Back to Journals » Journal of Hepatocellular Carcinoma » Volume 8
Phase I Trial on Arterial Embolization with Hypoxia Activated Tirapazamine for Unresectable Hepatocellular Carcinoma
Authors Abi-Jaoudeh N , Dayyani F , Chen PJ, Fernando D , Fidelman N , Javan H, Liang PC, Hwang JI, Imagawa DK
Received 3 February 2021
Accepted for publication 20 April 2021
Published 17 May 2021 Volume 2021:8 Pages 421—434
DOI https://doi.org/10.2147/JHC.S304275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmed Kaseb
Video abstract of "Tirapazamine embolization for unresectable hepatocellular carcinoma" [ID 304275].
Views: 194
Nadine Abi-Jaoudeh,1 Farshid Dayyani,2 Pei Jer Chen,3 Dayantha Fernando,1 Nicholas Fidelman,4 Hanna Javan,1 Po-Chin Liang,5 Jen-I Hwang,6 David K Imagawa7
1Department of Radiological Sciences, University of California Irvine, Orange, CA, USA; 2Chao Comprehensive Digestive Disease, University of California Irvine, Orange, CA, USA; 3Hepatitis Research Center, National Taiwan University, Taipei City, Taiwan; 4Department of Radiology, University of California San Francisco, San Francisco, CA, USA; 5Department of Medical Imaging, National Taiwan University, Taipei City, Taiwan; 6Department of Radiology, Taichung Veteran General Hospital, Taichung, Taiwan; 7Surgery Services, University of California Irvine, Orange, CA, USA
Correspondence: Nadine Abi-Jaoudeh
University of California Irvine, Department of Radiological Sciences, 101 The City Drive South Route, 140 Rm 115, Orange, CA, 92868, USA
Tel +1-540-292-6567
Email [email protected]
Background: Tirapazamine (TPZ) is a hypoxia activated drug that may be synergistic with transarterial embolization (TAE). The primary objective was to evaluate the safety of combining TPZ and TAE in patients with unresectable HCC and determine the optimal dose for Phase II.
Methods: This was a Phase 1 multicenter, open-label, non-randomized trial with a classic 3+3 dose escalation and an expansion cohort in patients with unresectable HCC, Child Pugh A, ECOG 0 or 1. Two initial cohorts consisted of I.V. administration of Tirapazamine followed by superselective TAE while the remaining three cohorts underwent intraarterial administration of Tirapazamine with superselective TAE. Safety and tolerability were assessed using NCI CTCAE 4.0 with clinical, imaging and laboratory examinations including pharmacokinetic (PK) analysis and an electrocardiogram 1 day pre-dose, at 1, 2, 4, 6, 10, and 24 hours post-TPZ infusion and an additional PK at 15- and 30-minutes post-TPZ. Tumor responses were evaluated using mRECIST criteria.
Results: Twenty-seven patients (mean [range] age of 66.4 [37– 79] years) with unresectable HCC were enrolled between July 2015 and January 2018. Two patients were lost to follow-up. Mean tumor size was 6.53 cm ± 2.60 cm with a median of two lesions per patient. Dose limiting toxicity and maximum tolerated dose were not reached. The maximal TPZ dose was 10 mg/m2 I.V. and 20 mg/m2 I.A. One adverse event (AE) was reported in all patients with fatigue, decreased appetite or pain being most common. Grade 3– 5 AE were hypertension and transient elevation of AST/ALT in 70.4% of patients. No serious AE were drug related. Sixty percent (95% CI=38.7– 78.9) achieved complete response (CR), and 84% (95% CI=63.9– 95.5) had complete and partial response per mRECIST for target lesions.
Discussion: TAE with TPZ was safe and tolerable with encouraging results justifying pursuit of a Phase II trial.
Keywords: phase I trial, hypoxia activated agent, hepatocellular carcinoma, image guided locoregional therapies, transarterial chemoembolization
The Barcelona Clinic Liver Cancer (BCLC) staging system recommends transarterial chemoembolization (TACE) for intermediate stage hepatocellular carcinoma (HCC), as level 1A evidence has shown TACE extends survival.1,2 TACE is the most common palliative treatment offered to patients with intermediate or advanced HCC.3 The overall response rate of TACE is 52% in a recent systematic review of 10,000 patients.4 Several technical iterations have been attempted to improve outcomes.5,6 Prospective randomized controlled trials (RCT) comparing drug-eluting bead chemoembolization (DEB-TACE) vs conventional TACE (cTACE) failed to show improved response or survival with beads.7–9 Prospective RCT comparing TACE vs transarterial bland embolization (TAE) also failed to show improved response or survival with the addition of cytotoxic chemotherapy.10 Although survival outcomes of TACE have improved in the past decade, these modest gains are attributed to improvements in imaging and microcatheter technologies enabling superselective treatment.4,11,12 TACE is considered palliative because TACE induces ischemia but it is not uniform with areas of non-lethal hypoxia. The latter activates hypoxia induced factor 1α and vascular endothelial growth factor (VEGF), survival and natural selection of cancer stem cells that lead inevitably to relapse.12–14 Several prospective RCT combining sorafenib, an anti-VEGF agent (Sorafenib, Bayer Leverkusen, Germany) with TACE yielded negative results.12,15–17 However, local delivery of a hypoxia-activated agent would potentially be synergistic with embolization compared to cytotoxic agents currently used in TACE. Tirapazamine (TPZ) is a prodrug that is selectively toxic to hypoxic cells in solid tumors. In a hypoxic environment, TPZ undergoes an enzymatic reduction into hydroxyl free radicals that non-selectively induce DNA damage and cell necrosis. TPZ in a hypoxic environment can induce cell death nonselectively, even in cancer stem cells which have been implicated as a mechanism of TACE failure.18–21 Some safety data is available on TPZ as several Phase III RCT studied its effect when administered intra-venously (I.V.) with chemotherapy in patients with cervical, head, and neck and non-small cell lung cancers.22–24 Those studies all failed to show any benefit to adding TPZ without using an approach to ensure sustained tumor hypoxia to activate it. However, preliminary in-vivo studies combining TPZ and hepatic artery ligation in a mouse model demonstrated encouraging results.18 We performed a Phase I dose escalation study combining TPZ with TAE in patients with unresectable HCC.
Materials and Methods
All regulatory and ethics approvals were obtained from the Food and Drug Administration with an investigational new drug filed. In addition, approval from the hepatobiliary disease-oriented team (DOT) as well as the University of California Irvine’s institutional review board (IRB), University of California San Francisco IRB, Taichung Veteran General Hospital IRB, and National Taiwan University IRB were obtained. This trial was registered on ClinicalTrials.gov identifier: NCT02174549 and performed in accordance with the Declaration of Helsinki. Strobe statement is provided in Supplemental Table S1. This was a multicenter open label non-randomized Phase I study with a classic three + three dose escalation design and an expansion cohort conducted at four tertiary care centers in the United States and Taiwan. All participants provided written informed consent. The goal of the study was to determine the safety, tolerability, toxicity, and pharmacokinetics of TPZ followed by TAE. The study was performed to determine the recommended dose of TPZ for the Phase II trial as well as obtain preliminary tumor response data.
Patients recruited on the trial had HCC according to American Association for the Study of Liver Disease or biopsy.1,25 Five patients (18.5%) were BCLC A and 22 patients (81.5%) were BCLC B. The BCLC A patients were not operative nor ablation candidates per tumor board decision because of severe portal hypertension, severe comorbidities, or location of the lesion. Patients could not have more than four lesions, with the largest measuring less than 10 cm in diameter. Tumor volume could not exceed 50% of the liver volume. Patients were Child-Pugh A, ECOG 0 or 1, with platelets ≥75,000/µL, t-bilirubin≤ 2 mg/dL, and AST/ALT<5-times the upper limit of normal. Patients with clinical hypoxia defined as O2 saturation less than 92% on room air were excluded; as were patients who suffered from arterial insufficiency or microangiopathy as evidenced by gangrenous changes, since TPZ may lead to deterioration. Patients with a history of stroke or acute myocardial infarction (AMI) within the past 6 months were excluded due to concern that TPZ may trigger a cardiovascular event. Patients with remote history (>6 months) of stroke or AMI or stable angina were allowed since TPZ’s half-life is short, ~40 minutes, and patients were monitored overnight to ensure safety. Patients with prolonged QTc (>470 ms), patients with a known diagnosis of cancer other than HCC, and pregnant women were also excluded.
Two initial cohorts were performed with I.V. administration of TPZ (5 and 10 mg/m2) followed by TAE. Intra-venous IV administration of TPZ cohorts were performed at the request of the FDA. The remaining three cohorts consisted of intra-arterial (I.A.) administration of TPZ at 5 mg/m2, 10 mg/m2, and 20 mg/m2 doses followed by TAE with an expansion cohort performed at 20 mg/m2 or rounded to a fixed dose of 35 mg per treatment. TPZ was administered first to allow homogenous distribution throughout the tumor followed by TAE to induce hypoxia and activate the TPZ. The intra-arterial administration of TPZ was the preferred route due to several advantages including greater local concentration throughout the tumor when TPZ is administered directly in the tumor supplying artery vs systemically where it would have to circulate throughout the body. Secondly, the half-life of TPZ is only 40 minutes. With systemic administration, enough time must be given for the TPZ to circulate into the tumor but, at the same time, embolization must be performed quickly to induce hypoxia and activate the TPZ within 40 minutes. This becomes challenging when patients have several vessels supplying tumors that must each be catheterized and embolized within 40 minutes of TPZ administration. The concern with mixing TPZ with TAE was the water–oil emulsion stability and the potential of heterogenous distribution of TPZ throughout the tumor cells. Heterogenous distribution was seen in a VX2 rabbit tumor model treated with conventional TACE.26
The majority of procedures were performed under general anesthesia (42 procedures), while 19 were performed under conscious sedation. General anesthesia was chosen based on comorbidities, patient preference, and the inability to hold still or cooperate with breathing instructions. Patients were placed supine on an angiographic table, access was obtained from the common femoral artery using the Seldinger technique with standard upsize to a 5Fr sheath. Selective catheterization of the celiac artery was performed using a standard 5Fr catheter with a dual phase cone beam computed tomography (Clarity FD20, Philips, NL) using contrast administration of 2 mL/s for a total of 20 mL with a 5 second acquisition delay. A microcatheter was inserted co-axially and used for superselective catheterization of each tumor feeding artery. For the initial six patients, once the microcatheter was positioned in the tumor feeding artery, TPZ was injected I.V. from a venous access line at a rate of 6 mL/min. After TPZ injection, the operator waited 5 min to allow drug circulation before starting the embolization in a superselective fashion using a Lipiodol and Gelfoam slurry. If multiple tumor supplying vessels were present, then selective catheterization and embolization was performed sequentially. In the remaining three cohorts, once superselective catheterization of a tumor vessel had occurred, TPZ was injected I.A. through the microcatheter at a rate of 6 mL/min. Five minutes post-TPZ infusion, embolization was performed using a Lipiodol and Gelfoam slurry. If the tumor was supplied by multiple arteries, the TPZ volume was divided by the number of feeding vessels.
If more than one lesion was present, tumors were segmented, total tumor volume was obtained, and the proportion of each lesion relative to the total tumor volume was calculated. The TPZ volume was divided between the tumors proportionally to each tumor’s volume.
Blood samples were collected for pharmacokinetic analysis 1 day pre-dose, at 15, and 30 minutes, 1, 2, 4, 6, 10, and 24 hours after the end of TPZ infusion. An electrocardiogram was obtained at 1 day pre-dose, 1, 2, 4, 6, 10, and 24 hours post-TPZ infusion. Patients remained under observation for at least 24 hours post-procedure. Patients were discharged if they did not experience significant adverse events (AE) and laboratory tests did not demonstrate grade three or higher elevation of AST/ALT/total-bilirubin. A complete review of systems and physical examination, complete metabolic panel, complete blood count, alpha fetoprotein (AFP), and adverse events (AE) per CTCAE v 4.0 were assessed daily during hospitalization, then on days 8, 15, 22, 56, and every 56 days after that. Repeat TPZ and TAE was done “on demand” when viable tumor was detected, and the patient was considered suitable for embolization. Viable tumor included residual or recurrent tumor or new tumor in untreated area as embolization is a localized treatment performed in a superselective manner in this study. If patients underwent a second embolization, assessment as detailed above was performed daily while in hospital, then on days 8, 29, 56, and then every 56 days post-procedure. Repeat embolization was also performed with TPZ followed by embolization as described above. Patients remained in their initial assigned cohort in terms of administration route and dose of TPZ. Meaning, a patient assigned to the 5 mg/m2 TPZ I.V. administration cohort would receive that same dose and administration route for the initial procedure and any repeat procedure required.
Clinic visits including a review of systems and physical examination, imaging, complete metabolic panel, complete blood count, AFP, as well as hepatitis testing for positive patients were performed every 56 days. Imaging consisted of dynamic contrast-enhanced magnetic resonance imaging with subtraction to avoid the ambiguity related to Lipiodol accumulation within the tumor.
RECIST and mRECIST were evaluated on every scan by an independent diagnostic radiologist at the same institution. Efficacy was evaluated using mRECIST and RECIST criteria to determine complete response rate (CR), overall response rate (ORR) defined as CR and partial response, duration of CR and duration of ORR. Target lesion response was measured in the TPZ with TAE treated lesions and de novo lesions were evaluated and assessed in the overall response. ORR is defined as the best overall response rate by either mRECIST or RECIST regardless of when it was achieved during the study. The duration of response is defined as the first time CR or PR was achieved until documented mRECIST progression either by a 20% increase in the tumor size or the appearance of new lesions. Progression free survival (PFS) and overall survival (OS) were also evaluated. PFS is defined by the first documented progression by mRECIST or RECIST criteria, ie, tumor size increase by 20% or appearance of new lesions. Although TPZ embolization is a superselective localized therapy, PFS was censored at progression of an existing lesion or at the first appearance of a new lesion. Meaning a new lesion in an untreated area of the liver was considered as progression even if the initial lesion still demonstrated complete response and the second lesion also achieved complete response after treatment.
Statistical Analysis
The dose escalation study was planned as a 3+3 classic design. In addition, an expansion cohort was planned. The sample size was calculated based on the anticipated improvement of 30% in complete response compared to the historic control, ie, TACE. With 15 patients in the expansion cohort, the study had approximately 80% power to rule out the null hypothesis at the 5% type I error (one-sided). Descriptive tables that summarize the number and percentage of patients that experienced toxicity as categorized in the NCI CTCAE version 4.0 were generated for the overall patient population and by patient-specific dose level. For both dose escalation and expansion cohorts, CR rate and ORR by the modified RECIST criteria within the territory of embolization were used as the endpoints for efficacy assessment of the benefit of TPZ in HCC patients. A 90% confidence interval based on the binomial distribution was provided for both endpoints. Time to progression, PFS, and OS were calculated based on Kaplan–Meier method and log-log transformation for the pointwise confidence interval. The OS was based on intent to treat. Waterfall plot and spider plots were used to assess response and duration of response.
Results
Twenty-seven participants (17 men and 10 women, median [range] age=68 [37–79] years) were enrolled between July 2015 and January 2018. All patients were reviewed at tumor board in each of the respective institutions and deemed unresectable by the hepatobiliary surgeons. A flow chart was provided with the patients (see Figure 1). Twelve participants were Asian, nine Caucasian, and six Hispanic. All patients were Child Pugh A, with 20 having a score of 5, the remaining seven had a score of 6. The ALBI score was 1 in 19 patients, 2 in six patients, and 3 in two patients. The mean tumor size was 6.53±2.60 cm. Median number of target lesions per patient was two.
 |
Figure 1 Flow chart of patients. |
AFP levels were above 20 ng/mL (upper limit of normal) in 16 patients and greater than 200 ng/mL in six patients (shown in Table 1).
 |
Table 1 Demographics of the Patients Enrolled in the Phase I Trial |
All patients were treatment naïve. All patients received at least one embolization cycle. Eight patients received two cycles, eight received three cycles, two received four cycles, and one had 5 cycles during the course of their follow-up. The average number of treatments per patient was 2.2 over a median follow-up of 303 days (range=23–856). Six patients were assigned to I.V. TPZ cohorts, three received a 5 mg/m2 dose and three a 10 mg/m2 dose. Three patients were assigned to I.A. TPZ cohort at 5 mg/m2, another three at 10 mg/m2, and the remaining participants were assigned to 20 mg/m2 I.A.
All patients tolerated the transarterial Tirapazamine embolization (TATE) therapy well without dose limiting toxicity. No dose reduction was needed. The maximum tolerated dose was not established. Mean TPZ exposures increased proportionally with the dose for both I.V. and I.A. administration routes. The dose escalation was stopped as the concerns of increasing TPZ injection volume beyond 50 mL (20mg/m2) of a pH 4 solution in one session outweighed the residual margin for potential improvement in the response.
The adverse events affecting more than 5% of patients included fatigue (37%), abdominal pain (29.6%), nausea (25.9%), vomiting (22.2%), decreased appetite (25.9%), fever (22.2%), and increased AST and ALT (14.8%) that renormalized within 10 days consistent with post-embolization syndrome (provided in Table 2). Seven patients (25.9%) experienced very transient hypertension. All episodes of hypertension were intraprocedural and coincided with TPZ administration. Initial patients under conscious sedation expressed discomfort and those under general anesthesia demonstrated an increased heart rate and blood pressure during TPZ administration. In subsequent patients, an analgesic bolus was given before TPZ injection. Serious AEs (SAE) are listed in Table 3, although none were considered as drug related. One patient experienced chest pain post-procedure attributed to dyspepsia as the electrocardiogram and troponins were negative, and the patient’s symptoms resolved with proton pump inhibitors and anti-nausea medications. One patient did not respond to treatment, demonstrated progressive disease, and eventually died from progression.
 |
Table 2 Subjects with Post-Treatment AEs by CTCAE Grades in Descending Frequency (All with Frequency Above 5%) |
 |
Table 3 Serious Adverse Events (SAE) |
Severe cirrhosis and portal hypertension were present at screening on imaging in 59% of cases. None of the patients experienced worsening varices or variceal bleeding during follow-up. There was no statistical difference between AST, ALT, bilirubin, albumin at screening, day 56 of cycle 1 or last follow-up. All patients but three remained Child Pugh A until their final follow-up on study. Two patients had decreased albumin from 2.8 to 2.7 and 2.5 respectively at their day 56 cycle 1 visit. Neither had further deterioration in their liver function. The third patient had stable liver function at day 56 of cycle 1 but during follow-up developed increased bilirubin and ascites attributed to disease progression and portal vein invasion.
Twenty-five patients had a response assessment. One patient came to the initial three clinical weekly follow-ups but moved abroad before the first imaging follow-up. A second patient had a microwave ablation at an outside hospital after his procedure before his imaging follow-up and was excluded from the response analysis. The target and overall lesion responses by mRECIST and RECIST are show in Table 4, as well as overall response rate (CR+PR) and the disease control rate (DCR). Sixty percent (95% CI=38.7–78.9) of the treated patients achieved target lesion CR by mRECIST criteria and 84% (95% CI=63.9–95.5) achieved ORR by mRECIST (shown in Table 4 and Figure 2).
 |
Table 4 Detailed Responses for Target Lesions and Overall Lesions Including Overall Response Rate and Disease Control Rate (by mRECIST and RECIST Criteria) |
All but four patients achieved CR after the first treatment. Of the four patients requiring two procedures to achieve CR, two patients were in the I.V. cohort with lesions in both lobes. Since TPZ has a half-life of 40 minutes, its effect may have dissipated by the time the superselective embolization of the lesions in one lobe were complete and catheter repositioned to treat the lesions of the other lobe. Therefore separate procedures were planned for the right and left lobes in I.V. cohort patients. Two patients had residual tumor and required two procedures to achieve CR. The majority (86.6%) of additional procedures in CR patients were due to the appearance of new lesions in untreated areas.
If assessed by RECIST criteria, ORR was 72% (95% CI=50.2–88.2) in target lesions and 68% (95% CI=46.5–85.1) overall, as shown in Table 4. One patient with CR in target lesion developed a new lesion in non-embolized territory, explaining the difference between target and overall lesion responses.
The duration of response for target lesions by mRECIST in patients with a response (complete or partial) was not reached. The duration of response for overall lesions by mRECIST in patients who achieved CR and CR+PR were 323 days (95% CI=101–680) and 282 days (95% CI=162–680) (shown in Table 5 and Figure 3), respectively.
 |
Table 5 Duration of Response for Complete Response and Overall Response for Target Lesions and Overall Lesions (by mRECIST) |
Among patients with elevated baseline AFP, 15/16 (93.8%) had a decrease in their AFP by at least 30% post-treatment with normalization of AFP values in 9/16 (56.3%). This result is consistent with observed CR and ORR.
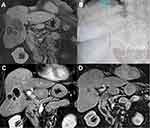
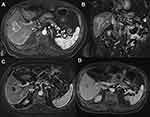
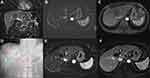
A dose/response correlation in I.V cohorts was seen with 5 mg/m2 having only a 33% CR rate compared to the 10 mg/m2 I.V. cohort. The number of patients was too small for statistical significance. The 5 mg/m2, 10 mg/m2, and 20 mg/m2 intra-arterial dose cohorts including the expansion cohort all showed a 60% CR response rate without a dose/response correlation, implying that the tumor was likely completely saturated. Table 6 details the response rate by cohort. There was no difference in response between tumors greater or smaller than 5 cm (Figure 2). An analysis of response by tumor volume in cm3 is shown in Figure 4, although the sample is too small for statistical significance, larger lesions may have a better response with TATE. The time to progression per mRECIST and RECIST was 324 days for both, with 95% CI=205–723 days and 240–723 days, respectively. The PFS at 6-months per RECIST and per mRECIST were 90.5% (95% CI=67.0–97.5) and 80.5% (95% CI=54.7–92.5), respectively. The median overall survival was 52 months (95% CI=21–not reached). Figure 5 illustrates the Kaplan–Meier curves for PFS per mRECIST and RECIST with the median PFS per mRECIST and RECIST with its 95% confidence interval (CI), respectively. Figure 6, Figure 7, Figure 8 illustrates the Kaplan–Meier curves for OS with its median and 95% CI. Figures 7 and 8 provide additional patient examples.
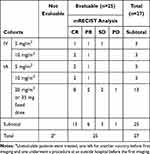 |
Table 6 Comparison of Target Lesion Response by mRECIST Criteria by Dose Cohort |
 |
Figure 4 Details the best percentage change from baseline in the target lesion size as a function of the baseline target lesion volume by cm3. |
 |
Figure 5 (A) Kaplan–Meier curve of progression free survival by mRECIST. (B) Kaplan–Meier curve of progression free survival by RECIST. Median PFS and 95% CI are provided in each case. |
 |
Figure 6 Kaplan–Meier with the overall survival with median and 95% CI. |
Discussion
Tirapazamine combined with transarterial embolization (TATE) was safe and well tolerated in patients with unresectable HCC. Our patient population consisted of non-operative candidates due to portal hypertension, cirrhosis, and number, size, or location of tumors. In our study, there was no dose limiting toxicity and the maximum tolerated dose was not reached. Dose escalation was not pursued further as the injection volume of TPZ at a dose of 20 mg/m2 (35 mg total) was 50 mL of a pH4 solution. The goal of intra-arterial administration was to saturate the tumor cells with TPZ and then induce hypoxia. At the FDA’s request, the trial was started with intravenous systemic administration of TPZ. A dose/response correlation in I.V cohorts was seen with 5 mg/m2 having a worse response compared to the 10 mg/m2 I.V. cohort; however, the sample is small for statistical analysis. Due to systemic circulation, the local tumor concentration in the intravenous cohort is estimated at no more than 5%. From the cell line studies, depending on the cell type and the level of cellular hypoxia, a dose of 50 nM to 10 µM of TPZ is needed to induce cell death.18,21 In the intra-arterial administration cohorts, the TPZ was delivered locally in the tumor-supplying artery, likely resulting in a much higher tumor concentration. The 5 mg/m2, 10 mg/m2, and 20 mg/m2 intra-arterial dose cohorts all showed a 60% CR rate without a dose/response correlation, implying that the tumor was likely completely saturated, which was further confirmed by the same 60% CR rate in the fixed dose cohort of 20 mg/m2 or 35 mg. The 20 mg/m2 or 35 mg dose of TPZ was chosen because it would enable operators to saturate large tumors without safety concerns. Indeed, TPZ was studied extensively in Phase III clinical trials where it was administered I.V. in conjunction with chemotherapy and/or chemoradiation for non-small cell lung cancer, head and neck cancer, and cervical cancer at a dose range of 260–330 mg/m2, which is much higher than the dose used in this study.22–24 Most common AE in our study were consistent with post-embolization syndrome.4,5 All SAE were unrelated to the study drug. There was no deterioration in liver function in our study without differences in AST, ALT, bilirubin, and albumin levels between screening and final follow-up, even in patients with portal hypertension. TATE is performed in a super-selective manner sparing as much normal liver as possible. Only one patient developed ascites during follow-up attributed to disease progression with portal vein invasion.
Previous studies failed to show a benefit to adding TPZ to standard chemotherapy or chemoradiation. However, these studies administered TPZ systemically and did not ensure sustained hypoxic conditions necessary for its activation. Consistently, pre-clinical studies using the HBx transgenic mouse HCC model demonstrated that TPZ followed by hepatic artery ligation induced complete tumor necrosis vs minimal tumor necrosis with TPZ alone or hepatic artery ligation alone.18 In the same model, doxorubicin with hepatic artery ligation resulted in less than 10% tumor necrosis. Therefore, combination of TPZ and TAE appeared to be synergistic. Preliminary efficacy from this Phase I study was encouraging with an overall response rate for all lesions of 80% by mRECIST and 68% per RECIST. The high concordance between mRECIST and RECIST may be explained by the fact that when a response is achieved with TATE, the tumor is truly necrotic. This claim is supported by the sustained duration of response (CR and CR+PR) in target lesions (not reached in both) and all lesions (282 days for ORR and 323 days for CR group). PFS at 6-months per RECIST and per mRECIST were 90.5% (95% CI=67.0–97.5) and 80.5% (95% CI=54.7–92.5), respectively, if appearance of a new lesion is counted as a PFS event. PFS can be calculated without counting the appearance of a new lesion as a PFS event, as done in the TACTICS trial.17 However, during our end of Phase 1 meeting, the FDA insisted that the definition of PFS include the appearance of a new lesion. Therefore, in the analysis of PFS duration, a new lesion was censored as a PFS event.
Trans-arterial chemoembolization is the standard of care for unresectable HCC1,25,27 as it confers an increase in OS.4,27 TACE is considered palliative. Moreover, intermediate HCC encompasses a large pool of patients and depending on tumor size and technique, response varies widely. Indeed, superselective TACE was associated with decreased local recurrence.28 In a recent review, the authors reported that superselective TACE for HCC smaller than 4 cm is associated with aCR of 66%; however, for lesions greater than 5 cm, CR drops to 25% only.29 However, the OS of unresectable HCC increased from 13.9 months with best supportive therapies to 18.1 months with cTACE, 20.6 months with DEB-TACE, and 20.8 months with TAE in a 2017 meta-analysis of RCT on transarterial therapies for HCC.9 Therefore, from the current literature available, the tumor size and procedural technique affect outcome more than the chemotherapy regimens which have, thus far, failed to provide additional survival or efficacy benefits.6,9 We combined a novel hypoxia-activated agent with TAE that would be synergistic with sustained hypoxia produced by embolization. Our study demonstrated an improved CR rate in target lesions with TATE vs rate reported in the literature with cTACE (60% vs 26%) for the tumor size3,12,27 reported in this study (ie, 6.53 cm). The majority of patients achieved CR after one procedure and there was no difference in response between lesions smaller or greater than 5 cm. In fact, the response per volume of tumor had a trend of improved response with larger tumors; however, the sample size is too small for statistical significance. That being said, it does confirm that the response remains high in larger lesions with TATE. Although some weak publications exist, data and guidelines have not endorsed hepatic arterial infusion or stereotactic body radiotherapy in the treatment of HCC.1 Lobar transarterial radioembolization (TARE) is generally reserved for patients who have progressed with TACE and lobar TARE failed to demonstrate an advantage compared to sorafenib in multiple prospective randomized trials.30,31 Radiation segmentectomy has emerged in recent years as a more effective therapeutic option; however, the recently presented retrospective Legacy trial included only patients with solitary tumors, less than 8 cm.32 Our patient population had multiple lesions up to 10 cm. The Legacy trial recommends a minimum of 400 Gy dose to the treated area basically “ablating” the entire treated area.32 Delivering over 400 Gy in multiple areas in cirrhotic patients may precipitate liver failure. Our patients had multiple lesions in multiple segments. Patients with multiple larger lesions currently treated with TACE have an unmet need for improved therapy. In the great majority of patients achieving CR with TATE, repeat procedures were due to new lesions in untreated areas of the liver discovered during follow-up. Moreover, there was an improved ORR with TATE vs TACE reported in a large meta-analysis (80% vs 52%).4 PFS at 6-months in that meta-analysis was 57.2%, while PFS at 6 months was 90.5% with TATE.4 This is consistent with previous data demonstrating that CR results in prolonged PFS and OS.33–35 Prior reports have shown that TPZ is capable of killing hypoxia-induced cancer stem cells,36 further prolonging the duration of CR compared to cTACE. These results should be confirmed in a prospective randomized trial.
Apart from the inherent limitations associated with Phase I, ie, small number of patients, and strict recruitment criteria, limitations of this study include the lack of a mandatory biopsy to determine grade of the HCC treated, although some patients did have biopsies and various tumor grades were seen on pathology. The dose escalation was stopped, and the maximum tolerated dose was never reached. It was not possible to determine in-vivo the level of drug needed for cell death, although it seems that the drug levels were sufficient to cause tumor necrosis.
Conclusion
This Phase I study demonstrated that I.A. TPZ with bland embolization for unresectable intermediate HCC was safe, tolerable without liver function deterioration. Moreover, tumor response appears encouraging with a CR of 60% and ORR of 84% per mRECIST for target lesion (NCT02174549).
Data Sharing Statement
De-identified participant data will be shared. The exact details of what data and how it will be shared have not been fully determined as much of our research staff and operations were completely suspended with COVID-19 and have only partially resumed. The UC Regents has confirmed an onsite return of July 1, 2021. We apologize for the lack of details, but the de-identified participant level data will be shared. Please contact the UCI PI site Dr Nadine Abi-Jaoudeh after that date for more details at [email protected]. She will be coordinating with the other PI sites.
Statement of Ethics
All regulatory and ethics approvals were obtained from the Food and Drug Administration with an investigational new drug and the approval from the hepatobiliary disease-oriented team (DOT) as well as the University of California Irvine’s institutional review board (IRB), University of California San Francisco IRB, Taichung Veteran General Hospital IRB and National Taiwan University IRB. This trial was registered on ClinicalTrials.gov identifier: NCT02174549. All participants provided written informed consent. The trial was performed in accordance with the Declaration of Helsinki.
Acknowledgments
We would like to acknowledge Dr Fa Chyi Lee, M.D. Kari Nelson, M.D. Dr Zeljka Jutric, and James Katrivesis, M.D. for their contributions to the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was sponsored by Teclison Inc, the manufacturer of the drug who also held the IND with the FDA. The data was collected by the investigators into an electronic database that the sponsor had access to. The manuscript was written by the authors and the decision to submit for publication was the sole decision of the authors.
Disclosure
Dr Abi-Jaoudeh is principal investigator on a research agreement between the University of California Irvine and Teclison Inc, Philips Medical Systems Inc. Dr. Abi-Jaoudeh is principal investigator on sponsored research by Sillajen Inc., SIRTEX Inc., Dr. Abi-Jaoudeh has shares in Bruin Biosciences Inc and has served on an advisory board with Genentech, Medtronic, Eisai, and QED therapeutics Inc.
Dr. Dayyani was paid by the speaker’s bureau of Amgen, Eisai, Exelixis, Taiho, Merck, BMS, Deciphera, Signatera, Ipsen, and Sirtex. He is a consultant for Eisai, Array, Exelixis, Genentech, Foundation Medicine, and Ipsen.
Dr. Fernando, Dr. Hwang, Dr Liang, and, Dr. Javan have not declared any conflicts of interest.
Dr. Fidelman has reported grants from Merck, SIRTEX and Boston Scientific.
Dr Chen has declared grants from Bristol Myers Squibb and personal fees from Roche.
Dr. Imagawa has shares and is part owner of Bruin Biosciences Inc., is consultant for Bayer Inc, and is principal investigator on sponsored research by Beigene and Eisai. He is also paid by the speaker’s bureau of Eisai.
References
1. European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
2. Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi:10.1016/S0140-6736(18)30010-2
3. White JA, Gray SH, Li P, et al. Current guidelines for chemoembolization for hepatocellular carcinoma: room for improvement? Hepatol Commun. 2017;1(4):338–346. doi:10.1002/hep4.1046
4. Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: a systematic review of efficacy and safety data. Hepatology. 2016;64(1):106–116. doi:10.1002/hep.28453
5. de Baere T, Arai Y, Lencioni R, et al. Treatment of liver tumors with lipiodol TACE: technical recommendations from experts opinion. Cardiovasc Intervent Radiol. 2016;39(3):334–343. doi:10.1007/s00270-015-1208-y
6. Imai N, Ishigami M, Ishizu Y, et al. Transarterial chemoembolization for hepatocellular carcinoma: a review of techniques. World J Hepatol. 2014;6(12):844–850. doi:10.4254/wjh.v6.i12.844
7. Lammer J, Malagari K, Vogl T, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi:10.1007/s00270-009-9711-7
8. Golfieri R, Giampalma E, Renzulli M, et al. Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma. Br J Cancer. 2014;111(2):255–264. doi:10.1038/bjc.2014.199
9. Katsanos K, Kitrou P, Spiliopoulos S, Maroulis I, Petsas T, Karnabatidis D. Comparative effectiveness of different transarterial embolization therapies alone or in combination with local ablative or adjuvant systemic treatments for unresectable hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. PLoS One. 2017;12(9):e0184597. doi:10.1371/journal.pone.0184597
10. Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34(17):2046–2053. doi:10.1200/JCO.2015.64.0821
11. Trevisani F, Golfieri R. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: where are we now? Hepatology. 2016;64(1):23–25. doi:10.1002/hep.28554
12. Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64(5):1090–1098. doi:10.1016/j.jhep.2016.01.012
13. Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ, Cao GW. Increased expression of vascular endothelial growth factor in hepatocellular carcinoma after transcatheter arterial chemoembolization. Acta Radiol. 2008;49(5):523–529. doi:10.1080/02841850801958890
14. Xiao EH, Guo D, Bian DJ. Effect of preoperative transcatheter arterial chemoembolization on angiogenesis of hepatocellular carcinoma cells. World J Gastroenterol. 2009;15(36):4582–4586. doi:10.3748/wjg.15.4582
15. Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology. 2014;60(5):1697–1707. doi:10.1002/hep.27290
16. Kudo M, Arizumi T. Transarterial chemoembolization in combination with a molecular targeted agent: lessons learned from negative trials (post-TACE, BRISK-TA, SPACE, ORIENTAL, and TACE-2). Oncology. 2017;93(Suppl 1):127–134. doi:10.1159/000481243
17. Kudo M, Ueshima K, Ikeda M, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69(8):1492–1501. doi:10.1136/gutjnl-2019-318934
18. Lin WH, Yeh SH, Yeh KH, et al. Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice. Proc Natl Acad Sci U S A. 2016;113(42):11937–11942. doi:10.1073/pnas.1613466113
19. Guise CP, Mowday AM, Ashoorzadeh A, et al. Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia. Chin J Cancer. 2014;33(2):80–86. doi:10.5732/cjc.012.10285
20. Chang L, Liu X, Wang D, et al. Hypoxia-targeted drug Q6 induces G2-M arrest and apoptosis via poisoning topoisomerase II under hypoxia. PLoS One. 2015;10(12):e0144506. doi:10.1371/journal.pone.0144506
21. Costa AK, Baker MA, Brown JM, Trudell JR. In vitro hepatotoxicity of SR 4233 (3-amino-1,2,4-benzotriazine-1,4-dioxide), a hypoxic cytotoxin and potential antitumor agent. Cancer Res. 1989;49(4):925–929.
22. DiSilvestro PA, Ali S, Craighead PS, et al. Phase III randomized trial of weekly cisplatin and irradiation versus cisplatin and tirapazamine and irradiation in stages IB2, IIA, IIB, IIIB, and IVA cervical carcinoma limited to the pelvis: a Gynecologic Oncology Group study. J Clin Oncol. 2014;32(5):458–464. doi:10.1200/JCO.2013.51.4265
23. Lim AM, Rischin D, Fisher R, et al. Prognostic significance of plasma osteopontin in patients with locoregionally advanced head and neck squamous cell carcinoma treated on TROG 02.02 phase III trial. Clin Cancer Res. 2012;18(1):301–307. doi:10.1158/1078-0432.CCR-11-2295
24. Rischin D, Peters LJ, O’Sullivan B, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans-Tasman Radiation Oncology Group. J Clin Oncol. 2010;28(18):2989–2995. doi:10.1200/JCO.2009.27.4449
25. Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi:10.1002/hep.29086
26. Gaba RC, Baumgarten S, Omene BO, et al. Ethiodized oil uptake does not predict doxorubicin drug delivery after chemoembolization in VX2 liver tumors. J Vasc Interv Radiol. 2012;23(2):265–273. doi:10.1016/j.jvir.2011.10.022
27. Geschwind JF, Kudo M, Marrero JA, et al. TACE treatment in patients with sorafenib-treated unresectable hepatocellular carcinoma in clinical practice: final analysis of GIDEON. Radiology. 2016;279(2):630–640. doi:10.1148/radiol.2015150667
28. Miyayama S, Matsui O, Yamashiro M, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007;18(3):365–376. doi:10.1016/j.jvir.2006.12.004
29. Golfieri R, Renzulli M, Mosconi C, et al. Hepatocellular carcinoma responding to superselective transarterial chemoembolization: an issue of nodule dimension? J Vasc Interv Radiol. 2013;24(4):509–517. doi:10.1016/j.jvir.2012.12.013
30. Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled Phase 3 trial. Lancet Oncol. 2017;18(12):1624–1636. doi:10.1016/S1470-2045(17)30683-6
31. Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: selective internal radiation therapy versus sorafenib in asia-pacific patients with hepatocellular carcinoma. J Clin Oncol. 2018;36(19):1913–1921. doi:10.1200/JCO.2017.76.0892
32. Lewandowski R, Johnson GE, Kim E, et al. Use of yttrium-90 (Y90) glass microspheres (TheraSphere) as neoadjuvant to transplantation/resection in hepatocellular carcinoma: analyses from the LEGACY study. J Clin Oncol. 2021;39(3_suppl):300. doi:10.1200/JCO.2021.39.3_suppl.300
33. Kwan SW, Fidelman N, Ma E, Kerlan RK, Yao FY. Imaging predictors of the response to transarterial chemoembolization in patients with hepatocellular carcinoma: a radiological-pathological correlation. Liver Transpl. 2012;18(6):727–736. doi:10.1002/lt.23413
34. Miyayama S, Yamashiro M, Hashimoto M, et al. Comparison of local control in transcatheter arterial chemoembolization of hepatocellular carcinoma </=6 cm with or without intraprocedural monitoring of the embolized area using cone-beam computed tomography. Cardiovasc Intervent Radiol. 2014;37(2):388–395. doi:10.1007/s00270-013-0667-2
35. Iwazawa J, Ohue S, Hashimoto N, Muramoto O, Mitani T. Survival after C-arm CT-assisted chemoembolization of unresectable hepatocellular carcinoma. Eur J Radiol. 2012;81(12):3985–3992. doi:10.1016/j.ejrad.2012.08.012
36. Govaert KM, Emmink BL, Nijkamp MW, et al. Hypoxia after liver surgery imposes an aggressive cancer stem cell phenotype on residual tumor cells. Ann Surg. 2014;259(4):750–759. doi:10.1097/SLA.0b013e318295c160
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.