Back to Journals » Infection and Drug Resistance » Volume 16
Pharmacokinetics of Efavirenz 600mg in Combination with Rifampicin in Chinese HIV/TB Co-Infection Patients
Authors Wang T, Liu Y , Zhu C, Yang S , Yang D, Xiao J, Gao G
Received 4 April 2023
Accepted for publication 1 July 2023
Published 17 July 2023 Volume 2023:16 Pages 4659—4666
DOI https://doi.org/10.2147/IDR.S415749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Tongtong Wang,1,* Yingchu Liu,2,* Chunyu Zhu,1,* Siyuan Yang,3 Di Yang,4 Jiang Xiao,4 Guiju Gao4
1Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 2School of General Practice and Continuing Education, Capital Medical University, Beijing, People’s Republic of China; 3Laboratory of Infectious Diseases Center, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China; 4Clinical and Research Center of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Guiju Gao, Professor of Medicine Clinical and Research Center of Infectious Diseases, Beijing Ditan Hospital, Capital Medical University, No. 8, Jingshun Dongjie, Chaoyang District, Beijing, 100015, People’s Republic of China, Email [email protected]
Background: Rifampicin is a known inducer of the cytochrome P450 (CYP2B6) enzyme, which can lead to a decrease in the concentration of efavirenz. Therefore, we conducted a study to evaluate the effect of daily rifampicin intake on efavirenz 600mg pharmacokinetics and HIV-1 virological suppression.
Methods: Patients receiving antiretroviral therapy containing efavirenz (600mg daily), and we collected efavirenz concentration at four visit points: ART day 14 (PK1), ART day 42 (PK2), ART day 140 (PK3), and ART day 336 (PK4), and performed pharmacokinetics analysis.
Results: From February 2017 to November 2020, 29 HIV/TB co-infection patients were included. Ninety percent of patients had a concentration of ≥ 1000ng/mL of efavirenz during the study. All patients had efavirenz Cmax ≥ 1000ng/mL, 86% patients showed good virology response.
Conclusion: Our study shows that the use of rifampicin in HIV/TB co-infection patients does not affect efavirenz drug concentrations, that virological suppression is good and that no efavirenz dose adjustment is required.
Keywords: HIV/TB, rifampicin, efavirenz, pharmacokinetics
Introduction
Tuberculosis (TB) is still one of the main causes of death in people living with human immunodeficiency virus (PLWH). According to the statistics of the World Health Organization (WHO), the probability of PLWH suffering from tuberculosis is 15–21 times that of HIV negative individuals.1 In 2020, about 820,000 PLWH suffered from tuberculosis, of which 214,000 died of tuberculosis.2 The double burden of HIV-related tuberculosis is particularly serious in developing countries, with 81% of tuberculosis patients complicated with HIV infection.3
Efavirenz is an effective non-nucleoside reverse transcriptase inhibitor (NNRTI), which is the main first-line treatment drug in China and other countries with high-burden of HIV/TB co-infection: Tenofovir disoproxil fumarate (TDF)+Lamivudine (3TC)+ Efavirenz (EFV).2 At present, the appropriate efavirenz dose for HIV-infected patients receiving combination rifampicin therapy remains controversial. With regard to the intensive PK data collected mainly in developed countries, the United States Food and Drug Administration (FDA) recently approved the revised efavirenz package insert, suggesting that the standard daily dose of efavirenz should be increased from 600 mg to 800 mg for patients who weigh more than 50 kg and take rifampicin at the same time.4 However, there is little clinical evidence about the efficacy and safety of 600 mg and 800 mg. Moreover, the WHO does not recommend increasing efavirenz dose according to the weight of tuberculosis patients.5 It is important to determine the appropriate dose of efavirenz during tuberculosis treatment, because too high efavirenz concentration may increase drug-related toxicity, while too low efavirenz concentration may lead to treatment failure and HIV resistance.
Rifampicin is a known inducer of cytochrome P450 (CYP2B6) enzyme, while efavirenz is a substrate of CYP regulated by CYP2B6. When used in conjunction with rifampicin, it may result in decreased efavirenz concentration, increasing the risk of virologic failure.6 For the other three TB drugs (Isoniazid, Pyrazinamide, Ethambutol), isoniazid has been reported to reduce efavirenz concentrations in some studies, but isoniazid induces mainly CYP2A6, CYP3A4, not CYP2B6, and efavirenz is mainly metabolized by CYP2B6, so isoniazid has little effect on efavirenz. A study in Taiwan, China on ethnic Chinese showed no effect of pyrazinamide and ethambutol on efavirenz concentrations.7 Currently, there is limited pharmacokinetic research in China on the combined use of these drugs. Therefore, we designed a study to evaluate the effect of daily rifampicin intake on efavirenz 600mg pharmacokinetics and HIV-1 virological suppression.
Methods
Study Design and Population
This study was conducted at Beijing Ditan Hospital and was initiated in February 2017, and the study complies with the Declaration of Helsinki. Informed consent was obtained from patients before the study was conducted. All patients enrolled in the screening were male, 29 patients signed informed consent forms before enrollment. Subject signed an informed consent form before enrollment and patients were aged >18 years. Patients were ensured to be on anti-tuberculosis treatment for at least 2 weeks with the following anti-tuberculosis: Isoniazid+Rifampicin+Pyrazinamide+Ethambutol. Patients weighing ≥50 kg were given rifampicin 600 mg and if weighing <50 kg, rifampicin 450 mg.
Entry criteria:
Exclusion criteria:
Withdraw criteria:
Sample Collection
Four PK follow-up visits were carried out, and the sampling of each PK at 0 hours or before oral administration. The blood samples collected through veins were stored in EDTA tubes and stored at -80°C after centrifugation. Finally, all samples were sent to the experimental institution for testing. PK1 (ART day 14+RIF day 28). PK2 (ART day 42+RIF day 56). PK3 (ART day 140+RIF day 154). PK4 (ART day 336+RIF day 350).
Efavirenz Concentrations
Plasma concentrations of efavirenz were quantified with a validated (ultra) high-performance liquid chromatography bio-quantification method, the lower limit of detection is 0.1 ng/mL. The therapeutic range of efavirenz was considered to be 1000–4000 ng/mL.
Plasma HIV-1 RNA Test
M2000TM (Abbott, ABI 7500, Chicago, USA) was used for quantitative detection of plasma HIV-1 RNA (copies/mL), the lower limit of detection is 40 copies/mL. The plasma HIV-1 RNA was monitored one month after initiation of combination antiretroviral therapy in antiretroviral-naive patients, or change of regimens in the presence of virological failure; and every three to six months thereafter according to the national HIV treatment guidelines.
Safety, Tolerability and Treatment Compliance
We pay attention to the adverse reactions and clinical manifestations of patients after taking drugs, such as severe skin rash, severe gastrointestinal symptoms, and some central nervous system toxicities. We monitor the patient’s blood routine, liver and kidney function. During follow-up visits to patients, we count the number of medications remaining and reinforce patient education on medication adherence.
Statistical Analysis
Continuous variables are reported as median and range. For efavirenz concentrations at the same sampling time point, mean was performed. Curves were fitted to the mean EFV concentrations using Origin2021R (OriginLab, Northampton, Massachusetts, USA), using non-compartmental modeling techniques (Monolix, Version2021R1, Paris, France) and were applied to plasma efavirenz concentration–time data to obtain C18h, maximum plasma concentration (Cmax), and area under the plasma concentration–time curve from 0 to 18 hours (AUC0-18). Descriptive statistics, including geometric means (GM) and 95% confidence intervals (CI), were calculated for all parameters.9 HIV-1 RNA was log-transformed using the Chi-square test or Fisher’s exact test, as appropriate, and compared to selected variables, with P value <0.05 considered statistically significant.
Results
Study Population
A total of 40 HIV/TB patients were screened for the study. The patients had been receiving anti-tuberculosis treatment for 2 weeks and they will start ART at the same time.11 patients were not included in the study (6 patients fail screening, 4 patients did not have the required amount of venous blood collected, 1 patient had liver injury >5ULN). Twenty-nine patients were considered eligible to participate in the study (PK1). Twelve patients were lost to follow-up, 1 patient suffered liver injury, 1 patient withdrew from the study, 2 samples were withdrawn from the test due to hemolysis. Finally, 13 patients completed (PK4) (See Figure 1, Table 1).
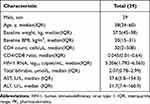 |
Table 1 Clinical Characteristics of 29 HIV-Infected Patients |
 |
Figure 1 Flow diagram of the study. Abbreviations: HIV/TB, human immunodeficiency virus/tuberculosis; ART, antiretroviral therapy; PK, pharmacokinetics; ULN, upper limit of normal value. |
Pharmacokinetics of Efavirenz 600mg
Efavirenz drug concentrations are shown in (Figure 2). In 29 patients, the average efavirenz concentration of all patients >1000ng/mL, with measurements above the threshold in all other periods. The efavirenz PK parameters measured on PK1, PK2, PK3 and PK4 are shown in (Table 2). Efavirenz Cmax and AUC0-18 decreased gradually, but tended to stabilize at PK4; C18h decreased at PK2, but rose to the maximum at PK4.
 |
Table 2 Plasma Efavirenz Pharmacokinetics |
 |
Figure 2 EFV600mg concentration–time curve. The black line represents the effective value of EFV concentration (1000ng/mL). The red arrows indicate mean EFV concentrations <1000ng/mL in 1 patient. |
HIV Virological Suppression
In the 29 patients receiving efavirenz 600mg, HIV-RNA decreased significantly at ART 2 weeks, which was significantly different from the baseline period (P < 0.001); There was no significant difference between ART 6 weeks and ART 2 weeks (P = 0.182); HIV-RNA continued to decline in ART 20 weeks, with a significant difference compared with ART 6 weeks (P < 0.001); There was no significant difference between ART 48 weeks and ART 20 weeks (P = 0.829). One patient failed ART at 6 weeks and 3 patients failed ART at 20 weeks. The ART regimen was subsequently changed (see Figures 1 and 3).
 |
Figure 3 HIV viral suppression status. |
Safety and Tolerability
During co-administration, efavirenz 600mg was well tolerated and no serious adverse events occurred. According to the withdrawn criteria, one patient withdrawn from the group due to liver injury related to efavirenz/rifampicin, and he will be required to follow up until the liver function test is completely normal. Due to the exclusion of patients with viral hepatitis infection and autoimmune liver disease, one patient withdrew from the trial due to an unexplained increase in ALT (>5ULN) before baseline, and the rest did not report drug-related adverse events.
Discussion
In this pharmacokinetic study of efavirenz 600mg, we found all patients the average concentration of efavirenz ≥1000ng/mL. We observed changes in HIV virologic suppression during follow-up, with 86% of patients showing excellent virologic response.
Our pharmacokinetic study showed that efavirenz Cmax remained in the range of 1000–4000ng/mL effective therapeutic concentrations overall when used with rifampicin. Despite a 25% reduction in efavirenz C18h during PK2, efavirenz concentrations remained above the effective therapeutic threshold. Although a decreasing trend in efavirenz Cmax and AUC0-18 was observed in PK2, a 29% increase in C18h and a 13% increase in AUC0-18 during PK3 and a maximum concentration and maximum area measured in PK4 were sufficient to maintain effective virological suppression during combination therapy. Notably, we also observed changes in efavirenz concentrations in four patients who failed antiviral therapy, only one of whom had a C18h <1000 ng/mL and the other three had a C18h >1000 ng/mL. We believe that even if patients can maintain effective efavirenz concentrations, treatment failure may still occur. Achieving the minimum effective therapeutic level of efavirenz concentration may only be the foundation for successful treatment but not the only determining factor.
Pharmacogenomics studies have shown that the concentration of efavirenz is regulated by CYP2B6, which converts efavirenz into inactive metabolites, while rifampicin is a potent CYP450 inducer that can widely induce drug-metabolizing enzymes and transporters.10 In addition, patients with HIV/TB co-infection receive multiple drugs, leading to more complex drug interactions. Clinical studies on the combination of efavirenz 600mg and rifampicin for tuberculosis treatment have shown different results, with a reduction in efavirenz concentration observed in studies on healthy volunteers.11 Lopez-Cortes et al found that the exposure to efavirenz was reduced by about 25% whether measured by area under the concentration–time curve (AUC) or peak and trough concentration,6 which is consistent with our research results. However, several studies have shown that during anti-tuberculosis treatment based on RIF, there is an abnormal increase in efavirenz concentration.12–14 Although our pharmacokinetic results of efavirenz 600mg have been validated in HIV/TB patients, in this study, we have demonstrated for the first time that efavirenz 600mg can be co-administered with rifampicin in the Chinese HIV/TB population and maintain effective virological suppression.
The packaging instructions for efavirenz approved by the US Food and Drug Administration suggest that patients with a weight of ≥50 kilograms should receive a dose of 800mg per day during treatment with rifampicin.15,16 However, some clinical trials have shown that the virological suppression effect remains good during treatment with standard doses of efavirenz and rifampicin,17–19 this is similar to the 86% virological suppression results in our study. Several studies have shown that the increasing dose of rifampicin during anti-tuberculosis treatment is safe,20–22 however, these studies were almost entirely conducted in HIV-1 negative tuberculosis patients or in HIV/TB co-infected patients without severe immune suppression who had not received highly effective antiretroviral therapy (HAART). In addition, we found that efavirenz concentration levels fluctuated greatly between the ART6 and ART24 periods, which may not support the conclusion that efavirenz dosage should be adjusted based on patient weight during anti-tuberculosis treatment with rifampicin.23,24
There are some limitations to our study, the most obvious of which is the insufficient sample size, which did not yield typical data characteristics. Finally, we did not investigate genetic polymorphism, which may have some impact on the experimental conclusions.
Conclusion
In this study, rifampicin did not affect efavirenz pharmacokinetics and virological suppression was good. Even if the concentration of EFV decreases at a certain point in time, it will not fall below the effective treatment threshold. Among Chinese patients receiving treatment for HIV/TB co-infection, most patients could achieve therapeutic efavirenz concentrations, with or without combination with rifampicin, when receiving a standard 600 mg of efavirenz daily.
Ethical Approval
The study was approved by the Committee of Ethics at Beijing Ditan Hospital, Capital Medical University, Beijing, China (Registration No.201704002).
Acknowledgments
We thank patients for their participation and dedication to HIV/TB research. We thank the staff of Beijing Ditan hospital for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Clinical characteristic applied research and achievement promotion in the capital, Name: Study on the effect of rifampicin on the blood concentration of efavirenz in HIV/TB patients (Z171100001017053).
Disclosure
The authors have no competing interests to declare.
References
1. World Health Organization. Global Tuberculosis Reports 2021. Geneva, Switzerland: World Health Organization; 2021. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021.
2. World Health Organization. Tuberculosis. Geneva, Switzerland: World Health Organization; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/tuberculosis.
3. Coelho L, Cardoso SW, Amancio RT, et al. Trends in AIDS-defining opportunistic illnesses incidence over 25 years in Rio de Janeiro, Brazil. PLoS One. 2014;9:e98666. doi:10.1371/journal.pone.0098666
4. Borand L, Madec Y, Laureillard D, et al. Plasma concentrations, efficacy and safety of efavirenz in HIV-infected adults treated for tuberculosis in Cambodia (ANRS 1295-CIPRA KH001 CAMELIA trial). PLoS One. 2014;9:e90350. doi:10.1371/journal.pone.0090350
5. Habtewold A, Makonnen E, Amogne W, et al. Is there a need to increase the dose of efavirenz during concomitant rifampicin-based antituberculosis therapy in sub-Saharan Africa? The HIV-TB pharmagene study. Pharmacogenomics. 2015;16:1047–1064. doi:10.2217/pgs.15.35
6. López-Cortés LF, Ruiz-Valderas R, Viciana P, et al. Pharmacokinetic interactions between efavirenz and rifampicin in HIV-infected patients with tuberculosis. Clin Pharmacokinet. 2002;41:681–690. doi:10.2165/00003088-200241090-00004
7. Lee KY, Lin SW, Sun HY, et al. Therapeutic drug monitoring and pharmacogenetic study of HIV-infected ethnic Chinese receiving efavirenz-containing antiretroviral therapy with or without rifampicin-based anti-tuberculous therapy. PLoS One.2014;9(2):e88497 doi:10.1371/journal.pone.0088497.
8. Acquired Immunodeficiency Syndrome and Hepatitis C Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention. Chinese Guidelines for Diagnosis and Treatment of Human Immunodeficiency Virus Infection/Acquired Immunodeficiency Syndrome(2021 edition). Medical Journal of Peking Union Medical College Hospital. 2022;13(2):203–226. doi:10.12290/xhyxzz.2022-0097
9. Keizer RJ, Zandvliet AS, Beijnen JH, Schellens JH, Huitema AD. Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses. AAPS J. 2012;14:601–611. doi:10.1208/s12248-012-9373-2
10. Zhang J, Hayes S, Sadler BM, et al. Population pharmacokinetics of dolutegravir in HIV-infected treatment-naive patients. Br J Clin Pharmacol. 2015;80:502–514. doi:10.1111/bcp.12639
11. Dooley KE, Denti P, Martinson N, et al. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis. 2015;211:197–205. doi:10.1093/infdis/jiu429
12. Dooley KE, Flexner C, Andrade AS. Drug interactions involving combination antiretroviral therapy and other anti-infective agents: repercussions for resource-limited countries. J Infect Dis. 2008;198:948–961. doi:10.1086/591459
13. Luetkemeyer AF, Rosenkranz SL, Lu D, et al. Relationship between weight, efavirenz exposure, and virologic suppression in HIV-infected patients on rifampin-based tuberculosis treatment in the AIDS Clinical Trials Group A5221 STRIDE Study. Clin Infect Dis. 2013;57:586–593. doi:10.1093/cid/cit246
14. Gengiah TN, Holford NH, Botha JH, Gray AL, Naidoo K, Abdool Karim SS. The influence of tuberculosis treatment on efavirenz clearance in patients co-infected with HIV and tuberculosis. Eur J Clin Pharmacol. 2012;68:689–695. doi:10.1007/s00228-011-1166-5
15. Orrell C, Cohen K, Conradie F, et al. Efavirenz and rifampicin in the South African context: is there a need to dose-increase efavirenz with concurrent rifampicin therapy. Antivir Ther. 2011;16:527–534. doi:10.3851/IMP1780
16. Liu J, Chan-Tack K, Jadhav P, et al. Why did the FDA approve efavirenz 800 mg when co-administered with rifampin? Int J Clin Pharmacol Ther. 2014;52:446–453. doi:10.5414/CP202079
17. Brennan-Benson P, Lyus R, Harrison T, Pakianathan M, Macallan D. Pharmacokinetic interactions between efavirenz and rifampicin in the treatment of HIV and tuberculosis: one size does not fit all. AIDS. 2005;19:1541–1543. doi:10.1097/01.aids.0000183519.45137.a6
18. McIlleron HM, Schomaker M, Ren Y, et al. Effects of rifampin-based antituberculosis therapy on plasma efavirenz concentrations in children vary by CYP2B6 genotype. AIDS. 2013;27:1933–1940. doi:10.1097/QAD.0b013e328360dbb4
19. Manosuthi W, Sungkanuparph S, Thakkinstian A, et al. Efavirenz levels and 24-week efficacy in HIV-infected patients with tuberculosis receiving highly active antiretroviral therapy and rifampicin. AIDS. 2005;19:1481–1486. doi:10.1097/01.aids.0000183630.27665.30
20. Manosuthi W, Mankatitham W, Lueangniyomkul A, Chimsuntorn S, Sungkanuparph S. Standard-dose efavirenz vs. standard-dose nevirapine in antiretroviral regimens among HIV-1 and tuberculosis co-infected patients who received rifampicin. HIV Med. 2008;9:294–299. doi:10.1111/j.1468-1293.2008.00563.x
21. Stott KE, Pertinez H, Sturkenboom M, et al. Pharmacokinetics of rifampicin in adult TB patients and healthy volunteers: a systematic review and meta-analysis. J Antimicrob Chemother. 2018;73:2305–2313. doi:10.1093/jac/dky152
22. Svensson EM, Svensson RJ, Te Brake L, et al. The Potential for Treatment Shortening With Higher Rifampicin Doses: relating Drug Exposure to Treatment Response in Patients With Pulmonary Tuberculosis. Clin Infect Dis. 2018;67:34–41. doi:10.1093/cid/ciy026
23. Bertrand J, Verstuyft C, Chou M, et al. Dependence of efavirenz- and rifampicin-isoniazid-based antituberculosis treatment drug-drug interaction on CYP2B6 and NAT2 genetic polymorphisms: ANRS 12154 study in Cambodia. J Infect Dis. 2014;209:399–408. doi:10.1093/infdis/jit466
24. Berhan A, Berhan Y, Yizengaw D. A meta-analysis of drug resistant tuberculosis in Sub-Saharan Africa: how strongly associated with previous treatment and HIV co-infection. Ethiop J Health Sci. 2013;23:271–282. doi:10.4314/ejhs.v23i3.10
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
