Back to Journals » Clinical Ophthalmology » Volume 16
Photorefraction Screening Plus Atropine Treatment for Myopia is Cost-Effective: A Proof-of-Concept Markov Analysis
Authors Hong CY , Boyd M , Wilson G, Hong SC
Received 17 February 2022
Accepted for publication 18 May 2022
Published 13 June 2022 Volume 2022:16 Pages 1941—1952
DOI https://doi.org/10.2147/OPTH.S362342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Chuen Yen Hong,1 Matt Boyd,2 Graham Wilson,3 Sheng Chiong Hong4
1Dunedin School of Medicine, University of Otago, Dunedin, New Zealand; 2Adapt Research Ltd, Reefton, New Zealand; 3Matai Medical Research Institute, Gisborne, New Zealand; 4oDocs Eye Care, Dunedin, New Zealand
Correspondence: Chuen Yen Hong, Dunedin School of Medicine, University of Otago, Dunedin, New Zealand, Tel +6421 209 1230, Email [email protected]
Purpose: The prevalence of myopia is increasing globally, putting individuals at risk of myopia-associated visual impairment. Low-dose atropine eye drops have been found to safely reduce the risk of progression from myopia to higher levels of myopia and pathological states. In New Zealand, school children have an eye check at age 11. In this study, we aimed to estimate the cost-effectiveness of introducing photorefractive screening for myopia at age 11 in the New Zealand context, with atropine 0.01% eye drops treatment for those screening positive.
Patients and Methods: A Markov cohort simulation was used to model the impact of screening plus atropine compared to usual care across a lifetime horizon and societal perspective with a 3% discount rate. Cost-effectiveness was determined by the incremental cost-effectiveness ratio (ICER), with utility measured in quality-adjusted life-years (QALYs). Multivariate sensitivity analyses were carried out to investigate factors influencing cost-effectiveness.
Results: The ICER for screening plus atropine was NZ$1590 (95% CI 1390, 1791) per QALY gained, with 7 cases of lifetime blindness prevented per 100,000 children screened.
Conclusion: Screening for myopia with photorefraction at age 11 and atropine 0.01% eye drop treatment of children screening positive is likely to be cost-effective. These results suggest that a real-world trial and cost-effectiveness analysis would be worth considering in New Zealand.
Keywords: cost-benefit analysis, photorefractive screening, myopia, atropine
Introduction
Myopia is defined as a refractive error causing light to focus in front of the retina when ocular accommodation is relaxed. This can be due to elongation of the eyeball, an overly curved cornea, and/or a lens with increased optical power.1 Myopia usually first occurs in school-age children, progressing in the absence of intervention until adulthood.2 Predisposing factors include parental myopia, East Asian ethnicity, excessive near work, limited outdoor time, and existing refractive error.3,4 The World Health Organization (WHO) defines myopia and high myopia as a spherical equivalent of ≤ −0.50D and ≤ −5.00D, respectively, in either eye. Pathologic myopia is high myopia with myopia-related fundus abnormalities such as myopic macular degeneration.1 A proportion of patients with myopia will progress and may have high myopia or pathological myopia. Myopia increases the risk of associated ocular pathology and subsequent visual impairment. Even low myopes are twice as likely as those with normal vision to develop myopic maculopathy, glaucoma or posterior subcapsular cataract and at least three times as likely to develop retinal detachment.1,5,6 The risks significantly increase with high myopia.7 With the increasing prevalence of myopia worldwide,8 there will be a rise in myopia-associated visual impairment.
The economic burden of myopia on patients, public health systems and wider society is significant, with international analyses showing that the cost of refractive correction is higher than the cost for other ocular diseases,9 and myopia is responsible for an annual US$202 billion global loss of gross domestic product (GDP).10 Individual costs can be US$709 per person per year, with lifetime costs of US$17,020.11 Successful prevention of myopia would reduce vision impairment, blindness, and costs to individuals and wider society.
Methods to ameliorate myopia progression include behavioral (reduced near work12–16,18 and increased outdoor activity12,16–18), optical (special glasses or contact lenses and orthokeratology contact lenses to reshape the cornea) and pharmacological interventions. Key among pharmacologic interventions is atropine eye drops, which is among the most effective interventions for progressive myopia.19
With an effective screening program in place, interventions can be targeted to those most likely to benefit systematically and efficiently. Vision checks already occur in New Zealand in year 7 (age 11). Children in whom myopia is discovered are generally referred for corrective lenses. However, the New Zealand Myopia Action Group is currently refining New Zealand-specific guidelines adapted from international guidelines20 and is advocating for public funding of low-dose atropine.
The current monthly user cost of low-dose atropine (0.01%) in New Zealand is approximately NZ$50/month per individual.20 There are no commercially viable atropine drops available in New Zealand, and these eye drops are specially prepared by two compounding pharmacies in Auckland. The wisdom of offering low-dose atropine as a general first-line treatment strategy for a myopia screening program rests upon the appropriateness of this screening and management strategy, as well as the cost-effectiveness of the approach. A recent study has shown that a screening program in New Zealand for myopia may perform well.20
Given the interest in new guidance for myopia prevention in New Zealand, we aim to evaluate the cost-effectiveness of a hypothetical myopia screening program in the New Zealand setting, where children are screened in year 7 (age 11), and those who screen positive are offered low-dose atropine treatment alongside current standard care (optical interventions).
Materials and Methods
A hybrid decision tree Markov model was developed to compare the long-term clinical and economic outcomes of a myopia screening program in New Zealand. The study setting was the New Zealand public healthcare system with a societal perspective. A state-transition Markov model simulated the clinical path of two hypothetical cohorts of individuals from age 11 until assumed death at age 80 (current life expectancy of New Zealand population rounded to nearest 10-years21). Cohorts were assigned to either usual care with corrective lenses and optometry follow-up or to a screening strategy for myopia at age 11 with those screening positive treated with atropine.
Model Structure
Markov models have been widely used in medical decision-making since they were first introduced by Beck and Pauker22 almost four decades ago. A Markov cohort state-transition model is often used to simulate the prognosis of a group of patients following an intervention as they move between different health states over time. The average number of individuals in each health state in the next time step is calculated as the sum of current state-configuration and the change in the state-configuration during that time step.23,24 In order to perform a cost-effectiveness analysis, we need to assign QALYs and costs to each state.25
The Markov state transition model with one-year cycles consists of five exclusive health states (represented by ovals in Figure 1). The initial prevalence of myopia (assumed to be the same in both cohorts) was obtained from international data and based on the ethnic composition of the local population. Individuals with screen-detected myopia were simulated to receive two years of treatment with atropine 0.01% eye drops with one further year of treatment if they failed to respond. Response to treatment was defined as mean annual progression of <0.50D over two years. The potential effect of preventative treatment in terms of reduced progression to high myopia, pathological myopia, and blindness were simulated in the model. The model assumed that there was full compliance with treatment.
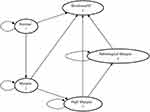 |
Figure 1 Markov model structure – ovals represent health states, arrows represent state transitions. |
At the end of the simulation, accumulated costs and health outcomes (QALYs) were summarized for each cohort. The standard care cohort was subjected to optometry and corrective lens costs. The atropine cohort was subjected to screening costs, optometry and corrective lens costs, along with the excess costs of atropine treatment and monitoring. Both cohorts accumulated excess costs of disease states for those differentially progressing to high myopia, pathological myopia and blindness. Willingness to pay (WTP) was defined using the WHO GDP per capita method, resulting in WTP of NZ$58,000 per QALY.
Disease Parameters
Available prevalence data for myopia in New Zealand were old (a single study from 1988).26,27 Therefore, data from countries with similar demographics to New Zealand were used. Markov state transition probabilities for normal vision to myopia were derived from 5 to 6 year incidence and prevalence data for myopia in Australia28 and set so that the model reproduced prevalence estimates of myopia across a range of ages. The state transition probabilities are age-dependent and non-linear, taking into account the ages of peak progression between states. To estimate the probability of transition from myopia to high myopia, high myopia to pathological myopia and from any state to blindness, prevalence data for New Zealand (or Australia) were used whenever these were available.
The transition probabilities were validated by running the model’s standard care pathway to generate outputs comparable to available prevalence data. These known prevalence data include a lifetime prevalence of myopia of 43.8%,29 and the final prevalence of myopic blindness in the modelled population at age 80 years calibrated to 0.11% as derived for the Australasian population at age 80 in the year 2050 from a systematic review of global prevalence of blindness due to myopic macular degeneration.8
Screening Efficacy
The modelled sensitivity and specificity of screening with photorefraction were estimated by averaging results from previously reported studies.30–33 In ophthalmology, photorefraction is a method to estimate the refractive state of the eye where light is projected into the eye during flash photography (co-axial or eccentric) and the path of light as it is reflected back from the retina is captured using a camera or other image-capturing device.34
Response to Treatment
We modelled 0.01% atropine as the intervention because that is the formulation currently prescribed in the New Zealand context for myopia. Studies have shown that approximately 80% of participants responded to atropine 0.01% treatment.35,36 Of the studies with the longest follow-up, the 5-year ATOM2 randomized, double-masked, controlled trial reported that 24% of children overall required re-treatment, and only 8% of those aged 10–13 required re-treatment.35
Efficacy of Treatment
The efficacy of 0.01% atropine has been confirmed by meta-analysis.37 A meta-analysis of other doses indicates that higher doses may have more effect,38 but studies also show greater rebound at higher doses,39 as well as more adverse effects.
The ATOM2 RCT found that 0.01% atropine led to a reduction of 50% in the rate of progression of myopia across five years (two years treatment, one year washout, two further years treatment for non-responders).35,40 The mean myopia progression at five years (−1.38D) in children initially randomized to atropine 0.01% was similar to that in placebo eyes at 2.5 years (–1.40D), suggesting that atropine 0.01% slowed myopia progression by 50%. Reduction in the rate of myopia progression by 50% reduces the incidence of high-risk myopia above 5D by 90% or more (see Supplementary Material for details).41
Utilities
Utility was measured in quality-adjusted life years (QALYs). Crude QALYs were obtained by inverting the disability weights from the Global Burden of Disease Study for minor, moderate, and severe visual impairment, as well as for blindness.42 There is controversy around the correct disability weights to apply when capturing the disutility of visual impairment,43 so we have erred on the side of the most cautious approach (ie, low Global Burden of Disease estimates for blindness and visual impairment, rather than alternative published high ones).44
Costs
Costs were evaluated over the lifetimes of individuals, with death in the base case assumed to be at age 80 years with a 3% annual time preference discount applied to both costs and utilities. Costs were attributed to each of the health states as follows (see Table 1): Normal (not screened): zero, comparator. Normal (screened): cost of screening. Myopia (screened): cost of screening, cost of the initial consultation (optometry), costs of atropine treatment, cost of monitoring while on atropine. Myopia (both arms): cost of optometry, cost of lenses. High myopia: cost of optometry, cost of transition lenses. Pathological myopia: annual excess cost of “low vision” from a societal perspective applied to 50% of those with pathological myopia. Blindness: annual excess cost of “low vision” from a societal perspective.45 Efficacy and safety costs were included as annual comprehensive optometry visits to monitor axial length and refraction (as well as alter lens prescription if photophobia is problematic), as well as intraocular pressure testing and assessment of pupil function.
 |
Table 1 Model Parameters and Values |
All costs were inflated to NZ$2021 using the online Reserve Bank of New Zealand consumer price index calculator. Costs modelled excluded initial setup costs of the screening program, ie, costs of databases, photo-screeners, and personnel training.
No additional costs were included for any adverse effects of atropine based on a meta-analysis of ten studies, which identified no serious complications at any dose of atropine used for myopia.38 The ATOM2 study found that “0.01% dose appears to offer an appropriate risk-benefit ratio, with no clinically significant visual side effects balanced against a reasonable and clinically significant 50% reduction in myopia progression”.35
Model Assumptions
The model assumes that the reduction in the rate of progression from myopia to high myopia due to the intervention persists lifelong following the cessation of successful treatment for treatment responders. One year of re-treatment was assumed to have only half the response rate as the initial two years of treatment.
The model assumes the prevalence of myopia at age 11 equivalent to that described in a 2016 global prevalence study of ten-year-olds.3 This is a conservative assumption because 11-year-olds will have a higher prevalence than 10-year-olds.
Following screening, all children eligible for atropine are assumed to take up treatment. Scenario analysis explored the impact of a discount rate of 3% versus 6%, screening at age five rather than 11, as well as life expectancy of 95 rather than 80.
The Markov model was constructed using TreeAge R2.1 and study findings were reported in accordance with the EQUATOR Network CHEERS checklist for health economic analyses, see checklist in the Supplementary Material.
Results
All model input parameters are listed in Table 1, along with sources of information. Additional detail is given in Supplementary Table 1.
The main results are presented in Table 2. The incremental gain in QALYs is 0.0129 (95% CI 0.0127, 0.0131) per individual screened. This effect came at an additional incremental cost of NZ$17.70 (15.40, 20.00) per individual screened, resulting in an ICER of NZ$1590.42 (95% CI 1390.10, 1790.74) per QALY gained for the strategy of photorefractive screening plus atropine treatment compared to usual care. Assuming all who screen positive take up atropine treatment, then for every 100,000 screened, it is expected that 7 cases of blindness can be prevented (as well as 462 cases of pathological myopia and 816 cases of high myopia).
Sensitivity Analysis
A sensitivity analysis showed that uncertainty around the costs associated with pathological myopia (NZ$1200–2700 per annum), the discount rate (3% vs 6%), and the efficacy of atropine 0.01% in reducing the transition to high myopia (0.5–1.0) were the factors that had the most substantial influence on cost-effectiveness, with costs associated with pathological myopia being the most important variable. The effect of variations in the different parameters on ICER is presented in a Tornado diagram (Figure 2). The cost-effectiveness plane produced from a probabilistic sensitivity analysis (Figure 3) shows that the incremental cost is well below the WTP threshold. A proportion of simulations produced cost-saving results.
 |
Figure 2 Tornado plot: showing the impact of univariate change in input parameters to their 95% confidence limits. |
 |
Figure 3 Cost-effectiveness plane: showing results of Monte Carlo simulation drawing model parameters at random from their distributions, with replacement, n = 1000 cycles. |
Scenario Analysis
When using a 6% time preference discount on costs and utilities, the outcome is less cost-effective with an ICER of NZ$17,877 per QALY gained. When screening at age five years, the ICER is NZ$1409 per QALY gained. Assuming a life expectancy of 95 years, the ICER is NZ$475 per QALY gained.
Discussion
Myopia screening with photorefraction followed by treatment for two years with 0.01% atropine is likely to be cost-effective, with an ICER of NZ$1590 per QALY gained which is well below the GDP per capita (NZD$58,000). Furthermore, 7 cases of lifetime blindness are averted per 100,000 children screened.
Sensitivity analysis found that the intervention is more cost-effective if individuals are assumed to have a life expectancy of 95 rather than 80 because the benefits (in terms of pathology and blindness prevented) accumulate later in life. The current life expectancy of New Zealanders is 82 years and is expected to increase; hence, although the magnitude of ICER reduction may not be as significant, the intervention is still expected to be cost-effective. The intervention is also slightly more cost-effective if children are screened at age five rather than 11. This is likely because of the near-term benefits of reducing progression to high-myopia, which are sustained life-long. However, the prevalence of myopia at age five is only 2.27% vs 9.24% at age 11, so considerably fewer individuals will benefit if screening is at age five. If a 6% discount rate is preferred, the intervention still falls below the WTP threshold with an ICER of NZ$17,877 per QALY gained.
Our results describe steady-state costs of the screening program and exclude initial one-off setup costs such as the purchase of photo-screener devices, training of technicians, and database setup costs.
While there is debate about the optimal approach for preventing the progression of childhood myopia, we chose to assess a proof-of-concept Markov model of screening plus atropine. There are three reasons for choosing 0.01% atropine in the model. Firstly, this is the concentration most commonly in use in New Zealand. Secondly, the ATOM2 study demonstrated a 50% reduction in the rate of progression of myopia with 0.01% atropine across 5 years, beyond the point at which treatment rebound effects are seen at higher doses.35 Thirdly, this low concentration exhibits fewer adverse effects than higher doses. We acknowledge the recent three-year results of the LAMP study, which concludes that 0.05%, 0.025%, and 0.01% atropine eye drops were all well tolerated and 0.05% was the most effective in controlling spherical equivalence progression.53 Following the findings from the Phase 1–3 reports of the LAMP study, there has been an anecdotal increased use of 0.05% atropine in New Zealand. More recently, a randomized control trial found that to achieve a similar reduction in myopic progression as that for older children on the lower concentration of atropine, younger children require a concentration of 0.05%.54 If this screening program was implemented at age five as part of B4 School Check, use of 0.05% atropine may be considered, given the increased risk of high myopia development with young age at onset and better tolerance against accommodation loss as an adverse effect.54
For optimal results, long-term treatment compliance and adherence is crucial. Our model assumed full compliance to treatment which is unlikely to be true in the real-world setting. Atropine 0.01% has been shown to have fewer adverse effects (photophobia, poor near vision acuity, allergies, headache, chalazion, systemic effects) while being as effective as higher doses for slowing the progression of myopia.37,55,56 For treatment duration between 12 and 60 months, a meta-analysis comparing atropine 0.01% eye drops with the control found that the odds ratio of adverse events was 0.26 (95% CI 0.11, 0.61).37 One study with five-year follow-up reported that only 2% of subjects reported side effects (photophobia, reading difficulty, mydriasis and headaches) that required treatment cessation.57 With atropine 0.01% having less side effects, compliance has been found to be high. The ATOM2 RCT defined compliance as >75% expected use and found that 98.8% of those in the atropine 0.01% were compliant within the two-year period.40 Using the same definition, the LAMP Phase 3 study reported that 84.7% of those in the atropine 0.01% group were compliant within the three-year period.53 Another study in European children found that 78% of children adhered to high-dose atropine for the one-year of treatment, with 90% of the children adhering to treatment more than 6 times a week.58
Our model assumed that those considered treatment responders have a reduced lifetime risk of progression to high myopia and pathological myopia. It will be important to follow the results of ongoing studies to understand the duration of effect of low-dose atropine treatment and revise the model accordingly. Our results suggest that a real-world cost-effectiveness trial is warranted given the long delay while awaiting life-long follow-up data.
Screening at age 11 is convenient in the New Zealand context because there is already a school-based vision check at this age. The prevalence of myopia in this age group was estimated to be approximately 10% in the model. Children developing myopia after this one-off screening point will not be detected by screening. The model did not include atropine treatment for children developing myopia later than age 11. However, incident (non-screen-detected) myopia at older ages could still be treated similarly.
Estimation of costs for the model were conservative with the use of the New Zealand Clear Focus Study mean population cost of “low vision” for pathological myopia (with low vision) and blindness.45 The Clear Focus study included health system costs, productivity losses, career opportunity costs, indirect costs such as disability aids, and deadweight losses in taxation and welfare. However, actual costs of blindness may be much greater than the average costs for visual impairment. European research has found direct medical costs alone were at least five times higher for pathological myopia (choroidal neovascularization) than for high myopia without choroidal neovascularization (EUR1985 vs EUR356).59 A German study found that people with blindness incurred direct medical, direct non-medical, and indirect 6-month costs per person of approximately EUR20,000.60 Any increase in the assumed cost of visual impairment and blindness will only tend to make the intervention more cost-effective.
Our study does not address the cost-effectiveness of atropine 0.01% versus other concentrations nor of atropine versus other non-pharmacological interventions (such as the new Defocus Incorporated Multiple Segments (DIMS), ie, MiYOSMART glasses,61–63 orthokeratology,64–67 or multifocal soft contact lenses68–70). Although the initial differences in the prevalence of myopia at age 11 by ethnicity were modelled, any potential differences in response to treatment and the impact of sex or genetic predisposition were not considered. The modelled population was effectively a homogeneous population beyond age 11. Furthermore, the model assumed that non-responders to atropine treatment had the same probability of transitioning to high myopia as the normal population. This may not be the case in reality.
The reduction in the rate of myopia progression was assumed to persist, so those who are treated and responded have a reduced lifetime-likelihood of progressing to high myopia. This assumption seems justified given the observation that the probability of transitioning to high myopia reduces with age, peaking in the teens and reducing dramatically over the life course. However, we acknowledge that the ATOM2 study only provides follow-up data for a 5-year period. All individuals were assumed to live to 80 years (the life expectancy in New Zealand to the nearest 10-years). However, with 3% and 6% discounting the QALYs accumulated in later years of life make only slight contribution to model results.
There is clearly a need to mitigate the approaching epidemic of myopia and its complications. Meta-analysis of global epidemiological studies predicts a five-to-six-fold increase in myopic macular degeneration related visual impairment and blindness by 2050.8 It is essential to act now to prevent this surge. New Zealand is well placed to act with an existing childhood vision screening program at age 11, and this research highlights the cost-effectiveness of preventing childhood myopia progression.
Future research should focus on confirming the duration of benefits of atropine treatment. For example, whether the 50% reduction in the rate of progression persists beyond the 5-years follow-up period. Also, research should seek to establish the optimal therapeutic concentration of atropine. However, the cost-effectiveness of atropine in New Zealand will be driven mainly by the cost of the pharmacist’s time in preparing the solution rather than the cost of atropine itself. Currently, the only commercially available atropine eye drops is the MyopineTM eye drops developed by the Singapore Eye Research Institute. This costs between NZ$25–30 for a month supply, roughly half of what we pay in New Zealand.
Treatment with atropine eye drops can slow myopia progression in many children, thereby averting the costs associated with high and pathological myopia or blindness. Results from our Markov model suggest that screening children at age 11 for myopia using photorefraction results in an average of 0.0129 QALYs gained per individual screened, at the cost of NZ$1590 per QALY gained. This compares very favorably with other screening programs in New Zealand.71–73 The costs and benefits of active screening to identify and treat children with myopia are largely unknown. Mathematical modeling to assess the cost-effectiveness of screening for uncorrected refractive errors in children found that for countries in the WHO Western Pacific Region A (which includes New Zealand), cost per QALY averted was I$1232 (NZ$1780, using 2021 PPP exchange rate of 1.44574), when all school children between age 5–15 years are screened annually.75 Although there remain many unknowns, which were identified as limitations and areas for further research, this proof-of-concept calculation suggests that a real-world trial and cost-effectiveness analysis would be worthwhile to establish the actual screening cost and its impact.
Acknowledgments
The authors acknowledge Dr. Renoh Chalakkal for his support. CYH Received Funding as a Summer Student from oDocs Eye Care Research Institute. MB received funding from oDocs Eye Care Research Institute. This work was supported by HRC Health Delivery Research Activation Grant-2 (20/1258).
Disclosure
Dr Chuen Yen Hong reports personal fees, non-financial support from oDocs Eye Care Research Institute, grants from Health Research Council, during the conduct of the study. Dr Graham Wilson is a shareholder in oDocs Eye Care Ltd., during the conduct of the study. Dr Sheng Chiong Hong reports grants from oDocs Eye Care Ltd., during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Flitcroft D. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31(6):622–660.
2. Yu L, Li Z-K, Gao J-R, et al. Epidemiology, genetics and treatments for myopia. Int J Ophthalmol. 2011;4(6):658.
3. Rudnicka AR, Kapetanakis VV, Wathern AK, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. 2016;100(7):882–890.
4. Lin Z, Gao TY, Vasudevan B, et al. Near work, outdoor activity, and myopia in children in rural China: the Handan offspring myopia study. BMC Ophthalmol. 2017;17(1):1–8.
5. Mitchell P, Hourihan F, Sandbach J, et al. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010–2015.
6. Vongphanit J, Mitchell P, Wang JJ. Prevalence and progression of myopic retinopathy in an older population. Ophthalmology. 2002;109(4):704–711.
7. Morgan IG, Ohno-Matsui K, Saw S-M. Myopia. Lancet. 2012;379(9827):1739–1748.
8. Fricke TR, Jong M, Naidoo KS, et al. Global prevalence of visual impairment associated with myopic macular degeneration and temporal trends from 2000 through 2050: systematic review, meta-analysis and modelling. Br J Ophthalmol. 2018;102(7):855–862.
9. Frick KD, Gower EW, Kempen JH, et al. Economic impact of visual impairment and blindness in the United States. Arch Ophthalmol. 2007;125(4):544–550.
10. Fricke T, Holden B, Wilson D, et al. Global cost of correcting vision impairment from uncorrected refractive error. Bull. 2012;90:728–738.
11. Zheng Y-F, Pan C-W, Chay J, et al. The economic cost of myopia in adults aged over 40 years in Singapore. Invest Ophthalmol Vis Sci. 2013;54(12):7532–7537.
12. Huang PC, Hsiao YC, Tsai CY, et al. Protective behaviours of near work and time outdoors in myopia prevalence and progression in myopic children: a 2-year prospective population study. Br J Ophthalmol. 2020;104(7):956–961.
13. Ku PW, Steptoe A, Lai YJ, et al. The associations between near visual activity and incident myopia in children: a nationwide 4-year follow-up study. Ophthalmology. 2019;126(2):214–220.
14. French AN, Morgan IG, Mitchell P, Rose KA. Risk factors for incident myopia in Australian schoolchildren: the Sydney adolescent vascular and eye study. Ophthalmology. 2013;120(10):2100–2108.
15. Huang HM, Chang DS, Wu PC. The association between near work activities and myopia in children—a systematic review and meta-analysis. PLoS One. 2015;10(10):e0140419.
16. French AN, Ashby RS, Morgan IG, Rose KA. Time outdoors and the prevention of myopia. Exp Eye Res. 2013;114:58–68.
17. Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119(10):2141–2151.
18. Saw SM, Matsumura S, Hoang QV. Prevention and management of myopia and myopic pathology. Invest Ophthalmol Vis Sci. 2019;60(2):488–499.
19. Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708.
20. Wilkinson B, Wilson G. Does screening for myopia in New Zealand meet screening programme criteria? N Z Med J. 2020;133(1509):9.
21. Stats NZ [Homepage on the Internet]. Available from: https://www.stats.govt.nz/topics/life-expectancy.
22. Beck JR, Pauker SG. The Markov process in medical prognosis. Med Decis Making. 1983;3(4):419–458.
23. Iskandar R. Adding noise to Markov cohort state‐transition model in decision modeling and cost‐effectiveness analysis. Stat Med. 2020;39(10):1529–1540.
24. Iskandar R. A theoretical foundation for state-transition cohort models in health decision analysis. PLoS One. 2018;13(12):e0205543.
25. Komorowski M, Raffa J. Markov models and cost effectiveness analysis: applications in medical research. In: Secondary Analysis of Electronic Health Records. Springer; 2016:351–367.
26. Petty A, Wilson G. Reducing the impact of the impending myopia epidemic in New Zealand. N Z Med J. 2018;131(1487):80–85.
27. Williams SM, Sanderson GF, Share DL, et al. Refractive error, IQ and reading ability: a longitudinal study from age seven to 11. Dev Med Child Neurol. 1988;30(6):735–742.
28. French AN, Morgan IG, Burlutsky G, et al. Prevalence and 5-to 6-year incidence and progression of myopia and hyperopia in Australian schoolchildren. Ophthalmology. 2013;120(7):1482–1491.
29. Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–1042.
30. Paff T, Oudesluys-Murphy AM, Wolterbeek R, et al. Screening for refractive errors in children: the plusoptiX S08 and the Retinomax K-plus2 performed by a lay screener compared to cycloplegic retinoscopy. J AAPOS. 2010;14(6):478–483.
31. Rajavi Z, Sabbaghi H, Baghini AS, et al. Accuracy and repeatability of refractive error measurements by photorefractometry. J Ophthalmic Vis Res. 2015;10(3):221.
32. Yan X-R, Jiao W-Z, Li Z-W, et al. Performance of the Plusoptix A09 photoscreener in detecting amblyopia risk factors in Chinese children attending an eye clinic. PLoS One. 2015;10(6):e0126052.
33. Sanchez I, Ortiz-Toquero S, Martin R, et al. Advantages, limitations, and diagnostic accuracy of photoscreeners in early detection of amblyopia: a review. Clin Ophthalmol. 2016;10:1365.
34. Howland HC. Photorefraction of eyes: history and future prospects. Optom Vis Sci. 2009;86(6):603–606.
35. Chia A, Lu Q-S TD. Five-year clinical trial on atropine for the treatment of myopia 2: myopia control with atropine 0.01% eyedrops. Ophthalmology. 2016;123(2):391–399.
36. Sacchi M, Serafino M, Villani E, et al. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol Scand. 2019;97(8):e1136–e40.
37. Zhao Y, Feng K, Liu R-B, et al. Atropine 0.01% eye drops slow myopia progression: a systematic review and meta-analysis. Int J Ophthalmol. 2019;12(8):1337.
38. Zhao C, Cai C, Ding Q, et al. Efficacy and safety of atropine to control myopia progression: a systematic review and meta-analysis. BMC Ophthalmol. 2020;20(1):1–8.
39. Chia A, Chua W-H, Wen L, et al. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(2):451–7. e1.
40. Chia A, Chua W-H, Cheung Y-B, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347–354.
41. Brennan NA. Predicted reduction in high myopia for various degrees of myopia control. Cont Lens Anterior Eye. 2012;35:e14–e5.
42. Salomon JA, Haagsma JA, Davis A, et al. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3(11):e712–e23.
43. Braithwaite T, Taylor HR, Bourne RR, et al. Does blindness count? Disability weights for vision loss. Clin Exp Ophthalmol. 2017;45(3):217–220.
44. World Health Organization. Age-specific disability weights for untreated and treated forms of sequelae included in the Global Burden of Disease Study. Available from: https://www.who.int/healthinfo/global_burden_disease/tools_national/en/.
45. Access Economics. Clear Focus - the Economic Impact of Vision Loss in New Zealand; 2009.
46. StatsNZ. Available from: https://www.stats.govt.nz/infographics/major-ethnic-groups-in-new-zealand.
47. Matsumara S, Ching-Yu C, Saw SM. Global Epidemiology of Myopia. In: Ang M, Wong TY, editors. Updates on Myopia: A Clinical Perspective. Springer Nature; 2020:27–51.
48. Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9–25. e12.
49. Payscale.com. Available from: https://www.payscale.com/research/NZ/Job=Audio%2FVisual_Technician/Salary.
50. ocula.co.nz. Available from: https://www.ocula.co.nz/my-treatment/atropine-for-myopia-control/.
51. Optimus Healthcare. Atropine Costs. Optimus Healthcare; 2021.
52. nvision.co.nz. Available from: https://www.nvision.nz/our-eyewear/spectacles-pricing.
53. Yam JC, Zhang XJ, Zhang Y, et al. Three-Year Clinical Trial of Low-Concentration Atropine for Myopia Progression (LAMP). Study: Continued Versus Washout. Phase 3 Report. Ophthalmology. 2021;1:548.
54. Li FF, Zhang Y, Zhang X, et al. Age Effect on Treatment Responses to 0.05%, 0.025%, and 0.01% Atropine: low-Concentration Atropine for Myopia Progression Study. Ophthalmology. 2021;1:548.
55. Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135(6):624–630.
56. Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: a network meta-analysis. Ophthalmology. 2016;123(4):697–708.
57. Diaz-Llopis M, Pinazo-Durán MD. Superdiluted atropine at 0.01% reduces progression in children and adolescents. A 5 year study of safety and effectiveness. Arch Soc Esp Oftalmol. 2018;93(4):182–185.
58. Polling JR, Kok RG, Tideman JW, Meskat B, Klaver CC. Effectiveness study of atropine for progressive myopia in Europeans. Eye. 2016;30(7):998–1004.
59. Ruiz-Moreno J, Roura M. Cost of myopic patients with and without myopic choroidal neovascularisation. Arch Soc Esp Oftalmol. 2016;91(6):265–272.
60. Chuvarayan Y, Finger RP, Köberlein-Neu J. Economic burden of blindness and visual impairment in Germany from a societal perspective: a cost-of-illness study. Eur J Health Econ. 2020;21(1):115–127.
61. Lam CSY, Tang WC, Lee PH, et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br J Ophthalmol. 2021.
62. Lam CS, Tang WC, Lee PH, et al. Myopia control effect of defocus incorporated multiple segments (DIMS) spectacle lens in Chinese children: results of a 3-year follow-up study. Br J Ophthalmol. 2021;1:87.
63. Li Y, Fu Y, Wang K, Liu Z, Shi X, Zhao M. Evaluating the myopia progression control efficacy of defocus incorporated multiple segments (DIMS) lenses and Apollo progressive addition spectacle lenses (PALs) in 6-to 12-year-old children: study protocol for a prospective, multicenter, randomized controlled trial. Trials. 2020;21(1):1–11.
64. Si JK, Tang K, Bi HS, Guo DD, Guo JG, Wang XR. Orthokeratology for myopia control: a meta-analysis. Optom Vis Sci. 2015;92(3):252–257.
65. Cho P, Tan Q. Myopia and orthokeratology for myopia control. Clin Exp Optom. 2019;102(4):364–377.
66. Walline JJ, Jones LA, Sinnott LT. Corneal reshaping and myopia progression. Br J Ophthalmol. 2009;93:1181–1185.
67. Hiraoka T, Kakita T, Okamoto F, et al. Long‐term effect of overnight orthokeratology on axial length elongation in childhood myopia: a 5‐year follow‐up study. Invest Ophthalmol Vis Sci. 2012;53:3913–3919.
68. Anstice NS, Phillips JR. Effect of dual-focus soft contact lens wear on axial myopia progression in children. Ophthalmology. 2011;118(6):1152–1161.
69. Chamberlain P, Peixoto-de-Matos SC, Logan NS, Ngo C, Jones D, Young G. A 3-year randomized clinical trial of MiSight lenses for myopia control. Optom Vis Sci. 2019;96(8):556–567.
70. Ruiz-Pomeda A, Pérez-Sánchez B, Valls I, Prieto-Garrido FL, Gutiérrez-Ortega R, MiSight Assessment V-C-C. Study Spain (MASS). A 2-year randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2018;256(5):1011–1021.
71. Health Partners Consulting Group. Cost-Effectiveness of Newborn Screening for Severe Combined Immune Deficiency. A Report prepared for the National Screening Unit; 2014.
72. Love T, Poynton M, Swansson J. The Cost Effectiveness of Bowel Cancer Screening in New Zealand: A Cost-Utility Analysis Based on Pilot Results. Vol. 2. Wellington Sapere Research Group; 2016:548.
73. Lew JB, Simms K, Smith M, Lewis H, Neal H, Canfell K. Effectiveness modelling and economic evaluation of primary HPV screening for cervical cancer prevention in New Zealand. PLoS One. 2016;11(5):e0151619.
74. Purchasing power parities (PPP). Available from: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm.
75. Baltussen R, Naus J, Limburg H. Cost-effectiveness of screening and correcting refractive errors in school children in Africa, Asia, America and Europe. Health Policy (New York). 2009;89(2):201–215.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

