Back to Journals » International Journal of Nanomedicine » Volume 13
Phase-shift, targeted nanoparticles for ultrasound molecular imaging by low intensity focused ultrasound irradiation
Authors Li M, Luo H, Zhang W, He K, Chen Y, Liu J , Chen J, Wang D, Hao L , Ran H, Zheng Y, Wang Z , Li P
Received 22 February 2018
Accepted for publication 1 May 2018
Published 4 July 2018 Volume 2018:13 Pages 3907—3920
DOI https://doi.org/10.2147/IJN.S166200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Lei Yang
This paper has been retracted.
Maoping Li, 1,2 Hua Luo, 3 Weiyang Zhang, 1 Kunyan He, 4 Yong Chen, 3 Jianxin Liu, 2 Junchen Chen, 5 Dong Wang, 1 Lan Hao, 2 Haitao Ran, 2 Yuanyi Zheng, 2 Zhigang Wang, 2 Pan Li 2
1Department of Ultrasound, The First Affiliated Hospital, Chongqing Medical University, Chongqing 400016, China; 2Institute of Ultrasound Imaging, Chongqing Medical University, Chongqing 400010, China; 3Chongqing Protein way Biotechnology Co., Ltd., Chongqing 400039, China; 4The Fifth Affiliated Hospital of Sun Yat-sen University, Guangzhou, 519000, China; 5Hunan Key Laboratory of Skin Cancer and Psoriasis, Changsha, 410008, China
Purpose: Ultrasound (US) molecular imaging provides a non-invasive way to visualize tumor tissues at molecular and cell levels and could improve diagnosis. One problem of using US molecular imaging is microbubbles challenges, including instability, short circulation time, and poor loading capacity and penetrability. It is urgent to design new acoustic contrast agents and new imaging methods to facilitate tumor-targeted imaging. In this study, phase-shift poly lactic-co-glycolic acid (PLGA) nanoparticles modified with folate as an efficient US molecular probe were designed and the long–term targeted imaging was achieved by low-intensity focused US (LIFU) irradiation.
Methods: A new 5-step method and purification procedure was carried out to obtain uniform folic acid polyethylene glycol PLGA (PLGA-PEG-FA), the structure of which was confirmed by 1H nuclear magnetic resonance spectroscopy and thin-layer chromatography. Perflenapent (PFP) was wrapped in PLGA-PEG-FA by a double emulsion solvent evaporation method to obtain PFP/PLGA-PEG-FA nanoparticles. The targeted ability of the resulting nanoparticles was tested in vivo and in vitro. LIFU irradiation can irritate nanoparticle phase-shift to enhance tumor imaging both in vivo and in vitro.
Results: PLGA-PEG-FA was a light yellow powder with a final purity of at least 98%, the structure of which was confirmed by 1H nuclear magnetic resonance spectroscopy and thin-layer chromatography. Highly dispersed PFP/PLGA-PEG-FA nanoparticles with spherical morphology have an average diameter of 280.9± 33.5 nm, PFP load efficiency of 59.4%± 7.1%, and shells, thickness of 28± 8.63 nm. The nanoparticles can specifically bind to cells expressing high folate receptor both in vivo and in vitro. Ultrasonic imaging was significantly enhanced in vitro and in vivo by LIFU irradiation. The retention time was significantly prolonged in vivo.
Conclusion: Phase-shift PFP/PLGA-PEG-FA nanoparticles induced by LIFU can significantly enhance ultrasonic imaging, specifically targeting tumors expressing folate receptor. As a potential targeting acoustic molecular probe, PFP/PLGA-PEG-FA nanoparticles can be used to achieve targeted localization imaging.
Keywords: folic acid, targeted, phase-shift, nanoparticles, acoustic contrast agent
Corrigendum for this paper has been published
Introduction
Ultrasound (US) molecular imaging provides a non-invasive way to visualize tumor tissues at molecular and cell levels and could improve diagnosis and treatment. US molecular probes can serve as contrast agents for tumor imaging as well as drug vehicles for target therapy. Commonly used US molecular probes, such as targeted microbubbles and perfluorocarbon emulsions are limited by a conflict between US visualization and particle penetration. Targeted microbubbles challenges include instability, short circulation time, and poor loading capacity and penetrability, while perfluorocarbon nanoemulsions that effectively penetrate tumor tissue are limited by poor acoustic echogenicity.1
In recent years, phase-changeable perfluorocarbon nanoemulsions have been developed as novel US molecular probes or contrast agents for possible clinical translation. These phase-changes contrast agents based on an US-triggered phase transition has provided medical researchers with a “middle-ground” between the inert liquid emulsion and gas-based microbubbles contrast agent platforms.2 We previously developed perfluorohexane (PFH)@ poly lactic-co-glycolic acid (PLGA)/Fe3O4 nanocapsules in which phase transition could be induced to form microbubbles and demonstrated that they could serve as contrast agents to enhance US and magnetic resonance imaging (MRI).3 Furthermore, they have synergistic tumor ablation effects when used to enhance near infrared-induced photothermal therapy and high-intensity focused US (HIFU) irradiation, offering a dual-modal, imaging-guided protocol for tumor therapy.
Typical US molecular probes consist of a biodegradable shell, an acoustic core, and ligands on or within the shell targeting to specific sites.4 PLGA possesses characteristics of favorable biocompatibility, controllable degradation, and low toxicity,5 and has been widely used to form the shells of various nanoparticles. Liquid PFH can be converted to gas when the temperature is close to its boiling point (b.p.), thereby generating acoustic bubbles. This typical phase-shift material is often encapsulated into nanocapsules. Phase-changeable polymeric and lipid nanoparticles encapsulating PFH have been produced by many groups.6–8 They can pass through the vascular endothelium gap and penetrate tumor tissue where they are retained due to an enhanced permeability and retention effect. However, because of the relatively high boiling temperature of PFH (58°C–60°C), the phase transition of these nanoparticles requires heating or HIFU exposure, which could cause damage to normal tissue.9,10
In the present work, we prepared phase-shift PLGA nanoparticles encapsulating perflenapent (PFP) with a low b.p. (29°C) as a novel US molecular probe that requires phase transition by low-intensity focused US (LIFU). We added a folate-targeting group to the nanoparticle surface to improve penetrating and targeting abilities. It has been reported that folate receptors (FRs) are overexpressed on the surface of many epithelium-derived malignant tumor cells with good tumor tissue specificity. In contrast to monoclonal antibodies, folic acid (FA) has a low molecular weight and does not induce human immunogenicity, which allows it to penetrate tissue and target tumors.11,12 Here, we show that phase-shift PLGA nanoparticles modified with folate are an efficient US molecular probe. Their basic characteristics were determined, and the targeting effect and phase transition for US imaging were investigated in vitro and in vivo (Figure 1). Collectively, our findings suggest they could be useful for non-invasive tumor imaging and therapy.
Materials and methods
PLGA-polyethylene glycol (PEG)-FA preparation and structure confirmation
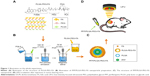
A 5-step method was performed to prepare FA-targeted PEG PLGA (PLGA-PEG-FA) (Figure 2).
Step 1: NH2-PEG-NH2 (MW = 3,500, JenKem, Beijing, China) and Di-Boc (Boc, TCI, Tokyo, Japan) reactions were performed for NH2-PEG-BOC, which was purified with reverse-phase chromatography (purification equipment [NP7030C; Hanbon Science & Technology, Huaian, China]). Step 2: FA (TCI) was mixed with N-hydroxysuccinimide (NHS, TCI) to obtain FA-NHS. Step 3: NH2-PEG-BOC and NHS-FA were mixed, and the products were purified by ion chromatography and reverse-phase chromatography to obtain uniform FA-PEG-NH2. Step 4: PLGA (PLGA-COOH, 50:50, MW = 25,000, Daigang, Jinan, China) was reacted with NHS to obtain PLGA-NHS. Step 5: PLGA-NHS and FA-PEG-NH2 were dissolved, and the mixture was then subjected to dialysis with a molecular weight cutoff of 10 kDa prior to ion chromatography purification and lyophilization to obtain PLGA-PEG-FA.
For 1H nuclear magnetic resonance spectroscopy (1H-NMR) analysis (AV-500, Bruker, Billerica, MA, USA), 20 mg PLGA-PEG-FA was dissolved in dimethyl sulfoxide-d6, with tetramethylsilane as an internal standard.
Thin-layer chromatography (TLC) detection of PEG groups in PLGA-PEG-FA: 10 mg each of PEG, PLGA, PLGA-NHS, PLGA-PEG-FA, FA-PEG-NH2, and FA were dissolved in DMSO, each with a concentration of 1 mg/mL. Each solution was applied on a thin-layer plate (Jiangyou, Yantai, China), sprayed with iodoacetic acid, and stained.
PFP/PLGA-PEG-FA nanoparticle preparation and characterization
PFP/PLGA-PEG-FA nanoparticles were prepared with a double emulsion solvent evaporation method. Briefly, 25 mg PLGA was dissolved in 3 mL dichloromethane (DCM) and added to 200 μL PFP (Sigma-Aldrich Chemical Co., St Louis, MO, USA), the resulting emulsion was obtained using an ultrasonic probe (VCX-130, Sonics & Materials Inc, Newtown, CT, USA) at a constant power output of 100 W for 1 min in a 4°C saline bath. Next, 75 mg PLGA-PEG-FA was dissolved in 2 mL DCM and added into the aforementioned emulsion, followed by shaking for 2.5 min. Next, 3 mL 5% poly (vinyl alcohol) (MW=25,000, Sigma) solution was slowly added with continuous agitation, followed incubation in a saline bath at 4°C with an ultrasonic probe (75 W, 2 min). Agitation was continued until the DCM completely evaporated. The precipitate was collected after centrifugation and washed 3 times, re-suspended with double distilled water to a nanoparticle concentration of 3.6×1015 particles/mL, and divided into 1-mL aliquots that were lyophilized and stored in the dark at −20°C.
To determine PFP load of PFP/PLGA-PEG-FA nanoparticles, we followed the same procedures which are mentioned above to prepare the nanoparticles. After these nanoparticles were collected, DCM was applied to dissolve PFP/PLGA-PEG-FA nanoparticles. Because PLGA-PEG-FA dissolved in DCM but PFP did not, they were separated by centrifugation at low temperature and then were determined. PFP load efficiency was calculated according to the obtained volume from centrifugation and the added volume during the preparation.
The PFP/PLGA-PEG-FA nanoparticles were observed by scanning electron microscopy (SEM; Hitachi S-3400N, Tokyo, Japan) and transmission electron microscopy (TEM; Hitachi H-7600, Japan). The size distribution and zeta potential of PFP/PLGA-PEG-FA nanoparticles were determined by dynamic light scattering with a Malvern Zetasizer Nano ZS unit (Malvern Instruments, Westborough, MA, USA). The pH value was measured, and nanoparticle US images were recorded by 5–12 MHz probe of US machine (Philips iU 22, Amsterdam, the Netherlands) with the parameter setting: 11 MHz, power 85%, Gain 38, Mechanical Index 0.47, Depth 4 cm, Focus Depth 3–4 cm.
Target specificity of PFP/PLGA-PEG-FA nanoparticles
In vitro target specificity
SKOV3 cells expressing high levels of FR and WISH cells not expressing FR were selected to assess target specificity. Using PLGA-PEG-FA-fluorescein isothiocyanate (FITC) and PLGA-PEG-FITC as shell materials, respectively, FITC-labeled nanoparticles (PFP/PLGA-PEG-FA-FITC and PFP/PLGA-PEG-FITC) were prepared according to the method described previously.
SKOV3 cells were cultured in Roswell Park Memorial Institute-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL of penicillin, and 100 U/mL of streptomycin. The cells were maintained in an incubator with a humidified atmosphere containing 5% CO2 at 37°C. Cells in an exponentially growth phase were used for the experiments. All cell culture reagents were purchased from Invitrogen (Carlsbad, CA, USA).
SKOV3 cells were seeded in culture dishes, and stable monolayer was formed 24 h post-seeding. The cells were collected and re-suspended to 106 particles/mL. SKOV3 cells were divided into Group A and B. For Group A: 200 μL of PFP/PLGA-PEG-FA-FITC nanoparticles was added to the cells at the concentrations described in Table 1 (64×104 particles/mL PFP/PLGA-PEG-FITC as a control). For Group B (antagonized group), 10 μL of FA was added to the cells with the concentrations listed in Table 2. Cells were incubated at 37°C for 30 min followed by the addition of 200 μL 64×104 particles/mL PFP/PLGA-PEG-FA-FITC nanoparticles.
For WISH cells, the concentration of PFP/PLGA-PEG-FA-FITC nanoparticles was the same as in Group A of SKOV3 cells (Table 3). Same experimental procedure was used in the aforementioned 3 groups following PFP/PLGA-PEG-FA-FITC nanoparticle treatment, and PBS was used as a negative control.
Different concentrations of these nanoparticles were incubated with SKOV3 or WISH cells at 37°C for 30 min. Then all the cells in each group were washed with PBS 3 times, centrifuged to pellet, and re-suspended for flow cytometer analysis to check the numbers of positive cells. Tubes were wrapped with foil to protect from light whenever possible.
In vivo target specificity
According to the method aforementioned, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI) was added at the same time as PFP to obtain DiI/PFP/PLGA-PEG-FA and DiI/PFP/PLGA-PEG nanoparticles at a concentration of 3.6×1015 particles/mL. All of our experimental protocols were approved by the Institutional Animal Care and Use Committee, Chongqing Medical University. The SKOV3 tumor-bearing nude mice model was described previously.13 Female immunodeficient BALB/c nude mice (6–8 weeks old, 18–24 g) were housed between 19°C and 22°C and humidity conditions. To induce solid tumors, 3×106 cells suspended in 100 μL PBS (pH =7.4) were subcutaneously injected into the right flank of mouse. Nine mice bearing xenograft SKOV3 tumors (10 mm in diameter) were randomly divided into 3 groups, including targeted group (0.2 mL of DiI/PFP/PLGA-PEG-FA injected via tail vein), non-targeted group (equivalent DiI/PFP/PLGA-PEG was injected), and antagonized group (injected with 80 μg/100 μL FA first, then with DiI/PFP/PLGA-PEG-FA 7.2×109 particles/mL for 0.2 mL). Tumor tissues were collected and fixed in 4% paraformaldehyde. Slides were prepared for fluorescent microscope (Olympus CKX41, Tokyo, Japan).
LIFU-induced phase-shift for US imaging
In vitro LIFU-induced phase-shift
Different concentrations of PFP/PLGA-PEG-FA nanoparticles were phase-shift induced by LIFU irritation. The LIFU device was specifically designed and developed in our laboratory (LM.SC051 ACA; Institute of Ultrasound Imaging of Chongqing Medical Sciences, Chongqing, China) with a driving frequency of 1.0 MHz, focal length of 1.5 cm, and focus area of 0.4 cm2. The acoustic intensity in a focal spot is 1.2 W/cm2. The parameter Duty Cycle was 50% and pulse repetition frequency was 0.5 kHz. The PFP/PLGA-PEG-FA nanoparticles with various concentrations were placed in a gel mold (3% agar w/v in distilled water). LIFU probe was placed perpendicular on the surface of the gel mold. Images of nanoparticles were obtained by US.
In vivo LIFU-induced phase-shift
Three concentrations of PFP/PLGA-PEG-FA nanoparticles were used to induce phase-shift by LIFU irritation. Mice were housed as aforementioned. Twelve mice bearing xenograft SKOV3 tumors (10 mm in diameter) were randomly divided into 4 groups: 3.6×1015 particles/mL as high concentration group, 3.6×1013 particles/mL as medium concentration group, 3.6×1011 particles/mL as low concentration group, and H2O/PLGA-PEG-FA nanoparticles as control. Point two of a millilitre of each nanoparticle was injected via tail vein. Five minutes after nanoparticle injection, tumors were irritated by LIFU with acoustic intensity of 1.20 W/cm2 for 2 min. US images were taken in fundamental and harmonic modes.
In vivo enhancement for US molecular imaging at different time
Six mice bearing xenograft SKOV3 tumors (10 mm in diameter) were randomly divided into 2 groups (n=3), and 0.2 mL injections were given via the tail vein. One group was injected with 3.6×1015 particles/mL PFP/PLGA-PEG-FA nanoparticles, and the other with SonoVue (Bracco International B.V., Milan, Italy) as a control. Then the tumors were irradiated by LIFU (1.20 W/cm2, 2 min) at 5 and 20 min, and 2 and 4 h after the injection. Finally, US images were observed in fundamental and harmonic modes.
Image analysis and statistical method
All images were analyzed by ImageJ (NIH, Bethesda, MD, USA). All data were presented as mean ± SD. Differences were considered statistically significant if P<0.05. The data were analyzed by Statistical Package for the Social Sciences software (SPSS; IBM, Armonk, NY, USA). One-way analysis of variance was used among multiple groups, and statistical analysis was performed between 2 groups if P<0.05. The least significant difference test was used for comparison between 2 groups if equal variances were assumed, and Dunnett T3 test was used if equal variances were not assumed.
Results
Preparation and structure confirmation of PLGA-PEG-FA
After several purifications, we produced a light yellow powder with a final purity of at least 98%. All the characteristic peaks were verified by 1H-NMR.
TLC results showed coloration in lanes 4 and 5, indicating that PEG groups were contained in PLGA-PEG-FA and FA-PEG-NH2 (Figure 3).
PFP/PLGA-PEG-FA nanoparticle preparation and characterization
The nanoparticles were a white emulsion and separated if left to stand. PFP load efficiency is obtained by demulsification. For the added volume during the preparation of 200 μL, the resulting PFP load efficiency was 59.4%±7.1%.
The diameter measured as a single peak with a mean of 280.9±33.5 nm, the surface was negatively charged with an electric potential of −56.9±14.8 mV, and the pH value was 7.2±0.6. Both SEM and TEM revealed smooth and uniform spherical morphology. TEM showed PFP/PLGA-PEG-FA nanoparticles texture clearly, the inside core was PFP, with high electronic density, and appears black whereas the polymeric shell seemed lighter. The nanoparticles polymer shell thickness was 28±8.63 nm and could be measured by TEM. US showed hyperechogenicity of dense, evenly distributed dots (Figure 4).
Target specificity of PFP/PLGA-PEG-FA nanoparticles
In vitro target specificity
After incubation with different concentrations of PFP/PLGA-PEG-FA-FITC nanoparticles, the amount of FITC-positive SKOV3 cells, which highly express FR, was increased in a dose-dependent manner in the range of 1–8×104 particles/mL concentration (P<0.05 or P<0.01, Table 1). The number of positive SKOV3 cells did not increase following treatment with concentrations of nanoparticles at and above 8×104 particles/mL (P>0.05, Table 1). Binding between PFP/PLGA-PEG-FA-FITC nanoparticles and FR followed an S-shape curve (Figure 5), which is consistent with the receptor-ligand interaction pattern. No positive cells were detected in the negative control group (PFP/PLGA-PEG-FITC) and in WISH cells that do not express FR, following incubation with different concentrations of PFP/PLGA-PEG-FA-FITC nanoparticles (P>0.05, Table 2). The result showed that compared with the control group, the positive cells rate were not statistically significant.
Different concentrations of FA were incubated with SKOV3 cells, which were then treated with the same concentration of PFP/PLGA-PEG-FA-FITC nanoparticles. In this case, the percentage of FITC-positive cells was negatively correlated with the FA concentration (P<0.01), and positive cells appeared to be undetectable under 200 pg/mL FA (Table 3).
The results which are mentioned above demonstrated that PFP/PLGA-PEG-FA nanoparticles can specifically bind to FR in vitro; FA can block those combinations.
In vivo target specificity
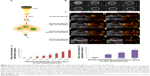
DiI is a red fluorescence dye. In targeted group, the mean fluorescence intensity was significantly higher than those in other groups (P<0.01, Table S1), which indicated that DiI/PFP/PLGA-PEG-FA nanoparticles bound to FRs. No difference was found in the fluorescence intensity between antagonized group and non-targeting group (P>0.05, Table S1), which confirmed the targeting mechanism via folate (Figure 6).
The result showed PFP/PLGA-PEG-FA nanoparticles can specifically bind with FR not only in vitro, but also in vivo.
LIFU-induced phase-shift for US imaging
In vitro LIFU-induced phase-shift
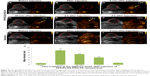
Different concentrations of PFP/PLGA-PEG-FA nanoparticles were used to induce phase-shift by LIFU irritation (Figure 7A). The results showed that nanoparticles could still be converted into gas bubbles at concentration as low as 3.6×103 particles/mL. However, the enhancement effect for US was positively correlated with the nanoparticle concentration after LIFU induction (P<0.05 or P<0.01, Table S2). The nanoparticles at higher concentration produced better enhancement for US imaging (Figure 7B and D).
In vivo LIFU-induced phase-shift
Three concentrations of PFP/PLGA-PEG-FA nanoparticles were used to induce phase-shift by LIFU irritation. When tumors were irradiated with LIFU (1.20 W/cm2, 2 min), the irradiated area exhibited enhanced echoes in harmonic mode. The results from 3 groups for enhancement of US imaging have significant difference compared with the control group. The enhancement effect is positively correlated to the concentration (P<0.01, Table S3), with the group with a high concentration having the best enhancement effect (Figure 7C and E).
In vivo enhancement for US molecular imaging at different times
Before and after PFP/PLGA-PEG-FA nanoparticle injection, there were no remarkable changes in tumor imaging for either mode. When the tumor was irradiated with LIFU (1.20 W/cm2, 2 min), the irradiated area exhibited slightly enhanced echoes in fundamental mode but distinct enhancement in harmonic mode. The irradiated area showed scattered dots that completely disappeared after ~5 min. The tumor was again irradiated with LIFU 20 min after injection and exhibited slightly enhanced echoes in fundamental mode and remarkably enhanced images in harmonic mode. These signals also disappeared completely about 5 min later. Two hours after injection, the tumor was irradiated by LIFU for a third time, and slightly enhanced images were noted. On the final LIFU application 4 h after injection, no enhancement was observed. (P<0.01, Table S4). For control group, 10 s after SonoVue injection into the tail vein of tumor-bearing nude mice, imaging in harmonic mode revealed inhomogeneous high enhancement with some unenhanced areas. After 2 min, the contrast agent had almost washed out, and no signal was observed. The tumor image did not change after LIFU irritation (Figure 8).
Discussion
Precise diagnosis and treatment planning require visualizing tumors at molecular and cellular levels. US molecular imaging technology provides a non-invasive method to identify malignant tumors at an early stage and quantitatively evaluate their development and evolution. Nanoscaled US contrast agents can penetrate tumor tissues and accumulate in the local area. In this work, phase-shift, folate-targeted PLGA nanoparticles were developed as a novel US molecular probe for tumor imaging. The PFP/PLGA-PEG-FA nanoparticles had a mean diameter of 280.9±33.5 nm, allowing them to extravasate into tumor tissue with defective vasculature, including enlarged endothelial gaps. In addition, the small molecule folate was bound to the nanoparticle surface to improve penetrability compared with monoclonal antibodies, which are commonly used to prepare US molecular probes. This natural ligand also allows nanoparticles to specifically accumulate near tumors. To improve nanoparticle quality and targeting efficiency, the shell material PLGA-PEG-FA was synthesized, purified, and confirmed. The most important step is obtaining uniform PLGA-PEG-FA, which we achieved with a 5-step synthesis method. These laid a good foundation for the preparation of targeting nanoparticles. Furthermore, ion chromatography and reverse-phase chromatography were employed to ensure high purity PLGA-PEG-FA.
PFP load is 59.4%±7.1% in our study. Since the particles are prepared by sonication, the energy input is very high, which will have a substantial impact on the final product.14 This was the main reason that parts of PFP were lost during this procedure.
PFP/PLGA-PEG-FA nanoparticle targeting requires that folate ligands are exposed on the exterior surface. We developed a new double-emulsion method based on that described by Esmaeili et al15 and Chen et al.16 Our in vitro results showed that PFP/PLGA-PEG-FA nanoparticles targeted to SKOV3 cells with high FR expression. The conjugation occurred in a dose-dependent way and was competitively inhibited by the FA addition, indicating specific binding between nanoparticles and FRs. Furthermore, the in vivo results confirmed red fluorescence accumulation in tumor tissue that was not observed in the non-targeted and antagonized groups, indicating high specificity of PFP/PLGA-PEG-FA nanoparticles.17
A number of studies have demonstrated that US can induce liquid-gas phase transformation to generate perfluorocarbon bubbles for enhancing US imaging.14,18,19 The mechanisms of acoustic droplet vaporation mainly include US-inherent biological effects, such as cavitation and mechanical effects and thermal effects, which trigger the phase transition of lipid perfluorocarbon encapsulated in the nanoparticle core, thus producing gas bubbles.20–23 Commonly used PFH is converted into gas under HIFU, while the liquid-gas phase shift in PFP can be induced by LIFU due to its much lower b.p.24,25 In the present work, PFP was encapsulated by biodegradable FA-modified PLGA materials to form phase-shift nanoparticles that could be transformed to gas bubbles to enhance US molecular imaging under LIFU.
The LIFU device was specially designed and developed in our laboratory with a driving frequency of 1.0 MHz, focal length of 1.5 cm, and focus area of 0.4 cm2. The acoustic intensity in a focal spot of 1.2 W/cm2 was applied for 2 min to induce phase transition. Our in vitro and in vivo results showed that PFP/PLGA-PEG-FA nanoparticles enhanced US imaging in tumor tissue following LIFU irradiation. Notably, enhancement was still possible 2 h after nanoparticle administration, demonstrating long retention time in tumor areas. More importantly, localized imaging was accomplished. Owing to the good penetrability of US and favorable focusing effect of LIFU, deeper sites can be viewed, but enhancement was not found in non-irradiated areas, indicating that gas bubbles were generated by LIFU-induced phase shift to enhance tumor imaging.
Conclusion
Here, we employed new methods to synthesize highly pure shell materials and to prepare the nanoparticles with the targeting ligand facing outward. Then, phase-shifted and folate-targeted PLGA nanoparticles encapsulating liquid perfluorocarbon with a low b.p. were successfully developed. Both in vitro and in vivo experiments suggested that the nanoparticles possessed favorable characteristics for use as a contrast agent, and their targeting ability and specificity to tumor tissue accumulation were verified. More important, phase shift was induced in these nanoparticles by LIFU irradiation for US imaging, indicating that the nanoparticles could serve as molecular probes for targeted tumor imaging. Combining folate-targeted PLGA nanoparticles with LIFU application provides a novel diagnostic protocol for tumors.
Acknowledgments
The authors are grateful to Chao Yu for technical support with flow cytometry and to Donghan Yang for providing helpful suggestions. This work was supported by the National Natural Science Foundation of China (Grant Nos 81227801, 81601513, 81630047, 31630026, 81371578, 81571688, 81371718), National Distinguished Young Scholars (Grant No 81425014), National Research Program of China (973 Program, Grant No 2011CB707905), Grant of Chongqing Medical University, China (Grant No CXTDG 201602007), and General Program of Health and Family Planning Commission of Chongqing, China (Grant No 2010-2-115).
Disclosure
The authors report no conflicts of interest in this work.
References
Rapoport N. Phase-Shift, Stimuli-responsive perfluorocarbon nanodroplets for drug delivery to cancer. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4:492–510. | ||
Sheeran PS, Dayton PA. Phase-change contrast agents for imaging and therapy. Curr Pharm Des. 2012;18(15):2152–2165. | ||
Zhao Y, Song W, Wang D, et al. Phase-shifted PFH@PLGA/Fe3O4 nanocapsules for MRI/US imaging and photothermal therapy with near-infrared irradiation. ACS Appl Mater Interfaces. 2015;7(26):14231–14242. | ||
Granja A, Vieira AC, Chaves LL, et al. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chem. 2017;237:803–810. | ||
Amiji MM. Nanotechnology for Cancer Therapy. Boca Raton: CRC; 2006:243–250. | ||
Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev. 2004;56(8):1127–1141. | ||
Leamon CP. Folate-targeted drug strategies for the treatment of cancer. Curr Opin Investig Drugs. 2008;9(12):1277–1286. | ||
Werner ME, Karve S, Sukumar R, et al. Folate-targeted nanoparticle delivery of chemo-and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32(33):8548–8554. | ||
Huang J, Xu JS, Xu RX. Heat-sensitive microbubbles for intraoperative assessment of cancer ablation margins. Biomaterials. 2010;31(6):1278–1286. | ||
Strohm E, Rui M, Gorelikov I, Matsuura N, Kolios M. Vaporization of perfluorocarbon droplets using optical irradiation. Biomed Opt Express. 2011;2(6):1432–1442. | ||
Moore JL. The significance of folic acid for epilepsy patients. Epilepsy Behav. 2005;7(2):172–181. | ||
Lazarou C, Kapsou M. The role of folic acid in prevention and treatment of depression: an overiew of existing evidence and implications for practice. Complement Ther Clin Pract. 2010;16(3):161–166. | ||
Sun Y, Zheng Y, Ran H, et al. Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablation. Biomaterials. 2012;33(24):5854–5864. | ||
Sheeran PS, Matsuura N, Borden MA, et al. Methods of generating submicrometer phase-shift perfluorocarbon droplets for applications in medical ultrasonography. IEEE Trans Ultrason Ferroelectr Freq Control. 2017;64(1):252–263. | ||
Esmaeili F, Ghahremani MH, Ostad SN, et al. Folate-receptor-targeted delivery of docetaxel nanoparticles prepared by PLGA-PEG-folate conjugate. J Drug Target. 2008;16(5):415–423. | ||
Chen J, Li S, Shen Q, He H, Zhang Y. Enhanced cellular uptake of folic acid–conjugated PLGA–PEG nanoparticles loaded with vincristine sulfate in human breast cancer. Drug Dev Ind Pharm. 2011;37(11):1339–1346. | ||
Kaneda MM, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37(10):1922–1933. | ||
Rapoport NY, Efros AL, Christensen DA, Kennedy AM, Nam KH. Microbubble generation in phase-shift nanoemulsions used as anticancer drug carriers. Bubble Sci Eng Technol. 2009;1(1–2):31–39. | ||
Pisani E, Tsapis N, Paris J, Nicolas V, Cattel L, Fattal E. Polymeric nano/microcapsules of liquid perfluorocarbons for ultrasonic imaging: physical characterization. Langmuir. 2006;22(9):4397–4402. | ||
Fabiilli ML, Haworth KJ, Sebastian IE, Kripfgans OD, Carson PL, Fowlkes JB. Delivery of chlorambucil using an acoustically-triggered perfluoropentane emulsion. Ultrasound Med Biol. 2010;36(8):1364–1375. | ||
Strohm E, Rui M, Gorelikov I, Matsuura N, Kolios M. Optical droplet vaporization of micron-sized perfluorocarbon droplets and their photoacoustic detection. Proc SPIE Int Soc Opt Eng. 7899:78993H–78993H-7. | ||
Cosco, D, Fattal E, Fresta M, Tsapis N. Perfluorocarbon-loaded micro and nanosystems for medical imaging: a state of the art. J Fluorine Chem. 2015;171:18–26. | ||
Strohm E, Gorelikov I, Matsuura N, Kolios M. Photoacoustic spectral characterization of perfluorocarbon droplets[C]//SPIE BiOS. International Society for Optics and Photonics. 2012;82232F-82232F-8. | ||
Díaz-López R, Tsapis N, Fattal E. Liquid perfluorocarbons as contrast agents for ultrasonography and 19F-MRI. Pharm Res. 2010;27(1):1–16. | ||
Zhang P, Porter T. An in vitro study of a phase-shift nanoemulsion: a potential nucleation agent for bubble-enhanced HIFU tumor ablation. Ultrasound Med Biol. 2010;36(11):1856–1866. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.








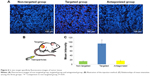
 ” Comparison to non-targeted group, P<0.01.
” Comparison to non-targeted group, P<0.01.