Back to Journals » Journal of Pain Research » Volume 11
Pharmacotherapeutic considerations for use of cannabinoids to relieve pain in patients with malignant diseases
Authors Darkovska-Serafimovska M, Serafimovska T, Arsova-Sarafinovska Z, Stefanoski S, Keskovski Z, Balkanov T
Received 21 December 2017
Accepted for publication 23 February 2018
Published 23 April 2018 Volume 2018:11 Pages 837—842
DOI https://doi.org/10.2147/JPR.S160556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Marija Darkovska-Serafimovska,1 Tijana Serafimovska,2 Zorica Arsova-Sarafinovska,1 Sasho Stefanoski,3 Zlatko Keskovski,3 Trajan Balkanov4
1Department of Pharmacology, Faculty of Medical Sciences, Goce Delcev University, Stip, Republic of Macedonia; 2Faculty of Pharmacy, Ss Cyril and Methodius University of Skopje, Skopje, Republic of Macedonia; 3NYSK Holdings, Skopje, Republic of Macedonia; 4Department of Pharmacology and Toxicology, Faculty of Medicine, Ss Cyril and Methodius University of Skopje, Skopje, Republic of Macedonia
Purpose: The aim of this review was to assess the efficacy of cannabis preparations for relieving pain in patients with malignant diseases, through a systematic review of randomized controlled trials (RCTs), which were predominantly double-blind trials that compared cannabis preparation to a placebo.
Methods: An electronic search of all literature published until June 2017 was made in MEDLINE/PubMed, Embase, The Cochrane Controlled Trials Register and specific web pages devoted to cannabis.
Results: Fifteen of the 18 trials demonstrated a significant analgesic effect of cannabinoids as compared to placebo. The most commonly reported adverse effects were generally well tolerated, mild to moderate. The main side effects were drowsiness, nausea, vomiting and dry mouth. There is evidence that cannabinoids are safe and modestly effective in neuropathic pain and also for relieving pain in patients with malignant diseases. The proportion of “responders” (patients who at the end of 2 weeks of treatment reported ≥30% reduction in pain intensity on a scale of 0–10, which is considered to be clinically important) was 43% in comparison with placebo (21%).
Conclusion: The target dose for relieving pain in patients with malignant diseases is most likely about 10 actuations per day, which is about 27 mg tetrahydrocannabinol (THC) and 25 mg cannabidiol (CBD), and the highest approved recommended dose is 12 actuations per day (32 mg THC/30 mg CBD). Further large studies of cannabinoids in homogeneous populations are required.
Keywords: cancer management, chronic pain, cannabidiol, tetrahydrocannabinol, medical marihuana, nabiximols, cannabinoid receptors
Introduction
Pain is a disagreeable sensorial and emotional experience that subjects associate with tissue damage and impairs quality of life.1 Effective therapeutic options for patients living with different forms of pain are limited. Opioids and anti-inflammatory drugs as first-line medications for the treatment of pain in patients with malignant diseases do not always give satisfactory results.
In traditional medicine, cannabis preparations have been used for thousands of years to treat disease or alleviate symptoms, but their efficacy for specific indications is not clear. Clinical use of cannabinoid substances is restricted, due to legal and ethical reasons, as well as limited evidence showing benefits. The medical use of cannabis is attractive to patients suffering from malignant diseases. However, scientific justification or positive experience of the use of cannabis in patients with malignant diseases has only been found for the following indications: alleviation of sickness, nausea and vomiting associated with the use of cytotoxic therapy, pain relief and stimulation of appetite (treatment of cachexia). Pain relief is the most commonly cited reason for the medical use of cannabis.2–5
Mechanism of action of cannabinoids
Cannabinoids bind to cannabinoid receptors and act as agonists. Cannabinoid receptors are cell membrane receptors, members of the G protein-coupled receptors. They are activated by three major groups of ligands: endocannabinoids, plant cannabinoids and synthetic cannabinoids. Four subtypes of these receptors have been identified. Two have been cloned (CB1 and CB2 cannabinoid receptors),6,7 while the other two, WIN and abnormal-cannabidiol (abn-CBD) receptors, have been characterized pharmacologically.8–11
The analgesic effect of cannabinoids as a result of binding of cannabinoids to cannabinoid receptors has been confirmed, and the role of the endocannabinoid system in pain relief has been verified in various types of pain: somatic, visceral and neuropathy.12 Classical analgesics, nonsteroidal anti-inflammatory drugs or opioids, paracetamol and antidepressants (with an analytical effect in some conditions) increase the activity of the endocannabinoid system.12
The discovery of the endocannabinoid system and the development of animal models with different forms of pain have recently demonstrated the synergism between the opiate and cannabinoid systems.12 There is a large amount of preclinical data in animal models on the analgesic effect of cannabinoids, predominantly Δ9-tetrahydrocannabinol (THC), nabilone and dronabinol, or combinations of THC and cannabidiol (CBD) and some other synthetic cannabinoids; and analgesic effects in the treatment of cancer-related pain without serious side effects have been shown.13–15
In humans, pharmacodynamic studies have demonstrated the effect of cannabinoids on provoked somatic pain (e.g., thermal stimulation), capsaicin-induced hyperalgesia, painful spasms in patients with multiple sclerosis (MS), and neuropathic pain in HIV/AIDS patients.12,26–28,30
Available cannabinoid analgesic agents
Two finished drug products – nabilone (Cesamet) and dronabinol (Marinol) – have been approved in many countries for the “prevention/treatment of chemotherapy induced nausea and vomiting”.15 The only pharmaceutical industry drug product carrying the cannabinoid therapeutic principle with regulatory approval (in some countries) for pain relief in patients with malignant diseases is nabiximols (Sativex spray). Sativex® (GW Pharmaceuticals, Cambridge, UK) is an oromucosal cannabis-based spray combining a CB1 partial agonist (THC) with a cannabinoid system modulator (CBD).16,17 It was approved by Health Canada in June 2005 for prescription for central neuropathic pain in MS, and in August 2007, it was additionally approved for the treatment of cancer pain, as an adjuvant analgesic in adults with advanced malignancy, who, despite the highest tolerated opioid dose, still feel moderate to severe chronic pain.18
In randomized controlled trials (RCTs), Sativex was adjunctively added to optimal drug regimens in patients with intractable symptoms, those often termed “untreatable”. The recommended maximum is 12 daily doses (32.4 mg THC and 30 mg CBD).12 Data on the analgesic effect of nabiximols in malignant patients are shown primarily as an illustration of the effects of different ratios of THC/CBD.
Approved ongoing clinical trials
An investigational new drug (IND) application to study Sativex in advanced clinical trials in the USA was approved by the FDA in January 2006 in patients with intractable cancer pain.15
Recently, the European Medicine Agency (EMEA) approved two double-blind, placebo-controlled safety and efficacy studies of Sativex as adjunctive therapy to opiates: the first one in pediatric patients from 8 to less than 18 years of age with cancer-related pain and the second one in pediatric patients from birth to less than 8 years of age with cancer-related pain (decision number P/0298/2014, PIP number EMEA-000181-PIP02-13). The completion date of the pediatric investigation plan is by July 2026.19
On February 17, 2016, orphan designation (EU/3/16/1621) was granted by the European Commission to GW Pharmaceuticals, for THC and CBD from extracts of Cannabis sativa for the treatment of glioma.20
Methods
An electronic search of all literature published until June 2017 was made in MEDLINE/PubMed, Embase, The Cochrane Controlled Trials Register and specific web pages devoted to cannabis. A systematic review of literature identified RCTs, evaluating the efficacy of cannabinoids in various chronic pain conditions that are not related to malignant diseases (including MS and HIV/AIDS neuropathies), compared with placebo and sometimes other active treatments.21–38 They demonstrated an analgesic effect of dronabinol, nabilone and natural THC and CBD in comparison with smoking marijuana. A detailed overview of preclinical and clinical data on the analgesic efficacy of cannabinoids is found in the document Health Canada2 and Ethnopharmacology.39 Only three RTCs evaluated the efficacy of cannabinoids compard to placebo in various pain conditions that are related to malignant diseases.40-42 The studies selected were double-blind RTCs with a crossover or parallel design.
Selection criteria
A systematic review of literature using the keywords cannabinoids, pain, malignant diseases, THC and RCTs identified 198 reports, of which 73 were potentially relevant RCTs, based on title and abstract screening. Seven of them had no relevant information obtained as full-text studies, five reports were duplicates (they contained data that had previously been published), 60 were in other clinical examinations (chemotherapy-induced nausea and vomiting, spasticity due to MS, sleep disorders and HIV/AIDS) and ten compared efficacy of cannabis for the treatment of chronic pain. Only three RCTs evaluated the efficacy of cannabinoids in pain conditions that are related to malignant diseases compared with synthetic THC and placebo (Figure 1).40–42
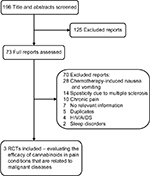  | Figure 1 Flow of studies through the review process. Abbreviation: RCTs, randomized controlled trials. |
Review of relevant research
In the intervention group, subjects were required to have received cannabis preparation, which at minimum contained the cannabinoids THC and CBD, applied by oral administration. Synthetic derivates of THC, such as dronabinol, nabilone or benzopyranoperidine, were likewise included. In the control group, subjects were required to have received a placebo treatment.40–42
The measure of efficacy chosen was the variable “intensity of pain” as scored by numeric analog scales. Patients at the end of 2 weeks of treatment reported ≥30% reduction in pain intensity on a scale of 0–10, which was considered as clinically important.
Double-blind, 2-week, multicenter RCT, placebo-controlled study
Respondents to the double-blind, 2-week, multicenter RCT, placebo-controlled study40 were adult patients with malignant diseases in terminal stage, who for at least a week used high (the most tolerated) doses of strong opiates, and despite this showed an intensity of pain ≥4 on a scale of 0–10.
For the treatment, patients were randomized into three groups. The first group received Sativex oral spray (nabiximols, 2.7 mg THC, 2.5 mg CBD per actuation) (n=60), the second group received THC as oral spray (2.7 mg THC per actuation) (n=58) and the third group received placebo spray (n=59). During the first week, the dose was titrated in patients based on tolerability and analgesia. The maximum permissible dose was 8 actuations in 3 hours (in intervals of at least 15 minutes between two doses) or at most 48 actuations for 24 hours (130 mg THC and 120 mg CBD).
The proportion of “responders” (patients who at the end of 2 weeks of treatment reported ≥ 30% reduction in pain intensity on a scale of 0–10, which is considered to be clinically important) in the Sativex group was 43% (statistically significantly compared with placebo; an improvement of –1.37 vs –0.69); in the THC group 23% (non-significant change compared with placebo; an improvement of –1.01 vs –0.69) and in the placebo group 21%.
Regarding side effects, in the Sativex group, 10/60 patients dropped out of treatment because of the side effects; in the second group where THC was orally administrated as spray, 7/58 patients dropped out of treatment because of the side effects and in the placebo group, 3/59 patients dropped out of treatment because of the side effects. The main side effects were drowsiness, nausea, vomiting and dry mouth.
Extension of the main double-blind, 2-week, multicenter RCT, placebo-controlled study
In 2013, an extension of the main study was opened.28 A total of 39 patients with chronic pain due to malignant disease, who had been treated with opioids in a previous study but with an inadequate analgesic response, were involved in a new extended open, randomized, controlled, multicenter study for 2 weeks in which patients continued treatment with Sativex.41 Doses remained unchanged. Of the 39 patients, 15 were treated for less than 2 weeks; others gradually withdrew from treatment during 1 year due to side effects (23/39), loss of efficacy (3/39) and a number of other reasons.
The study showed that the long-term use of THC/CBD spray is generally well tolerated without any loss of effect for pain relief because of long-term use. Moreover, patients who continued to use spray did not seek to increase the dose of spray or other medications for pain relief, suggesting that the adjuvant use of cannabinoids in cancer-related pain could be useful.
Multicenter, double-blind, placebo-controlled RCT, three different doses of Sativex
Respondents to this multicenter, double-blind, placebo-controlled RCT, with three different doses of Sativex,42 were adult patients with malignant diseases in terminal stage, who for at least a week used high (the most tolerated) doses of strong opiates, and despite this showed an intensity of pain ≥4 on a scale of 0–10.
For treatment, patients were randomized to receive three different doses of Sativex: group 1 received 4 actuations per day (10.8 mg THC, 10 mg CBD) (n=91), group 2 received 10 actuations per day (27 mg THC, 25 mg CBD) (n=88) and group 3 received 16 actuations per day (43 mg THC, 40 mg CBD) (n=90). A group receiving THC as oral spray and placebo spray (n=90) was also included. During the first 7 days, the dose was gradually increased (from 1 to 4, 10 or 16) and then adjusted for the next 2 weeks. From the 21st to the 35th day, there was a 14-day evaluation period.
Patients who at the end of 2 weeks of treatment reported ≥30% reduction in pain intensity on a scale of 0–10 were considered clinically important.
The results showed that with increasing doses, several patients dropped out of treatment because of side effects: 3/91 placebo, 5/91 lowest dose, 6/88 medium dose and 20/90 highest dose. Overall, the share of those with ≥30% reduction in pain did not differ for Sativex vs placebo. However, in an analysis that evaluated the average daily pain score during 14 days, the lowest and midpoint doses were better than placebo.
There is no RTC for relief of pain in malignant diseases that evaluates “smoking marijuana” or some other herbal preparation consisting of natural THC and CBD, except Sativex.
Discussion
The natural and synthetic agonists of the cannabinoid receptors have shown positive therapeutic results in the treatment of various pathological conditions, including pain as an inevitable symptom of tissue damage. The antinociceptive and anti-hyperalgesic effect of cannabinoids at the peripheral and central levels has been demonstrated and confirmed in various models of acute and chronic pain.43,44
The adverse effects (AEs) of cannabinoids on the central nervous system (CNS) are associated with abnormal psychomotor behavior, short-term memory impairment and intoxication.45
This review is prepared according to recommendations for systematic reviews.46,47 A systemic literature review identified more RCTs evaluating the efficacy of cannabinoids/cannabis in pain conditions, but only three double-blind controlled RTCs comparing the effectiveness of cannabinoids with synthetic THC and placebo in a variety of painful conditions that are associated with malignant disease.
In the first double-blind, 2-week multicenter RCT, placebo-controlled study,40 the proportion of responders in the Sativex group was 43%, in the THC group was 23% and in the placebo group was 21%. Extension of the main double-blind, 2-week, multicenter RCT, placebo-controlled study41 in 2013 showed that long-term use of THC/CBD spray is generally well tolerated without any loss of effect for pain relief because of long-term use. A multicenter double-blind, placebo-controlled RCT, in which three different doses of Sativex were used,42 showed that with increasing doses of THC/CBD, several patients dropped out of treatment because of side effects and those with ≥30% reduction in pain did not differ for Sativex vs placebo.
Thus, in two high-quality studies, the primary outcome of one can be regarded as positive and the other negative; however, both can be considered as evidence of the effectiveness of Sativex in specific painful conditions.
The inefficacy of the THC oral spray in one study, and variable results with relatively high doses of dronabinol, indicate that THC alone may not be sufficient for a good analgesic effect. This means that THC should be combined with CBD in order to achieve desire results.
Medicines that contain active ingredients that act as agonists of cannabinoid receptors are a promising therapeutic approach for treatment of various types of pain: neuropathic, inflammatory and oncological. This primarily refers to preparations containing exactly known similar amounts of THC and CBD, intended for the treatment of patients who do not respond to conventional therapy.11,41,42
In the studies of Sativex in patients with malignant diseases and pain despite opioid therapy, the target dose is most likely about 10 actuations per day (the average number of actuations in one RCT was 9; in the second, the “successful” dose was 6–10 actuations per day), which is about 27 mg THC and 25 mg CBD, and the highest approved recommended dose is 12 actuations per day (32 mg THC/30 mg CBD). The dose is introduced gradually, from 1 to 4–6 actuations during the day, for 5–7 days.12,37
Conclusion
There is evidence, although limited, to support the use of cannabis pharmacotherapy in the treatment of different forms of pain in patients. If a patient with chronic pain and their healthcare provider work together through first- and second-line treatment modalities without success, a trial of cannabis or a cannabinoid may be a reasonable next step. With increased use of medical cannabis as pharmacotherapy for pain comes a need for comprehensive risk–benefit discussions that take into account the significant possible AEs of cannabis.
Numerous randomized clinical trials have demonstrated the safety and efficacy of Sativex in central and peripheral neuropathic pain, rheumatoid arthritis and cancer pain. Common AEs included dizziness, dry mouth, nausea, fatigue, somnolence, euphoria and vomiting.
The recommended daily dose for the treatment of pain is a maximum of 32.4 mg THC and 30 mg CBD. Data on the analgesic effect of nabiximols in patients with malignant diseases are shown primarily as an illustration of the effects of different ratios of the THC/CBD combination. The degree to which cannabinoid analgesics will be adopted in adjunctive pain management practices remains to be determined.
Further large studies of cannabinoids in homogeneous populations are required.
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Classification of Chronic Pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–226. Available from: https://s3.amazonaws.com/rdcms-iasp/files/production/public/Content/ContentFolders/Publications2/FreeBooks/Classification-of-Chronic-Pain.pdf. Accessed March, 24, 2018. | ||
Bestrashniy J, Winters KC. Variability in medical marijuana laws in the United States. Psychol Addict Behav. 2015;29:639–642. | ||
Light MK, Orens A, Lewandowski B, et al. Market size and demand for marijuana in Colorado. The Marijuana Policy Group. 2014. Available from: http://www.cannabisconsumer.org/uploads/9/7/9/6/97962014/market_size_and_demand_study_july_9_2014%5B1%5D.pdf. Accessed November 17, 2016. | ||
Ilgen MA, Bohnert K, Kleinberg F, et al. Characteristics of adults seeking medical marijuana certification. Drug Alcohol Depen. 2013;132:654–659. | ||
Hill KP, Palastro MD, Johnson B, Ditre JW. Cannabis and pain: a clinical review. Cannabis Cannabinoid Res. 2017;2(1):96–104. | ||
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. | ||
Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. | ||
Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G-protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. | ||
Di Marzo V, Breivogel CS, Tao Q, et al. Levels, metabolism, and pharmacological activity of anandamide in CB1 cannabinoid receptor knockout mice, evidence for non-CB1, non-CB2 receptor-mediated actions of anandamide in mouse brain. J Neurochem. 2000;75:2434–2444. | ||
Hájos N, Ledent C, Freund TF. Novel cannabinoid sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. | ||
Manzanares J, Julian MD, Carrascosa A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr Neuropharmacol. 2006;4(3):239–257. | ||
Health Canada [webpage on the Internet]. Information for health care professionals. Cannabis (marihuana, marijuana) and the cannabinoids. Health Canada; 2013. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-use-marijuana/information-medical-practitioners/information-health-care-professionals-cannabis-marihuana-marijuana-cannabinoids.html. Accessed March, 24, 2018. | ||
Cridge BJ, Rosengren RJ. Critical appraisal of the potential use of cannabinoids in cancer management. Cancer Management Res. 2013;5:301–315. | ||
McAllister SD, Soroceanu L, Desperz PY. The antitumor activity of plant-derived non-psychoactive cannabinoids. J Neuroimmune Pharmacol. 2015;10(2):255–267. | ||
Abrams DI, Guzman M. Cannabis in cancer care. Clin Pharmacol Ther. 2015;97:575–586. | ||
McPartland JM, Russo EB. Cannabis and cannabis extracts: greater than the sum of their parts? J Cannabis Therapeut. 2001;1:103–132. | ||
Russo EB, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses. 2006;66:234–46. | ||
Russo EB. Cannabinoids in the management of difficult to treat pain. Ther Clin Risk Manag. 2008;4(1):245–259. | ||
European Medicines Agency [webpage on the Internet]. EMEA-001902-PIP01-15-M01. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/pips/EMEA-000181-PIP02-13/pip_001257.jsp&mid=WC0b01ac058001d129. Accessed March, 24, 2018. | ||
European Medicines Agency [webpage on the Internet]. EU/3/16/1621. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/orphans/2016/04/human_orphan_001722.jsp&mid=WC0b01ac058001d12b. Accessed March, 24, 2018. | ||
Wade DT, Collin C, Stott C, Duncombe P. Meta-analysis of the efficacy and safety of Sativex (nabiximols) on spasticity in people with multiple sclerosis. Mult Scler. 2010;16:707–714. | ||
Zajicek J, Ball S, Wright D, et al. Effect of dronabinol on progression in progressive multiple sclerosis (CUPID): a randomized, placebo-controlled trial. Lancet Neurol. 2013;12:857–865. | ||
Ball S, Vickery J, Hobart J, et al. The cannabinoid use in progressive inflammatory brain disese (CUPID) trial: a randomized dobule-blind placebo-controlled parallel-group multicenter trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess. 2015;19(12):vii–viii, xxv–xxxi, 1–187. | ||
Collin C, Davies P, Mutiboko IK, Ratcliffe S; Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14:290–296. | ||
Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel group study of Sativex in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32:451–459. | ||
Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex (nabiximols). Mult Scler. 2012;18:219–228. | ||
Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group enriched-design study of nabiximols (Sativex) as add-on therapy in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18:1122–1131. | ||
Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study of 160 patients. Mult Scler. 2004;10:434–441. | ||
Zajicek J, Fox P, Sanders H, et al. Cannabinoids for treatment of spasticity and other symptoms related to multiple sclerosis (CAMS study): multicenter randomized placebo-controlled trial. Lancet. 2003;362:1517–1526. | ||
Corey-Bloom J, Wolfson T, Gamst A, et al. Smoked cannabis for spasticity in multiple sclerosis: a randomized, placebo-controlled trial. CMAJ. 2012;184:1143–1150. | ||
Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology. 2005;65:812–819. | ||
Phillips TJC, Cherry CL, Cox S, Marshall SJ, Rice AS. Pharmacological treatment of painful HIV-associated sensory neuropathy: a systematic review and meta-analysis of randomized controlled trials. Plos One. 2010;5(12):e14433. | ||
Abrams DI, Hilton JF, Leiser RJ, et al. Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized placebo-controlled clinical trial. Ann Intern Med. 2003;139:258–266. | ||
Abrams DI, Jay CA, Shade SB, et al. Cannabis in painful HIV-associated sensory neuropathy. Neurology. 2007;68:515–521. | ||
Ellis RJ, Toperoff W, Vaida F, van den Brande G, et al. Smoked medicinal cannabis for neuropathic pain in HIV: a randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34:672–680. | ||
Ashton JC. Emerging treatment options for spasticity in multiple sclerosis – clinical utility of cannabinoids. Deg Neurol Neuromusc Dis. 2011;1:15–23. | ||
Lynch ME, Campbell F. Cannabinoids for treatment of chronic non-cancer pain; a systematic review of randomized trials. Br J Clin Pharmacol. 2011;72:735–744. | ||
Martín-Sánchez E, Furukawa TA, Taylor J, Martin JL. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med. 2009;10:1353–1368. | ||
Ben Amar M. Cannabinoids in medicine: a review of their therapeutic potential. J Ethnopharmacol. 2006;105:1–25. | ||
Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT. Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage. 2010;39:167–179. | ||
Johnson JR, Lossignol D, Burnell-Nugent M, Fallon MT. An open-label extension study to investigate the long-term safety and tolerability of THC/CBD oromucosal spray and oromucosal THC spray in patients with terminal cancer-related pain refractory to strong opioid analgesics. J Pain Symptom Manage. 2013;46:207–218. | ||
Portenoy RK, Banae-Motan ED, Allende S, et al. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized placebo-controlled dose-graded trial. J Pain. 2012;13:438–449. | ||
Iversen L, Chapman V. Cannabinoids, a real prospect for pain relief? Curr Opin Pharmacol. 2002;2:50–55. | ||
Pertwee RG. Cannabinoid receptors and pain. Prog Neurobiol. 2001;63:569–611. | ||
Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. | ||
Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions [webpage on the Internet]. Version 5.1.O (updated March 2011). Available from: http://handbook.cochrane.org/. Accessed June 23, 2017. | ||
Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York, UK: University of York; 2009. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf. Accessed June 23, 2017. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
