPD is based on inhaled pharmacotherapy to mitigate the symptoms of this disease.2
Furthermore, COPD is frequently accompanied by comorbidities, such as pulmonary hypertension, malnutrition, venous thromboembolism, anxiety, depression, osteoporosis, obesity, metabolic syndrome, diabetes, sleep disturbance and anemia, some of which being seen as systemic manifestations of this disease.3–5
In addition to the mitigation of clinical symptoms, COPD therapy aims to improve QoL (quality of life), whereby disease specific questionnaires for monitoring have been developed. The St. George’s Respiratory Questionnaire (SGRQ, with scores 0 – 100, with higher scores indicating lower QoL),6,7 where a validated COPD-specific version (SGRQ-C) is available,8 is frequently used to measure health status in COPD.
Integrated health assessment may further include other parameters to describe well-being in chronic diseases. In COPD symptoms of depression and pain are frequently described as related to quality of life9–12 and may even impact the risk of future exacerbations or death.13 Hence, monitoring depressive symptoms, using specific questionnaires like the Patient Health Questionnaire (PHQ-9),14 may be useful. Similarly, there is evidence of impaired physical activity in COPD,15,16 and it is well known that exercise training is associated with better exercise tolerance, skeletal and respiratory muscle functions, symptoms of dyspnea and fatigue and health-related quality of life.17
In 2018, the non-interventional
To address these previous findings,
Materials and Methods
Study Setting and Population
The population-based, non-interventional observational
Main inclusion criteria were selected to allow for a broad population of outpatients and consisted of (1) age ≥ 40 years and older, and (2) diagnosis of COPD according to GOLD criteria.1 Main exclusion criteria were (1) having undergone major lung surgery (eg, lung volume reduction, lung transplant), (2) reported moderate or severe exacerbation within the last four weeks, (3) lung cancer and (4) physical or cognitive impairment resulting in an inability to walk or to complete the questionnaires. No specific treatment was studied or required for participation.
Study Conduct and Endpoints
Eligible patients were offered to participate in
Questionnaire
The treating physicians provided spirometry data (FEV1%pred, FEV1/FVC, FVC), as well as data about the number of exacerbations during the last year and how often the patient was hospitalized for COPD exacerbations. Additionally, documentation of inhaled therapy and information on any previous COVID-19-infections and vaccinations were provided. Inhaled therapy was categorized as mono therapy (LABA or LAMA), dual therapy (LABA+ICS or LABA+LAMA) or triple therapy (LABA+LAMA+ICS). Furthermore, the last absolute blood eosinophil count was documented (if available in the patient`s medical record).
The patient provided information on demographics (age, sex, weight, height, smoking status) and comorbidities (arterial hypertension, diabetes mellitus, osteoporosis, and cardiac diseases).
Furthermore, the patient answered the SGRQ-C according to the current manual.21 The SGRQ–C is a well characterized, widely used tool to describe patient reported health status specific for COPD.8 It consists of a total score and the domains activity, symptoms, and impact on daily life. Each domain is given a score between 0 and 100, and higher scores indicate worse health status.
The PHQ-9 questionnaire was used to screen for depressive symptoms.22 As a self-administered tool it was filled by the patient. According to the questionnaire manual and current literature,14,23 we used the scores of the PHQ-9 to discriminate between no symptoms of depression (< 5), mild to moderate depression (5–14) and severe depression (≥ 15).
A 10-point scale for pain (ranging from 1 to 10, no pain to very severe pain) was used to capture the patient`s current general pain condition. Mild pain was defined as 2–7, severe pain as ≥ 8 points.
Two different items were used to assess the physical activity of patient. Firstly, out of the SGRQ-C two groups were constructed, using the following two questions asking how the chest trouble affects daily life activities: “Exercise is not safe for me.” and “I cannot play sports or games.”. Group 1 (“Impaired physical activity”) were all patients who answered affirmatively to both of the two questions of the SGRQ-C. Group 2 (“Preserved physical activity”) were all patients who answered in the negative to both questions from above.
Secondly, a 10-point scale answering the question “How much physical activity do you perform during a typical week?” and ranging from 1 (no physical activity) to 10 (a lot of physical activity) was used.
Data Management and Statistics
Categorical data were reported as absolute number and percentage, metric data by arithmetic mean, median, minimum, maximum, and standard deviation. Univariate and multivariate linear regression was used to study the relationships between PHQ-9 scores and demographic and disease specific characteristics. Fisher’s exact test and chi-square test were used to test for statistically significant relationships in categorical variables, T-test, Kruskal–Wallis test and Mann–Whitney-U-Test were used in metric variables. Statistical significance was defined as p ≤ 0.05. Data analysis was conducted in R (R: A Language and Environment for Statistical Computing; Version 4.3.0; https://www.R-project.org).
Results
A total of 89 pulmonologists contributed to this study and enrolled a total of 841 COPD patients. We excluded 28 patients because of missing data (eg, sex, age or FEV1). Furthermore, 183 patients were excluded because the spirometric criterion of airways obstruction was not met (FEV1/FVC <0.7).
Finally, 630 patients (62.5% men; mean age 66.8 ± 8.6 (SD) years; mean FEV1%pred. 54.3 ± 16.5 (SD)) were included in this study (see also Table 1). 89.0% were current or former smokers, and mean BMI was 26.6 kg/m² ± 5.5 (SD). Arterial hypertension, diabetes mellitus, heart disease or osteoporosis was reported in 46.0%, 16.0%, 16.7% and 11.6%, respectively.
 |
Table 1 Characteristics of Participants |
Severe airflow limitation (FEV1pred < 50%, GOLD grades 3 and 4) was seen in 39.7% of patients. Exacerbations in the previous year were reported by 46.9% of participants, 10.4% were therefore hospitalized. More than one exacerbation or a hospitalization due to an exacerbation was stated by 21.6% of patients (GOLD group E, previously C and D).
Female COPD patients were slightly younger, had lower BMIs, and better lung function (FEV1%pred). Compared to male patients, female COPD patients also reported less phlegm and were less likely to report diabetes mellitus and heart disease, but more frequently osteoporosis.
Inhaled triple therapy (LABA/LAMA/ICS) was used by 49.1% of all patients, while a combination therapy of LABA/LAMA or LABA/ICS was seen in 40.1% and 4.7%, respectively.
A previous COVID-19 infection was reported by 53 patients (6.6%) and 33 patients (5.2%) were not vaccinated against COVID-19. Unvaccinated patients tended to be younger (mean age 65.6 vs 66.8 years), more likely male, and had a numerically higher mean PHQ-9 score (mean total score 7.1 vs 5.3). No difference was seen in the SGRQ-C scores. Multivariate regression analysis confirmed that unvaccinated patients had significantly higher total PHQ-9 scores (OR 1.08 (1.1–1.2 CI, p = 0.029) compared to vaccinated patients.
Using the PHQ-9 questionnaire, no evidence of depressive symptoms was found in 53.8% (331 of 615 patients). Symptoms for severe depression (PHQ-9 score ≥ 15) were reported in 4.7%. Higher scores in the PHQ-9 questionnaire, indicating more severe depressive symptoms, were associated with lower quality of life (using the SGRQ-C), worse lung function (FEV1), more exacerbations, a higher pain score and more respiratory symptoms in crude analysis. Further details are presented in Table 2 and Figure 1. In a multivariate linear regression model, self-reported pain, dyspnea, and number of exacerbations were predictors of higher PHQ-9-scores, hence more depressive symptoms (Table 3).
 |
Table 2 Characteristics of Participants According to Their PHQ-9 Scores (Symptoms of Depression) |
 |
Table 3 Clinical Parameters and Their Associations with Symptoms of Depression - A Linear Regression Model with PHQ-9 as Dependent Variable (Range 0–27) |
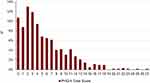 |
Figure 1 Percent distribution of PHQ-9 total scores (symptoms of depression). |
No pain was reported by 43.8% (269 of 630) of patients, whereas 2.9% indicated to suffer from severe pain (8–10 points; 10-point scale). Characteristics according to the current pain status are presented in Table 4. Percent distribution of the pain score is presented in Figure 2A. Patients reporting severe pain were more often female, reported more respiratory and depressive symptoms, were less physical active (self-reported) and had more exacerbations and a lower quality of life (using the SGRQ-C).
 |
Table 4 Characteristics of Participants According to General Pain (10-Point Scale - No Pain (1) vs Mild (2–7) to Severe Pain (8–10) |
 |
Figure 2 Percent distribution of a self-reported pain (A) and physical activity (B); data from 10-point scales. |
Severely impaired physical activity was reported by 45.9% (225 of 490) of patients. They were older, had more exacerbations, comorbidities (heart disease), pain, depressive symptoms, and a lower FEV1%pred. Furthermore, they reported less quality of life using the SGRQ-C (total score and all subscores). Further details are presented in Table 5. Percent distribution of the results of self-reported physical activity using a 10-point scale is presented in Figure 2B.
 |
Table 5 Characteristics of Participants According to Self-Reported Physical Activity (Impaired vs Preserved) |
In Figure 3 the relationship between self-reported physical activity (10-point scale) and depressive symptoms (total score PHQ-9) is visualized in a box plot. A higher self-reported physical activity was associated with a lower PHQ-9 score suggesting fewer depressive symptoms.
Discussion
In this work, we report real-world data of stable COPD outpatients in Austria, documenting an association of self-reported symptoms of depression and pain.
Using the PHQ-9 questionnaire, mild to moderate depressive symptoms were found in 41.5% of COPD patients, symptoms of severe depression in 4.7%. These symptoms were found closely associated with more exacerbations in the previous year, a lower lung function (FEV1), a higher pain score, and a higher degree of dyspnea (Table 3). In agreement, comparable symptom scores for depression (PHQ-9) were found in other COPD cohorts worldwide, suggesting an increased burden of co-existing depression in COPD patients.24–27 However, an analysis of the data from the German COPD cohort Cosyconet24 found an association between total PHQ-9 scores and age, sex and BMI, which we could not observe in our data. Symptoms of anxiety and depression have also been identified as predictors of mortality in the literature.28–30
In our cohort only 2.9% of subjects reported severe over-all pain, the remaining patients stated suffering only mild or no pain. Hence, self- reported pain was less likely reported here than other data suggest.31,32 We speculate that the lower pain scores in our cohort may be due to the selection of patients as only patients with stable disease in an outpatient setting were included. Furthermore, we could not confirm the association between lower FEV1 and a higher pain score. However, patients who reported more intense pain, tended to present with more exacerbations (Table 4).
Physical activity could be a relevant predictor for exacerbations, quality of life as well as depressive and respiratory symptoms (Table 5). These associations with a lower level of physical activity was also seen in previous publications.15,33 Moreover, exercise interventions have been effective to improve outcomes in COPD.34 Furthermore, a similar relationship between depressive symptoms and physical activity (Table 3 and Figure 1) was seen in a meta-analysis by Selzler et al.9
Strengths and limitations:
The study used a highly standardized protocol and included COPD patients from all nine Austrian federal states excluding those with a FEV1/FVC ratio > 0.7. Patient reported outcome included important domains addressing mental and physical well-being as well as disease specific health status (SGRQ-C). Selection of participating study sites was not at random, leaving the possibility of selection bias. The inclusion of COPD patients into the study was left to the investigator’s discretion. However, we were not able to address COPD patients, who were either undiagnosed or untreated for their COPD. Population-based random samples indicate that approximately 80% of COPD patients are non-diagnosed with the disease,35 and these patients experience different burden of disease. Furthermore, due to the cross-sectional design, we were not able to provide any longitudinal data. Due to the ongoing COVID-19 pandemic during the phase of data collection, some variables may have been biased.
Conclusions
In Austria, nearly half of stable COPD outpatients report symptoms indicative of mild to moderate or severe depression, which are associated with older age but not gender or smoking, lower FEV1, less physical activity, more pain, more respiratory symptoms and exacerbations, and heart disease as the only significant comorbidity. This suggests that depression may be an important therapy target in COPD patients.
Abbreviations
BMI, body mass index; CRF, case report form; COPD, chronic obstructive pulmonary disease; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, inhaled corticosteroid; PHQ-9, Patient Health Questionnaire-9; QoL, quality of life; SGRQ, St. George’s Respiratory Questionnaire; SGRQ-C, St. George’s Respiratory Questionnaire for COPD patients.
Ethics Approval and Consent to Participate
All participants provided written informed consent to participate in the study. The protocol was approved by the independent ethics committee of the Johannes Kepler University, Linz (vote 1129/2021). This study was conducted in accordance with the Declaration of Helsinki.
Acknowledgments
The authors wish to express their gratitude to all investigators who contributed to the conduct of the
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Funding of this study was provided by A. Menarini Pharma GmbH. The funding source had no role in data analysis, interpretation of the data, or decision to submit results.
Disclosure
Dr Horst Olschewski reports personal fees and/or non-financial support from Astra Zeneca, Boehringer, Menarini; personal fees from Chiesi, GSK and Novartis, outside the submitted work. Dr Sylvia Hartl reports grants and/or personal fees from GSK, Astra Zeneca, Menarini Pharma, Chiesi Farma, Roche Pharma, and MSD, outside the submitted work. Professor Arschang Valipour reports personal fees from Astra Zeneca, Boehringer Ingelheim, Chiesi, GSK, and Menarini, during the conduct of the study. Dr Georg-Christian Funk reports honorary for advisory board from Menarini Pharma. Dr Monika Merkle reports personal fees from Astra Zeneca, Boehringer Ingelheim, Chiesi, Menarini, Sanofi, and Novartis, outside the submitted work. Mrs Eva Wallner and Dr Stephan Brecht are employees of A. Menarini Pharma GmbH. The authors report no other conflicts of interest in this work.
References
1. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2023 REPORT). Available from: https://goldcopd.org/2023-gold-report-2/.
2. Barnes PJ. COPD 2020: new directions needed. Am J Physiol Lung Cell Mol Physiol. 2020;319(5):L884–L886. doi:10.1152/ajplung.00473.2020
3. Cavaillès A, Brinchault-Rabin G, Dixmier A, et al. Comorbidities of COPD. Eur Respir Rev. 2013;22(130):454–475. doi:10.1183/09059180.00008612
4. Agusti A, Ambrosino N, Blackstock F, et al. COPD: providing the right treatment for the right patient at the right time. Respir Med. 2023;207:107041. doi:10.1016/j.rmed.2022.107041
5. Divo M, Celli BR. Multimorbidity in patients with chronic obstructive pulmonary disease. Clin Chest Med. 2020;41(3):405–419. doi:10.1016/j.ccm.2020.06.002
6. Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85(Suppl B):25–31; discussion 33–7. doi:10.1016/s0954-6111(06)80166-6
7. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s respiratory questionnaire. Am Rev Respir Dis. 1992;145(6):1321–1327. doi:10.1164/ajrccm/145.6.1321
8. Meguro M, Barley EA, Spencer S, Jones PW. Development and validation of an improved, COPD-specific version of the St. George respiratory questionnaire. Chest. 2007;132(2):456–463. doi:10.1378/chest.06-0702
9. Selzler A-M, Ellerton C, Ellerton L, et al. The relationship between physical activity, depression and anxiety in people with COPD: a systematic review and meta-analyses. COPD. 2023;20(1):167–174. doi:10.1080/15412555.2023.2200826
10. Xia -J-J, Zou -X-X, Qiu Y, et al. Investigation and analysis of risk factors and psychological status of chronic obstructive pulmonary disease in permanent residents aged 40 or older in Hongyuan County, Aba Prefecture, Sichuan Province. Int J Chron Obstruct Pulmon Dis. 2023;18:827–835. doi:10.2147/COPD.S399279
11. Tanaka T, Okita M, Jenkins S, Kozu R. Clinical and psychological impact of chronic pain in people with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:893–903. doi:10.2147/COPD.S359223
12. Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345–349. doi:10.1183/09059180.00007813
13. Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766–777. doi:10.1378/chest.12-1911
14. Levis B, Benedetti A, Thombs BD. Accuracy of Patient Health Questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365(1476). doi:10.1136/bmj.l1476
15. Mihaltan F, Adir Y, Antczak A, et al. Importance of the relationship between symptoms and self-reported physical activity level in stable COPD based on the results from the SPACE study. Respir Res. 2019;20(1):89. doi:10.1186/s12931-019-1053-7
16. Tiller NB, Kinninger A, Abbasi A, et al. Physical activity, muscle oxidative capacity, and coronary artery calcium in smokers with and without COPD. Int J Chron Obstruct Pulmon Dis. 2022;17:2811–2820. doi:10.2147/COPD.S385000
17. Troosters T, Janssens W, Demeyer H, Rabinovich RA. Pulmonary rehabilitation and physical interventions. Eur Respir Rev. 2023;32(168):220222. doi:10.1183/16000617.0222-2022
18. Horner A, Burghuber OC, Hartl S, et al. Quality of life and limitations in daily life of stable COPD outpatients in a real-world setting in Austria - results from the CLARA project. Int J Chron Obstruct Pulmon Dis. 2020;15:1655–1663. doi:10.2147/COPD.S252033
19. Tsutsui M, Gerayeli F, Sin DD. Pulmonary rehabilitation in a post-COVID-19 world: telerehabilitation as a new standard in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:379–391. doi:10.2147/COPD.S263031
20. Halpin DMG, Vogelmeier CF, Agusti A. COVID-19 and COPD: lessons beyond the pandemic. Am J Physiol Lung Cell Mol Physiol. 2021;321(5):L978–L982. doi:10.1152/ajplung.00386.2021
21. St George’s Respiratory Questionnaire for COPD Patients (SGRQ-C) Manual; 2023 Available from: https://www.sgul.ac.uk/research/research-operations/research-administration/st-georges-respiratory-questionnaire/docs/sgrq-c-manual-april-2012.pdf.
22. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x
23. Instructions for Patient Health Questionnaire (PHQ) and GAD-7 Measures. Available from: https://www.phqscreeners.com/images/sites/g/files/g10016261/f/201412/instructions.pdf.
24. von SSM, Jörres RA, Behr J, et al. Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res. 2019;20(1):30. doi:10.1186/s12931-019-0997-y
25. Schuler M, Strohmayer M, Mühlig S, et al. Assessment of depression before and after inpatient rehabilitation in COPD patients: psychometric properties of the German version of the Patient Health Questionnaire (PHQ-9/PHQ-2). J Affect Disord. 2018;232:268–275. doi:10.1016/j.jad.2018.02.037
26. Matte DL, Pizzichini MMM, Hoepers ATC, et al. Prevalence of depression in COPD: a systematic review and meta-analysis of controlled studies. Respir Med. 2016;117:154–161. doi:10.1016/j.rmed.2016.06.006
27. Funk G-C, Kirchheiner K, Burghuber OC, Hartl S. BODE index versus GOLD classification for explaining anxious and depressive symptoms in patients with COPD - a cross-sectional study. Respir Res. 2009;10(1):1. doi:10.1186/1465-9921-10-1
28. Vikjord SAA, Brumpton BM, Mai X-M, Vanfleteren L, Langhammer A. The association of anxiety and depression with mortality in a COPD cohort. The HUNT study, Norway. Respir Med. 2020;171:106089. doi:10.1016/j.rmed.2020.106089
29. Papaioannou AI, Bartziokas K, Tsikrika S, et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823. doi:10.1183/09031936.00013112
30. Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171(4):453–462. doi:10.1176/appi.ajp.2013.13030325
31. van Dam Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898. doi:10.1136/bmjopen-2014-005898
32. Janssen DJA, Spruit MA, Uszko-Lencer NH, Schols JMGA, Wouters EFM. Symptoms, comorbidities, and health care in advanced chronic obstructive pulmonary disease or chronic heart failure. J Palliat Med. 2011;14(6):735–743. doi:10.1089/jpm.2010.0479
33. Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287–1295. doi:10.2147/copd.s101459
34. Priego-Jiménez S, Torres-Costoso A, Guzmán-Pavón MJ, Lorenzo-García P, Lucerón-Lucas-Torres MI, Álvarez-Bueno C. Efficacy of different types of physical activity interventions on exercise capacity in patients with chronic obstructive Pulmonary Disease (COPD): a network meta-analysis. Int J Environ Res Public Health. 2022;19(21):14539. doi:10.3390/ijerph192114539
35. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi:10.1378/chest.14-2535
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

