Back to Journals » Drug Design, Development and Therapy » Volume 17
Pharmacological and Clinical Studies of Medicinal Plants That Inhibit Dipeptidyl Peptidase-IV
Authors Rohani, Febrina E, Wahyuni IS, Levita J
Received 10 July 2023
Accepted for publication 24 October 2023
Published 23 November 2023 Volume 2023:17 Pages 3473—3491
DOI https://doi.org/10.2147/DDDT.S426870
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Rohani,1,* Ellin Febrina,2,* Indah Suasani Wahyuni,3,* Jutti Levita2,*
1Master Program in Pharmacy, Faculty of Pharmacy, Padjadjaran University, Sumedang, Indonesia; 2Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Padjadjaran University, Sumedang, Indonesia; 3Department of Oral Medicine, Faculty of Dentistry, Padjadjaran University, Sumedang, Indonesia
*These authors contributed equally to this work
Correspondence: Jutti Levita, Department of Pharmacology and Clinical Pharmacy, Faculty of Pharmacy, Padjadjaran University, Jl. Raya Bandung-Sumedang km 21, Sumedang, 45363, Indonesia, Tel +6222-84288888 Ext 3510, Email [email protected]
Abstract: Dipeptidyl peptidase IV (DPP-IV) is an enzyme responsible for the degradation of the incretin hormone glucagon-like peptide-1 (GLP-1). DPP-IV plays a significant role in regulating blood glucose levels by modulating the activity of GLP-1. In the context of diabetes, DPP-IV inhibitors effectively block the activity of DPP-IV, hence mitigating the degradation of GLP-1. This, in turn, leads to an extension of GLP-1’s duration of action, prolongs gastric emptying, enhances insulin sensitivity, and ultimately results in the reduction of blood glucose levels. Nonetheless, reported adverse events of DPP-IV inhibitors on T2DM patients make it essential to understand the activity and mechanism of these drugs, particularly viewed from the perspective of finding the effective and safe add-on medicinal plants, to be implemented in clinical practice. This review is intended to bring forth a thorough overview of plants that work by reducing DPP-IV activity, from computational technique, enzymatic study, animal experiments, and studies in humans. The articles were searched on PubMed using “Plants”, “DPP-IV”, “DPP-IV inhibitor”, “GLP-1”, “Type 2 diabetes”, “diabetes”, “in silico”, “in vitro”, “in vivo”, “studies in human”, “clinical study” as the query words, and filtered for ten years of publication period. Eighteen plants showed inhibition against DPP-IV as proven by in silico, in vitro, and in vivo studies; however, only ten plants were reported for efficacy in clinical studies. Several plant-based DPP-IV inhibitors, eg, Allium sativum, Morus Alba, Curcuma longa, Pterocarpus marsupium, and Taraxacum officinale, have established their functional role in inhibiting DPP-IV and have proven their effectiveness through studies in humans earning them a prominent place in therapeutic discovery.
Keywords: antidiabetics, antioxidants, diabetes mellitus, incretin hormone, medicinal plants
Introduction
As the world information society has grown, so has the number of the population with sedentary behavior. This term refers to any activity that requires a minimal amount of energy to maintain when a person is awake. This can include sitting, leaning, or lying down. Previous works have demonstrated that this less-active behavior is inextricably linked to all-cause mortality, eg, due to heart and blood vessel dysfunction, and type 2 diabetes mellitus (T2DM).1,2 The prevalence of T2DM in young people and adolescents is rising dramatically. The most predisposing causes of T2DM in elderly patients are being overweight with a lineage of DM, and a sedentary lifestyle.3 Adult-onset diabetes or non-insulin-dependent diabetes is a polygenic syndrome in which genetic and environmental health risks combine, leading to insulin resistance in the liver and muscles, and reducing the number of pancreatic β-cells. Most patients who suffer from T2DM are overweight, and the disease may go undetected for a long time as the patients move through the asymptomatic “pre-diabetes” stage.4 Chronic complications of DM lead to the impairment and malfunction of the organ.5 Therefore, achieving close to normal glucose levels is considered the main objective of DM management, which can be accomplished by the administration of oral hypoglycemic drugs.
Medications for DM fall into one of five categories, each with its unique mechanism of action: those that increase insulin secretion (eg, sulfonylureas and glinides), those that decrease the absorption of glucose in the intestine (eg, acarbose), drugs belonging thiazolidinediones and biguanides classes, drugs that decrease the reabsorption of glucose in the urine (eg, gliflozin), and drugs that target the incretin system (eg, DPP-IV inhibitors and GLP-1 analogs). Side effects of anti-DM medications include urinary tract infections (UTI), ketoacidosis, hypoglycemia, and weight gain. In addition to posing insurmountable obstacles to establishing effective dosing regimens in a clinical context, patients with DM may experience a decline in quality of life (QoL) due to side effects from their medication.6 Therefore, discovering plant-derived DPP-IV inhibitors is always challenging.
In this article, we discuss plants with DPP-IV inhibitory activity through in silico modeling, in vitro enzymatic, in vivo animal experiments, and studies in humans. The mechanism by which DPP-IV inhibitors affect glucagon-like peptide-1 (GLP-1) levels and their effect on insulin were also described.
Glucagon-Like Peptide-1 (GLP-1): The Incretin Hormone Secretion
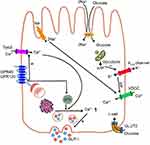
The rationale behind the increased production of insulin in response to meals when glucose is consumed orally, as opposed to intravenous administration, despite similar plasma glucose levels, can be attributed to the insulinotropic actions of incretin hormones. The enteroendocrine cells (EECs) in the digestive tract release incretin, which is crucial to the signalling process. EECs are part of the intestinal lining, which also includes mucus-secreting cells and absorptive epithelial cells. The EECs secrete hormones that function in food ingestion, abdominal peristaltic, stomach emptying, glucose balance, and regulation of hunger, among other functions. These hormones are released in response to nutrients and non-nutritional chemicals that either act on sensory transporters and receptors or on cellular metabolism. The primary secretions of EECs have allowed for their classification. GLP-1 is the incretin hormone that elevates insulin levels. GLP-1 and peptide YY (PYY) are secreted by L-cells, cholecystokinin (CCK) by I-cells, secretin by S-cells, glucose-dependent insulinotropic polypeptide (GIP) by K-cells, and gastrin by G-cells. I-cells and K-cells are duodenal EEC cells, L-cells are located in the ileum and colon.7 Intestinal and pancreatic GLP-1 receptors on vagal afferent neurons have revealed a pathway of communication between the intestines, the pancreas, and the brain. Similar to the GLP-1 EECs, the brain is capable of synthesizing GLP-1. The amazing scope of action of GLP-1 is highlighted by the fact that it influences eating behavior by communicating across the stomach, the brain, and the pancreas. Because of these roles, GLP-1 has been successful in the pharmacological control of T2DM.8,9 It has been extensively studied that the carbohydrates, proteins, and lipids of a meal may promote GLP-1 production from GLP-1 EECs.10
Moreover, the glucose uptake across cell membranes are affected by mediators, among those is sodium-coupled glucose transporters (SGLT), that play a crucial role in arbitrating glucose’s influence on GLP-1 release from EECs. Depolarization of the membrane, brought on by the influx of Na+ ions, activates the voltage dependent calcium channels (VDCC) and the exocytosis of GLP-1-containing vesicles.11 Amino acids affect the secretion of GLP-1 by a similar mechanism mediated by Na+ ions.12 G protein-coupled receptors (GPCRs) as GPR142 and CASR are activated by oligopeptides.13,14 Long-chain fatty acids are recognized by GPR40 and GPR120, whereas shorter fatty acids are recognized by other GPCRs.15 In pharmaceutical treatments for diabetes and obesity, one of the primary objectives is to increase the amount of GLP-1 that is secreted, and as a result, a lot of work has been put into figuring out the molecular processes that cause GLP-1 to be secreted. The decrease of stomach capacity and increase of stomach pressure in gastric bypass and other weight-loss surgeries are caused by GLP-1 secretion and stomach emptying (Figure 1).16,17
Most of GLP-1 in the lower brain stem occurs in the nucleus tractus solitarius (NTS), where it is produced by GLP-1 producing pre-proglucagon (PPG) neurons18 in respond to the release of GLP-1 in the peripheral. The vagal nerve is stimulated when the gastrointestinal GLP-1 binds to its receptor (GLP-1r), and eventually triggers the NTS PPG neurons to release GLP-1.19 Hence, there is a positive correlation between the secretion of GLP-1 in the periphery and the secretion of GLP-1 in the brain. As a whole, it shows that NTS PPG neurons and EECs respond differently to the same stimuli for GLP-1 release. Hormonal cues and vagal activity activate PPG neurons in the NTS, while dietary intake is the major activator for GLP-1 release from EECs. NTS PPG neurons integrate various inputs via excitatory, inhibitory, and neuro-modulatory influences before sending the signal to other central nodes. To further understand the mechanism on how GLP-1 work, the intracellular GLP-1 signaling pathway on insulin secretion is provided in the next section.
Pancreatic Insulin Secretion and the GLP-1 Signaling Pathway
Larger amounts of cyclic adenosine monophosphate (cAMP) are resulted from the stimulation of adenylate cyclase by GLP-1r via the activation of GPCRs (Gs).20 Accumulation of cAMP has been connected to intracellular signaling through protein kinase A (PKA) and other cAMP dependent pathways (eg, EPAC). GLP-1 may trigger a series of processes within the cell, including insulin synthesis, when it activates these pathways.21–23 Inhibition of ATP-regulated K+ channels, enhancement of L-type VDCC activity, and activating the non-specific cation channels via PKA and EPAC, are all effects of GLP-1 that have been well-established.24 These procedures activate the secretion of insulin in response to calcium and increase calcium influx. In instance, preventing the depolarization of the glucose-affected membrane by inhibiting ATP-regulated K+ channels increase a cell’s sensitivity to glucose. Stimulation of phospholipase C (PLC) and protein kinase C (PKC) is associated with heterotrimeric G protein subunit (Gq), which may be triggered by the GLP-1r. Strong evidence for a function for PLC/PKC in promoting insulin secretion can be obtained by imaging cytosolic/submembranous diacylglycerol (DAG), a PKC activator.25
It is confirmed that in patients with T2DM, the activity of pancreatic β-cells declines with time, thus, increasing this activity is essential to restore normal insulin secretion. GLP-1 can stimulate β-cells development from human precursor cells in rats, as well as stimulate their proliferation and inhibit their death.26,27 By activating the transcription and expression of insulin genes via both PKA-dependent and -independent signaling pathways, GLP-1 restores insulin storage and reduces β-cell depletion. Several kinases involved in cell signaling are activated in β-cells as a feedback to GLP-1 secretion. PKA activation in β-cells controls the insulin gene transcription factor of pancreatic and duodenal homeobox 1 (Pdx-1) by speeding its migration to the nucleus and subsequent uniting to the gene promoter in the nucleus. The GLP-1r activates the PKA pathway, which in turn upregulates the expression of the genes for cyclin D1 and insulin. T-cell factor-like 2 (TCFL2) and β-catenin are essential for this pathway.28 GLP-1-induced PKA activity controls NFAT transcription factor activation via a calcium/calcineurin mechanism.29 PKA is a nuclear enzyme that activates cAMP-response element binding protein (CREB), a transcription factor essential for the survival and growth of β-cells. GLP-1 also boosts the growth of β-cells via the CREB-mediated synthesis of insulin receptor substrate-2 (IRS-2), which in turn stimulates the PI3-kinase/Akt/PKB signaling pathway and cyclin D1 expression via cAMP and CREB.30,31 Moreover, the secretion of betacellulin via non-receptor cytoplasmic tyrosine kinase (Src) stimulates the epidermal growth factor (EGF) receptor, resulting in increased PI3-kinase and Akt/PKB activity and the proliferation of β-cells.32
Thus far we are aware that despite food, stimulation of nerve activity, and other hormones can modulate the release of GLP-1. The hormone somatostatin decreases the production of GLP- 1. GLP-1 is rapidly degraded by an enzyme, namely dipeptidyl peptidase-IV (DPP-IV).
Structure and the Active Site of DPP-IV
DPP-IV (Figure 2a) is a transmembrane glycoprotein serine dipeptidyl dipeptidase. This enzyme catalyzes the breakdown of GLP-1 to generate GLP-1 amide and the N-terminal histidine–alanine dipeptide. The primary structure of this enzyme reveals a short amine-terminal cytoplasmic domain of 6 amino acid residues, a longer transmembrane domain of 22 amino acid residues, and a larger portion of 738 amino acid residues. The catalytic domain (amino acid residues 506–766), a short flexible stalk (amino acid residues 29–39), and areas rich in glycosylation (amino acid residues 101–350) and cysteine (amino acid residues 55–100, 351–497) make up the extracellular domain.33 DPP-IV consists of a β-propeller and a catalytic α/β hydrolase, consists of the catalytic triad Ser630, Asp708, and His740, in its extracellular domains. The β-propeller is composed of highly glycosylated (where adenosine deaminase attached to) and cysteine-rich sections. Most of the DPP-IV activity in plasma comes from membrane-free DPP-IV protein, which can be cleaved by metalloproteases (MMPs).33,34
 |
Figure 2 (a) 3D structure of human dipeptidyl peptidase-IV in complex with a potent selective inhibitor, alogliptin (indicated by red arrow) (PDB ID 2ONC; PDB DOI https://doi.org/10.2210/pdb2ONC/pdb; Resolution 2.55 Å; deposited by Feng et al, 2007. Total structure weight: 346.99 kDa); (b) DPP-IV binding subsites for the inhibitors which are numbered from the cleavage point to the S2, S1, S1’, S2’, and S2 extensive subsites. Class I inhibitors bind to S1 and S2 subsites; Class II inhibitors to S2’, S1’, and S2 subsites; Class III inhibitors to S1, S2, and S2 extensive subsites. Adapted from Biochem Biophys Res Commun, 434(2), Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. 191–196, Copyright (2013), with permission from Elsevier.35 |
The active site of DPP-IV comprises of S2, S1, S’1, S’2 subsites and the S2 extended subsite (depicted in Figure 2b). S2 extensive subsite composed of Val207, Ser209, Phe357, and Arg358. S2 subsite composed of Arg125, Phe357, Arg358 as well as Glu205, Glu206, and Arg669. S1 subsite composed of Val711, Trp659, Tyr662, Ser630, Val656 Tyr666, and Asn710. S’1 subsite composed of Pro550, Ser630, Phe357, Tyr547, Tyr631, and Tyr666. S’2 subsite composed of Trp629, Tyr547, His740, and Ser630.
Because of these subsites, DPP-IV inhibitors were classified into: (1) class I inhibitors which establish interactions with the core S1 and S2 subsites and form a covalent bond with Ser630 in the catalytic triad; (2) class II inhibitors which occupy the S1’ and/or S2’ subsites in addition to the S2 subsite; and (3) class III inhibitors which build interactions with the S1, S2, and S2 extensive subsites. By creating salt bridges, DPP-IV inhibitors have a profound effect on the S2 subsite amino acids Glu206 and Glu205. Glu206 and Glu205 are the residues with which sitagliptin, an established DPP-IV inhibitor, forms the strongest bonds, through the amine group in the drug. In the S1 subsite, Tyr662 and Tyr666 interact weakly with the trifluorophenyl ring of sitagliptin. Interaction between the triazolopyrazine moiety of sitagliptin and Phe357 is strong due to the presence of π-π stacks interaction. There is also moderate interaction between the CF3 group of sitagliptin and Arg358.35–37
Adverse events (AEs) linked with DPP-IV inhibitors (sitagliptin, saxagliptin, linagliptin, vildagliptin, and alogliptin) extracted from the FDA Adverse Event Reporting System (FAERS) from 2004 to 2019 were defined as gastrointestinal nonspecific inflammation and dysfunctional conditions, hypersensitivity, severe cutaneous adverse reactions, and noninfectious diarrhoea.38 The reported adverse events of DPP-IV inhibitors have led to the perspective of finding effective and safe add-on medicinal plants.
Plant-Based DPP-IV Inhibitor with Antioxidant Properties
DM and its complications, including insulin resistance and insufficiency, have been linked to oxidative stress (OS), thus, this disease may be related to decreased levels of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px). Lack of these enzymes may give rise to the production of reactive oxygen species (ROS) and eventually, the development of diabetes complications via the increase of the susceptibility of tissues to oxidative stress.39 In patients with DM, glucose and free fatty acids are excessively oxidized to ROS, leading to the intrinsic apoptosis of β-cells. Cytochrome C is then liberated into the cytoplasm followed by the translocation of pro-apoptotic proteins, Bax and Bak, across the mitochondrial outer membrane. Apoptosis is triggered when caspase-9 is activated by cytochrome C, which also activates caspases 3 and 7.40,41
Plant-based antioxidants such as kinsenosides and flavonoids have demonstrated antidiabetic activity, and these compounds also help to maintain the health and function of pancreatic β-cells in animal models.40,41 The expression of pro-apoptotic genes increases in diabetics, while anti-apoptotic gene expression decreases. Flavonoids were reported to protect the growth of β-cells by reducing the expression of these genes.40 Antioxidants from plants that also inhibit DPP-IV, are thought to be the best way to maintain the function of β-cells thus treating DM.42
Plant-Derived DDP-IV Inhibitors from in silico, in vitro, in vivo, to Studies in Humans
Phytoconstituents with health benefits are generally utilized to treat potentially life-threatening diseases. Indigenous plants have been used to treat diabetes long before insulin and other synthetic anti-DM drugs were introduced, and they still become the focus of interest of anti-DM researches.43 Comprehensive studies of plants with inhibitory activity against DPP-IV are listed in Tables 1–4, respectively.
 |
Table 1 In silico Study |
 |
Table 2 In vitro Study Using Human DPP-IV Inhibitory Screening Kit |
 |
Table 3 In vivo Study |
 |
Table 4 Studies in Human |
Allium sativum
Three isolates of A. sativum (garlic) tubers, namely caffeic acid 3-glucoside, malonyl genistein, and calenduloside E (oleanolic acid 3-O--d-glucosiduronic acid), were computationally studied to examine their binding mode to DPP-IV. Of the three isolates, caffeic acid 3-glucoside revealed the weakest affinity with a docking score of −7.436 kcal/mol, compared to that of malonyl genistein and calenduloside E which was −7.438 kcal/mol and −10.172 kcal/mol, respectively. Moreover, the in vitro testing of the tuber extract revealed an inhibition towards DPP-IV (IC50 of 70.9 g/mL).44 In addition in vivo study in streptozotocin-induced rats showed a significant elevation of blood insulin and a reduction in blood glucose and HbA1c levels at week-8 post fresh garlic extract intervention.60 Randomized controlled trials involving sixty T2DM patients revealed a significant decrease of fasting plasma glucose (FPG), total cholesterol, LDL-C, triglycerides in 6 weeks after garlic powder tablets administration.69
Momordica charantia
The unripe fruit of M. charantia L. (Cucurbitaceae), generally known as bitter melon, is used as a vegetable in the tropics and subtropics countries. The aqueous extract of M. charantia fruits inhibited 28.15% of DPP-IV by in vitro assay.55 The number of pancreatic β-cells was reported to increase after oral doses of M. charantia in streptozotocin-induced diabetic Wistar rats. There was also a significant reduction in FPG.62 A randomized, placebo-controlled study involving 66 T2DM patients showed a reduction of FPG after 12 weeks of M. charantia supplements.70
Moringa oleifera
M. oleifera is abundant in both macronutrients and micronutrients, in addition to other bioactive constituents, all of which are essential for maintaining the body’s normal functioning and warding off certain diseases. In silico study of isothiocyanate which was found in the seed and leaf of M. oleifera indicated the formation of hydrogen bonds with His740 in S’2 subsite, Arg125 in S2 subsite, and Ser630, Asn710, and Tyr662 in S1 subsite of DPP-IV. The benzene ring of isothiocyanate builds a pi–pi interaction at with Phe357 in the S’1 subsite with binding energy of −81.10 kcal/mol. Isothiocyanate also inhibited DPP-IV with IC50 values of 157.694 µM.46 Furthermore, an in vivo study in alloxan-induced Sprague-Dawley rats exhibited diminished blood glucose levels after treatments with M. oleifera leaves.63 The hypoglycemic effect of M. oleifera leaves in seventeen T2DM patients has confirmed a reduction of blood glucose level.71
Morus alba
The IC50 of aqueous extract of M. alba leaves for DPP-IV inhibitory activity assay was 480 g/mL.57 Significant hypoglycemic effects, as measured by lower blood glucose and increased insulin concentration, were also reported in streptozotocin-induced Wistar rats.64 Obese people (BMI 25 kg/m2) with FPG between 100–140 mg/dL and/or 2 hours postprandial glucose between 140–199 mg/dL fared well in human study. FPG and HbA1c were shown to be lowered by consuming M. alba leaves.72
Eugenia jambolana
E. jambolana Lam., or black plum, has been proven for its inhibitory activity against DPP-IV enzyme by in vitro, having an IC50 value of 278.94 g/mL.58 In streptozotocin-induced diabetic rats a substantial decrease in FPG was announced after a treatment with α-hydroxy succinamic acid, an active component isolated from the fruit-pulp of E. jambolana.77 E. jambolana fruits extract in the form of polyherbal tablets (GlycaCare-II®) was shown to reduce HbA1c, postprandial blood glucose, and FPG by a statistically significant result in prediabetic and newly diagnosed diabetic patients for 120 days.73
Pterocarpus marsupium
The heartwood of the P. marsupium plant was reported for its DPP-IV inhibitory activity with an IC50 value of 273.73 g/mL.58 Supplementation with P. marsupium bark extracts significantly elevated insulin serum and reduced blood glucose in streptozotocin-induced Wistar rats.66 P. marsupium bark extract in the form of polyherbal tablets (GlycaCare-II®) was shown to significantly lower HbA1c, FPG, and postprandial blood glucose in both prediabetic and newly diagnosed diabetic patients when administered twice daily for 120 days.73
Gymnema sylvestre
G. sylvestre, which grows wild in many countries of Asia, Africa, and Australia, is still consumed as a nutritional supplement because of the numerous health benefits. It is widely used in both conventional medicine and alternative like Ayurveda to lower blood sugar levels.78 In vitro results of G. sylvestre leaves extracts confirmed a potential DPP-IV inhibitor with an IC50 value of 773.22 µg/mL.58 Tthe aqueous fraction of G. sylvestre ethanol extract significantly dropped the serum glucose and lipids in streptozotocin-induced, high-fat-induced obesity rats.65 As a supplement, G. sylvestre reduced glucose by 37%, cholesterol by 13%, transglutaminase by 5%, and low-density lipoproteins (LDL) by 19%, according to a human study including 32 adult patients with T2DM.74
Taraxacum officinale
In many countries, T. officinale is consumed as food, while in others, it is utilized in medicinal applications for the management and treatment of T2DM.79 The acetone extract of T. officinale showed the strongest inhibitory activity against DPP-IV followed by the ethanol extract by in vitro study. In streptozotocin-nicotinamide-induced diabetic rats, a polyherbal combination of T. officinale and M. charantia ethanol extracts was successful in decreasing plasma glucose comparable to glibenclamide and metformin.69 A single-center, unblinded, prospective interventional study conducted on 119 patients with T2DM treated with SR2004, containing the root extract of T. officinale in combination with other plants for 12 weeks, confirmed a reduction of HbA1c, blood glucose, total cholesterol, and serum triglycerides.80
Curcuma longa
The rhizomes of C. longa L. have been utilized in India and China as an effective treatment for diabetes. Curcumin, the major component in turmeric, exhibits strong anti-oxidative, anti-inflammatory, and anticancer properties.81 Docking scores of curcumin, which is targeted to DPP-IV for the purpose of lowering blood glucose, resulted a value of −66.765 kcal/mol indicating that the binding site for curcumin is most stable in S1, whereas S2 and S3 require stronger connections.48 Calebin A, another constituent of C. longa, interacts with the active site residues of DPP-IV, with the side-chain carboxylate oxygen and backbone carbonyl group of Glu206 in S2 subsite, Ser552, Cys551, and Tyr585 (docking score of −98.72 kcal/mol).47 An in vitro study towards the DPP-IV showed that curcumin had a higher inhibitory rate than that of P32/98 and resveratrol.48 The inhibitory rate of calebin A showed a maximum % inhibition of 55.9% at 26.3 mM.47 Moreover, a study in fifty-three T2DM patients treated with either 1500 mg of curcumin or a placebo capsule 3x/day for 10 weeks resulted in a considerably reduced mean values for BMI, abdominal circumference, and FPG in curcumin-treated patients. Homeostatic Model Assessment of Insulin Resistance or Pancreatic B Cell Function (HOMA-IR or HOMA-B) demonstrated no difference, as were HbA1c, insulin, malondialdehyde, total antioxidant capacity, or pancreatic β-cell function.75
Coptis chinensis
C. chinensis has been traditionally used to lower blood sugar in China. Berberine is its main active component.82 Berberine was reported could inhibit human recombinant DPP-IV (IC50 of 13.3 M).59 C. chinensis (80, 120, and 180 mg/kg) showed significant decrease in HbA1c, free fatty acid, total cholesterol, apolipoprotein B, and triglyceride of diabetic-induced animal models.67 Treatment with berberine 2x/day for 12 weeks resulted in a significant decrease in FPG and HbA1c in 98 T2DM patients.76
Conclusion
To prevent the breakdown of GLP-1 and maintain blood glucose levels, Allium sativum, Momordica charantia, Moringa oleifera, Morus alba, Eugenia jambolana, Pterocarpus marsupium, Gymnema sylvestre, Taraxacum officinale, Curcuma longa, and Coptis chinensis have established their functional role at molecular level, by in vitro, and in vivo studies. Moreover, these plants have proven their effectiveness through studies in humans. Based on our findings Allium sativum (caffeic acid 3-glucoside, malonylgenistin, calenduloside E as the active constituents), Morus Alba, Curcuma longa (calebin A and curcumin), Pterocarpus marsupium, and Taraxacum officinale have confirmed the best potential earning them a prominent place in DPP-IV inhibitor discovery. Regeneration of pancreatic β-cell mass and their mechanism to prevent oxidative stress in T2DM are additional benefits.
Abbreviations
A sativum, Allium sativum; ADA, adenosine deaminase; AE, Adverse effect; C. chinensis, Coptis chinensis. C. longa, Curcuma longa; cAMP, cyclic adenosine monophosphate; CAT, catalase; CCK, cholecystokinin; CREB, cAMP-response element binding protein; DAG, diacylglycerol; DM, diabetes mellitus; DPP-IV, dipeptidyl peptidase-IV; E. jambolana, Eugenia jambolana; EECs, enteroendocrine cells; EGF, epidermal growth factor; EPAC, exchange protein directly activated by cAMP; G. sylvestre, Gymnema sylvestre; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, Glucagon-like peptide 1; GSH-Px, glutathione peroxidase; HbA1c, glycated hemoglobin; M. oleifera, Moringa oleifera; M.alba, Morus alba; M.charantia, Momordica charantia; MMPs, metalloproteases; N/A, not applicable; NTS, nucleus tractus solitarii; OS, oxidative stress; Pdx-1, pancreatic and duodenal homeobox 1; P. marsupium, Pterocarpus marsupium; PKA, protein kinase A; PKC, protein kinase C; PLC, phospholipase C; PYY, peptide YY; RNS, reactive nitrogen species; ROS, reactive oxygen species; SAE, serious adverse effect; SGLT, sodium-coupled glucose transporters; SOD, superoxide dismutase; STZ, streptozotocin; T. officinale, Taraxacum officinale; T2DM, type 2 diabetes mellitus; TCFL2, T-cell factor-like 2; VDCC, voltage dependent calcium channels; Wnt, wingless/integrated; βTC, betacellulin.
Acknowledgments
The authors thank the Directorate of Research and Community Engagement of Padjadjaran University for facilitating the APC.
Disclosure
The authors state that they have no conflicts of interest.
References
1. Bellettiere J, LaMonte MJ, Evenson KR., et al. Sedentary behavior and cardiovascular disease in older women. Circulation. 2019;139(8):1036–1046. doi:10.1161/CIRCULATIONAHA.118.035312
2. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33(9):811–829. doi:10.1007/s10654-018-0380-1
3. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69–80. doi:10.1016/S2213-8587(17)30186-9
4. Kao KT, Sabin MA. Type 2 diabetes mellitus in children and adolescents. Aust Fam Physician. 2016;45(6):401–406.
5. White JR. A brief history of the development of diabetes medications. Diabetes Spectr. 2014;27(2):82–86. doi:10.2337/diaspect.27.2.82
6. Chaudhury A, Duvoor C, Reddy Dendi VS, et al. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol. 2017:8. doi:10.3389/fendo.2017.00006
7. Svendsen B, Pedersen J, Albrechtsen NJW, et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156(3):847–857. doi:10.1210/en.2014-1710
8. Egerod KL, Petersen N, Timshel PN, et al. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab. 2018;12:62–75. doi:10.1016/j.molmet.2018.03.016
9. Richards P, Parker HE, Adriaenssens AE, et al. Identification and characterization of GLP-1 receptor–expressing cells using a new transgenic mouse model. Diabetes. 2014;63(4):1224–1233. doi:10.2337/db13-1440
10. Hjørne AP, Modvig IM, Holst JJ. The sensory mechanisms of nutrient-induced GLP-1 secretion. Metabolites. 2022;12(5):420. doi:10.3390/metabo12050420
11. Ezcurra M, Reimann F, Gribble FM, Emery E. Molecular mechanisms of incretin hormone secretion. Curr Opin Pharmacol. 2013;13(6):922–927. doi:10.1016/j.coph.2013.08.013
12. Reimann F, Williams L, da Silva Xavier G, Rutter GA, Gribble FM. Glutamine potently stimulates glucagon-like peptide-1 secretion from GLUTag cells. Diabetologia. 2004;47(9):1592–1601. doi:10.1007/s00125-004-1498-0
13. Diakogiannaki E, Pais R, Tolhurst G, et al. Oligopeptides stimulate glucagon-like peptide-1 secretion in mice through proton-coupled uptake and the calcium-sensing receptor. Diabetologia. 2013;56(12):2688–2696. doi:10.1007/s00125-013-3037-3
14. Lin HV, Efanov AM, Fang X, et al. GPR142 controls tryptophan-induced insulin and incretin hormone secretion to improve glucose metabolism. PLoS One. 2016;11(6):e0157298. doi:10.1371/journal.pone.0157298
15. Ekberg JH, Hauge M, Kristensen LV, et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology. 2016;157(12):4561–4569. doi:10.1210/en.2016-1334
16. Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr. 2013;97(5):980–989. doi:10.3945/ajcn.112.047563
17. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. doi:10.1016/j.molmet.2019.09.010
18. Holt MK, Richards JE, Cook DR, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68(1):21–33. doi:10.2337/db18-0729
19. Williams DL. Minireview: finding the sweet spot: peripheral versus central glucagon-like peptide 1 action in feeding and glucose homeostasis. Endocrinology. 2009;150(7):2997–3001. doi:10.1210/en.2009-0220
20. Kaku K. New concept of the glucagon‐like peptide‐1 signaling pathway on pancreatic insulin secretion. J Diabetes Investig. 2020;11(2):265–267. doi:10.1111/jdi.13136
21. Baggio LL, Drucker DJ. Biology of Incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi:10.1053/j.gastro.2007.03.054
22. Cho YM, Fujita Y, Kieffer TJ. Glucagon-Like Peptide-1: glucose homeostasis and beyond. Annu Rev Physiol. 2014;76(1):535–559. doi:10.1146/annurev-physiol-021113-170315
23. Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi:10.1152/physrev.00034.2006
24. Shigeto M, Kaku K. Are both protein kinase A‐ and protein kinase C‐dependent pathways involved in glucagon‐like peptide‐1 action on pancreatic insulin secretion? J Diabetes Investig. 2014;5(4):347–348. doi:10.1111/jdi.12225
25. Shigeto M, Ramracheya R, Tarasov AI, et al. GLP-1 stimulates insulin secretion by PKC-dependent TRPM4 and TRPM5 activation. J Clin Invest. 2015;125(12):4714–4728. doi:10.1172/JCI81975
26. Ahrén B, Foley JE. Improved glucose regulation in type 2 diabetic patients with DPP-4 inhibitors: focus on alpha and beta cell function and lipid metabolism. Diabetologia. 2016;59(5):907–917. doi:10.1007/s00125-016-3899-2
27. Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates β-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52(3):741–750. doi:10.2337/diabetes.52.3.741
28. Liu Z, Habener JF. Glucagon-like peptide-1 activation of TCF7L2-dependent wnt signaling enhances pancreatic beta cell proliferation. J Biol Chem. 2008;283(13):8723–8735. doi:10.1074/jbc.M706105200
29. Lawrence MC, Bhatt HS, Easom RA. NFAT regulates insulin gene promoter activity in response to synergistic pathways induced by glucose and glucagon-like peptide-1. Diabetes. 2002;51(3):691–698. doi:10.2337/diabetes.51.3.691
30. Jhala US, Canettieri G, Screaton RA, et al. cAMP promotes pancreatic β-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17(13):1575–1580. doi:10.1101/gad.1097103
31. Kim MJ, Kang JH, Park YG, et al. Exendin-4 induction of cyclin D1 expression in INS-1 β-cells: involvement of cAMP-responsive element. J Endocrinol. 2006;188(3):623–633. doi:10.1677/joe.1.06480
32. Buteau J, Foisy S, Joly E, Prentki M. Glucagon-like peptide 1 induces pancreatic β-cell proliferation via transactivation of the epidermal growth factor receptor. Diabetes. 2003;52(1):124–132. doi:10.2337/diabetes.52.1.124
33. Sebastián-Martín A, Sánchez BG, Mora-Rodríguez JM, Bort A, Díaz-Laviada I. Role of Dipeptidyl Peptidase-4 (DPP4) on COVID-19 Physiopathology. Biomedicines. 2022;10(8):2026. doi:10.3390/biomedicines10082026
34. Zhong J, Kankanala S, Rajagopalan S. Dipeptidyl peptidase-4 inhibition: insights from the bench and recent clinical studies. Curr Opin Lipidol. 2016;27(5):484–492. doi:10.1097/MOL.0000000000000340
35. Nabeno M, Akahoshi F, Kishida H, et al. A comparative study of the binding modes of recently launched dipeptidyl peptidase IV inhibitors in the active site. Biochem Biophys Res Commun. 2013;434(2):191–196. doi:10.1016/j.bbrc.2013.03.010
36. Arulmozhiraja S, Matsuo N, Ishitsubo E, Okazaki S, Shimano H, Tokiwa H. Comparative binding analysis of dipeptidyl peptidase IV (DPP-4) with antidiabetic drugs – an ab initio fragment molecular orbital study. PLoS One. 2016;11(11):e0166275. doi:10.1371/journal.pone.0166275
37. Aulifa DL, Adnyana IK, Sukrasno S, Levita J. Inhibitory activity of xanthoangelol isolated from Ashitaba (Angelica keiskei Koidzumi) towards α-glucosidase and dipeptidyl peptidase-IV: in silico and in vitro studies. Heliyon. 2022;8(5):e09501. doi:10.1016/j.heliyon.2022.e09501
38. Huang J, Jia Y, Sun S, Meng L. Adverse event profiles of dipeptidyl peptidase-4 inhibitors: data mining of the public version of the FDA adverse event reporting system. BMC Pharmacol Toxicol. 2020;21(1):68. PMID: 32938499; PMCID: PMC7493367. doi:10.1186/s40360-020-00447-w
39. Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm J. 2016;24(5):547–553. doi:10.1016/j.jsps.2015.03.013
40. Ghorbani A, Rashidi R, Shafiee-Nick R. Flavonoids for preserving pancreatic beta cell survival and function: a mechanistic review. Biomed Pharmacother. 2019;111:947–957. doi:10.1016/j.biopha.2018.12.127
41. Oh YS. Plant-derived compounds targeting pancreatic beta cells for the treatment of diabetes. Evidence-Based Complement Altern Med. 2015;2015:1–12. doi:10.1155/2015/629863
42. Singh AK, Yadav D, Sharma N, Jin JO. Dipeptidyl Peptidase (DPP)-IV inhibitors with antioxidant potential isolated from natural sources: a novel approach for the management of diabetes. Pharmaceuticals. 2021;14(6):586. doi:10.3390/ph14060586
43. Ekor M, Lee SA, Parra KJ. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;5:4. doi:10.3389/fphar.2013.00177
44. Kalhotra P, Chittepu VCSR, Osorio-Revilla G, Gallardo-Velazquez T. Phytochemicals in garlic extract inhibit therapeutic enzyme DPP-4 and induce skeletal muscle cell proliferation: a possible mechanism of action to benefit the treatment of diabetes mellitus. Biomolecules. 2020;10(2):305. doi:10.3390/biom10020305
45. Purnomo Y, Soeatmadji DW, Sumitro SB, Widodo MA. Anti-diabetic potential of Urena lobata leaf extract through inhibition of dipeptidyl peptidase IV activity. Asian Pac J Trop Biomed. 2015;5(8):645–649. doi:10.1016/j.apjtb.2015.05.014
46. Yang Y, Shi CY, Xie J, Dai JH, He SL, Tian Y. Identification of potential dipeptidyl peptidase (DPP)-IV Inhibitors among moringa oleifera phytochemicals by virtual screening, molecular docking analysis, ADME/T-based prediction, and in vitro analyses. Molecules. 2020;25(1):189. doi:10.3390/molecules25010189
47. Chalichem NSS, Jupudi S, Yasam VR, Basavan D. Dipeptidyl peptidase-IV inhibitory action of Calebin A: an in silico and in vitro analysis. J Ayurveda Integr Med. 2021;12(4):663–672. doi:10.1016/j.jaim.2021.08.008
48. Huang PK, Lin SR, Chang CH, Tsai MJ, Lee DN, Weng CF. Natural phenolic compounds potentiate hypoglycemia via inhibition of Dipeptidyl peptidase IV. Sci Rep. 2019;9(1):15585. doi:10.1038/s41598-019-52088-7
49. Ram H, Kumar P, Purohit A, et al. Improvements in HOMA indices and pancreatic endocrinal tissues in type 2-diabetic rats by DPP-4 inhibition and antioxidant potential of an ethanol fruit extract of Withania coagulans. Nutr Metab. 2021;18(1):43. doi:10.1186/s12986-021-00547-2
50. Zabidi NA, Ishak NA, Hamid M, Ashari SE, Mohammad Latif MA. Inhibitory evaluation of Curculigo latifolia on α-glucosidase, DPP (IV) and in vitro studies in antidiabetic with molecular docking relevance to type 2 diabetes mellitus. J Enzyme Inhib Med Chem. 2021;36(1):109–121. doi:10.1080/14756366.2020.1844680
51. Quek A, Kassim NK, Lim PC, et al. α-Amylase and dipeptidyl peptidase-4 (DPP-4) inhibitory effects of Melicope latifolia bark extracts and identification of bioactive constituents using in vitro and in silico approaches. Pharm Biol. 2021;59(1):962–971. doi:10.1080/13880209.2021.1948065
52. Quek A, Kassim NK, Ismail A, et al. Identification of dipeptidyl peptidase-4 and α-amylase inhibitors from melicope glabra (Blume) T. G. Hartley (Rutaceae) using liquid chromatography tandem mass spectrometry, in vitro and in silico methods. Molecules. 2020;26(1):1. doi:10.3390/molecules26010001
53. Aulifa DL, Adnyana IK, Levita J, Sukrasno S. 4-hydroxyderricin isolated from the sap of angelica keiskei koidzumi: evaluation of its inhibitory activity towards dipeptidyl peptidase-IV. Sci Pharm. 2019;87(4):30. doi:10.3390/scipharm87040030
54. Shaikh S, Ali S, Lim JH, et al. Dipeptidyl peptidase-4 inhibitory potentials of Glycyrrhiza uralensis and its bioactive compounds licochalcone A and licochalcone B: an in silico and in vitro study. Front Mol Biosci. 2022:9. doi:10.3389/fmolb.2022.1024764
55. Perumal N, Nallappan M, Shohaimi S, Kassim NK, Tee TT, Cheah YH. Synergistic antidiabetic activity of Taraxacum officinale (L.) Weber ex F.H.Wigg and momordica charantia L. polyherbal combination. Biomed Pharmacother. 2022;145:112401. doi:10.1016/j.biopha.2021.112401
56. Ansari P, Flatt PR, Harriott P, Abdel-Wahab YHA. Anti-hyperglycaemic and insulin-releasing effects of Camellia sinensis leaves and isolation and characterisation of active compounds. Br J Nutr. 2021;126(8):1149–1163. doi:10.1017/S0007114520005085
57. Wang HJ, Chiang BH. Anti-diabetic effect of a traditional Chinese medicine formula. Food Funct. 2012;3(11):1161. doi:10.1039/c2fo30139c
58. Kosaraju J, Dubala A, Chinni S, Khatwal RB, Satish Kumar MN, Basavan D. A molecular connection of Pterocarpus marsupium, Eugenia jambolana and Gymnema sylvestre with dipeptidyl peptidase-4 in the treatment of diabetes. Pharm Biol. 2014;52(2):268–271. doi:10.3109/13880209.2013.823550
59. Al-masri IM, Mohammad MK, Tahaa MO. Inhibition of dipeptidyl peptidase IV (DPP IV) is one of the mechanisms explaining the hypoglycemic effect of berberine. J Enzyme Inhib Med Chem. 2009;24(5):1061–1066. doi:10.1080/14756360802610761
60. Al-Qattan KK, Thomson M, Ali M, Mansour MH. Garlic (Allium sativum) attenuate glomerular glycation in streptozotocin-induced diabetic rats: a possible role of insulin. Pathophysiology. 2013;20(2):147–152. doi:10.1016/j.pathophys.2013.04.001
61. Purnomo Y, Soeatmadji DW, Sumitro SB, Widodo MA. Incretin effect of Urena lobata leaves extract on structure and function of rats islet β-cells. J Tradit Complement Med. 2017;7(3):301–306. doi:10.1016/j.jtcme.2016.10.001
62. Ali AM, Moqbel MS, Al-Hizab FA. Effect of momordica charantia on insulin immune-reactive pancreatic beta cells and blood glucose levels in streptozotocin-induced diabetic rats. J Nutr Sci Vitaminol. 2022;68(5):438–445. doi:10.3177/jnsv.68.438
63. Villarruel-López A, López-de la Mora DA, Vázquez-Paulino OD, et al. Effect of Moringa oleifera consumption on diabetic rats. BMC Complement Altern Med. 2018;18(1):127. doi:10.1186/s12906-018-2180-2
64. Król E, Jeszka-Skowron M, Krejpcio Z, Flaczyk E, Wójciak RW. The effects of supplementary mulberry leaf (Morus alba) extracts on the trace element status (Fe, Zn and Cu) in relation to diabetes management and antioxidant indices in diabetic rats. Biol Trace Elem Res. 2016;174(1):158–165. doi:10.1007/s12011-016-0696-1
65. Kumar V, Bhandari U, Tripathi CD, Khanna G. Protective effect of gymnema sylvestre ethanol extract on high fat diet-induced obese diabetic Wistar rats. Indian J Pharm Sci. 2014;76(4):315–322.
66. Xu Y, Zhao Y, Sui Y, Lei X. Protective effect of Pterocarpus marsupium bark extracts against cataract through the inhibition of aldose reductase activity in streptozotocin-induced diabetic male albino rats. 3 Biotech. 2018;8(4):188. doi:10.1007/s13205-018-1210-6
67. Li JC, Shen XF, Shao JA, et al. The total alkaloids from Coptis chinensis Franch improve cognitive deficits in type 2 diabetic rats. Drug Des Devel Ther. 2018;12:2695–2706. doi:10.2147/DDDT.S171025
68. Zhang W, Jin Q, Luo J, Wu J, Wang Z. Phytonutrient and anti-diabetic functional properties of flavonoid-rich ethanol extract from Angelica Keiskei leaves. J Food Sci Technol. 2018;55(11):4406–4412. doi:10.1007/s13197-018-3348-y
69. Ashraf R, Khan RA, Ashraf I. Garlic (Allium sativum) supplementation with standard antidiabetic agent provides better diabetic control in type 2 diabetes patients. Pak J Pharm Sci. 2011;24(4):565–570.
70. Kim SK, Jung J, Jung JH, et al. Hypoglycemic efficacy and safety of Momordica charantia (bitter melon) in patients with type 2 diabetes mellitus. Complement Ther Med. 2020;52:102524. doi:10.1016/j.ctim.2020.102524
71. Leone A, Bertoli S, Di Lello S, et al. Effect of moringa oleifera leaf powder on postprandial blood glucose response: in vivo study on saharawi people living in refugee camps. Nutrients. 2018;10(10):1494. doi:10.3390/nu10101494
72. Thaipitakwong T, Supasyndh O, Rasmi Y, Aramwit P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement Ther Med. 2020;49:102292. doi:10.1016/j.ctim.2019.102292
73. Majeed M, Majeed A, Nagabhusahnam K, Mundkur L, Paulose S. A randomized, double-blind clinical trial of a herbal formulation (GlycaCare-II) for the management of type 2 diabetes in comparison with metformin. Diabetol Metab Syndr. 2021;13(1):132. doi:10.1186/s13098-021-00746-0
74. Li Y, Zheng M, Zhai X, et al. Effect Of-Gymnema Sylvestre, Citrullus Colocynthis And Artemisia Absinthium On Blood Glucose And Lipid Profile In Diabetic Human. Acta Pol Pharm. 2015;72(5):981–985.
75. Hodaei H, Adibian M, Nikpayam O, Hedayati M, Sohrab G. The effect of curcumin supplementation on anthropometric indices, insulin resistance and oxidative stress in patients with type 2 diabetes: a randomized, double-blind clinical trial. Diabetol Metab Syndr. 2019;11(1):41. doi:10.1186/s13098-019-0437-7
76. Zhang Y, Gu Y, Ren H, et al. Gut microbiome-related effects of berberine and probiotics on type 2 diabetes (the PREMOTE study). Nat Commun. 2020;11(1):5015. doi:10.1038/s41467-020-18414-8
77. Tanwar RS, Sharma SB, Prabhu KM. In vivo assessment of antidiabetic and antioxidative activity of natural phytochemical isolated from fruit-pulp of Eugenia jambolana in streptozotocin-induced diabetic rats. Redox Rep. 2017;22(6):301–307. doi:10.1080/13510002.2016.1229892
78. Khan F, Sarker MMR, Ming LC, et al. Comprehensive review on phytochemicals, pharmacological and clinical potentials of gymnema sylvestre. Front Pharmacol. 2019:10. doi:10.3389/fphar.2019.01223
79. Wirngo FE, Lambert MN, Jeppesen PB. The physiological effects of dandelion (Taraxacum officinale) in type 2 diabetes. Rev Diabet Stud. 2016;13(2–3):113–131. doi:10.1900/RDS.2016.13.113
80. Chatterji S, Fogel D. Study of the effect of the herbal composition SR2004 on hemoglobin A1c, fasting blood glucose, and lipids in patients with type 2 diabetes mellitus. Integr Med Res. 2018;7(3):248–256. doi:10.1016/j.imr.2018.04.002
81. Karlowicz-Bodalska K, Han S, Freier J, Smolenski M, Bodalska A. Curcuma Longa As Medicinal Herb In The Treatment Of Diabet- IC Complications. Acta Pol Pharm. 2017;74(2):605–610.
82. Zhao MM, Lu J, Li S, et al. Berberine is an insulin secretagogue targeting the KCNH6 potassium channel. Nat Commun. 2021;12(1):5616. doi:10.1038/s41467-021-25952-2
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.