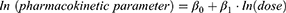Back to Journals » Drug Design, Development and Therapy » Volume 16
Pharmacokinetics and Safety of Single and Multiple Doses of Peficitinib (ASP015K) in Healthy Chinese Subjects
Authors Gao X, He X , Oshima H, Miyatake D, Otsuka Y, Kato K, Cai C, Wojtkowski T, Song N, Kaneko Y, Shi A
Received 25 January 2022
Accepted for publication 13 April 2022
Published 9 May 2022 Volume 2022:16 Pages 1365—1381
DOI https://doi.org/10.2147/DDDT.S359501
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Tuo Deng
Xin Gao,1 Xuemei He,1 Hiroyuki Oshima,2 Daisuke Miyatake,2 Yukio Otsuka,2 Kota Kato,3 Chunxiao Cai,4 Tomasz Wojtkowski,5 Nan Song,6 Yuichiro Kaneko,7 Aixin Shi1
1Clinical Trial Center, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Sciences, Beijing, People’s Republic of China; 2Clinical Pharmacology and Exploratory Development, Astellas Pharma Inc., Tokyo, Japan; 3Analysis & Pharmacokinetics Research Labs., Astellas Pharma Inc., Ibaraki, Japan; 4Development Medical Department, Astellas (China) Investment Co., Ltd, Beijing, People’s Republic of China; 5Data Science Development, Astellas Pharma US, Inc., Northbrook, IL, USA; 6Development Division Biostatistics and Statistical Programming, Astellas (China) Investment Co., Ltd, Beijing, People’s Republic of China; 7Biostatistics Group, Japan-Asia Data Science, Development, Astellas Pharma Inc, Tokyo, Japan
Correspondence: Aixin Shi, Clinical Trial Center, Beijing Hospital, National Center of Gerontology; Institute of Geriatric Medicine, Chinese Academy of Medical Science, Beijing, 100730, People’s Republic of China, Tel +86-10-85133632, Email [email protected]
Objective: To investigate the pharmacokinetics and safety of peficitinib (Janus kinase inhibitor for the treatment of rheumatoid arthritis) in healthy Chinese subjects following single and multiple doses.
Methods: This open-label, randomized study was conducted at one site in China. Subjects received peficitinib 50, 100 or 150 mg as a single dose on Day 1 (fasted) and once daily from Days 8 to 13 in the multiple-dose period (fed). Blood samples were collected before administration each day, and up to 72h post administration. Pharmacokinetic assessments included area under the concentration curve (AUC), half-life (t1/2), maximum concentration (Cmax), and time to maximum concentration (tmax) of peficitinib and its metabolites (H1, H2 and H4). Treatment-emergent adverse events (TEAEs) were evaluated.
Results: Thirty-six subjects were enrolled (12 per dose group). After a single dose of peficitinib, median tmax was 1.0– 1.5h and mean t1/2 was 7.4– 13.0h for all doses. In the multiple-dose period, median tmax was 1.5– 2.0h. Dose-proportional increases in Cmax and AUC24h were observed for peficitinib and its metabolites following single and multiple doses, with minimal drug accumulation. The major metabolite was H2, with a systemic exposure of > 150% of the parent AUC. Drug-related TEAEs were experienced by 5 (13.9%) and 12 (33.3%) subjects in the single- and multiple-dose periods, respectively. Following multiple doses of peficitinib, TEAEs were more frequent in higher than lower dose groups but were mild in severity with no related discontinuation or death.
Conclusion: Following single and multiple doses of peficitinib in healthy Chinese subjects, peficitinib demonstrated rapid absorption and was well tolerated at all doses.
Clinicaltrials.gov Identifier: NCT04143477.
Keywords: rheumatoid arthritis, treatment, dose-proportionality, tsDMARDs
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that causes systemic inflammation of the synovial tissues of various joints.1 The primary goal of RA therapy is to reduce disease activity and achieve remission by controlling typical complications of RA such as inflammation, loss of function, pain and reduced patient quality of life.2–4
For patients who have had an inadequate response to standard RA therapies, such as methotrexate (MTX), alternative treatment options are increasing.5 The 2019 EULAR recommendations revised the preference of biological disease-modifying antirheumatic drugs (bDMARDs) over targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs) in the treatment of RA because of new evidence supporting the long-term efficacy and safety of Janus kinase inhibitors (JAKis). As such, there is no preference in the guidelines between bDMARDs and tsDMARDs for early combination therapy with conventional DMARDs (such as MTX).5 However, the continued development of agents with alternative modes of action, such as tsDMARDs (including JAKis), is important to further expand upon the current treatment options available for patients with RA.4–9
Peficitinib is a novel oral tsDMARD that has pan-Janus kinase (JAK) inhibitory activity. It has been shown to be effective as a monotherapy in RA treatment in a randomized phase IIb study at doses of 25, 50, 100, and 150 mg/day,10 and in combination with other DMARDs or MTX in two randomized phase III studies at doses of 100 and 150 mg/day,11,22 with good tolerability over a 2-year treatment period.12 Peficitinib has been approved for the treatment of patients with RA in Japan (2019) and Taiwan (2020).13–15 It has also demonstrated rapid dose-proportional absorption after single- and multiple-dose administration;16 however, absorption is delayed by a food effect following a moderate- to high-fat meal.17
The pharmacokinetic (PK) profiles of peficitinib and its key plasma metabolites (H1, H2 and H4, shown to have very weak pharmacological activity in a cell-based assay of T-cell proliferation)13,18 have been examined extensively under fasted and fed conditions (for single- and multiple-dose administration, respectively), in two phase I randomized, placebo-controlled studies in the US19 and a phase I randomized, placebo-controlled study in Japan; with significant differences in plasma concentrations observed between subjects of different ethnicities.16,17 In the latter study, Cmax was 45.7–98.8% higher and AUCinf was 33.8–66.4% higher in Japanese versus Caucasian subjects.16 Additionally, peficitinib was well tolerated and dose-proportional exposure was demonstrated in each study.
This study was performed to expand upon the PK profile and safety data for peficitinib after single- and multiple-dose administration of 50, 100, and 150 mg peficitinib in healthy Chinese subjects.
Methods
Ethics
The study was reviewed and approved by the Ethics Committee of Beijing Hospital (2019BJYYEC-093-01), and conducted in accordance with applicable Chinese/International Conference on Harmonization (ICH) Good Clinical Practices (GCP) guidelines, applicable regulations and guidelines governing clinical study conduct, the ethical principles that have their origin in the Declaration of Helsinki, and CIOMS International Ethical Guidelines. All subjects provided written informed consent before undergoing any study-related procedures.
Study Design
This was an open-label, randomized study, conducted between December 2019 and December 2020 at one site in China. The aim of this study was to investigate the PK profile and safety of peficitinib in healthy Chinese subjects after single and multiple doses of 50, 100, and 150 mg peficitinib, using 50 mg tablets.
Study Subjects
Eligible subjects were healthy Chinese male or female adults aged ≥18–45 years, with a body mass index (BMI) >19 kg/m2 and ≤24 kg/m2, and a weight of ≥50 kg for males and ≥45 kg for females, at screening. Exclusion criteria included pregnancy (current or <6 months prior to screening); hypersensitivity to peficitinib (or formulation) and liver chemistry tests (aspartate aminotransferase [AST], alanine aminotransferase [ALT], alkaline phosphatase [ALP], gamma-glutamyl transferase [GGT] and total bilirubin [TBL]) above the upper limit of normal on Day −1.
Study Drug and Administration
Subjects were randomly allocated to one of three dose groups (50, 100, and 150 mg), with 6 male and 6 female subjects assigned to each group. Subjects were then sequentially enrolled into a single-dose period followed by a multiple-dose period. Study flow is described in Figure 1. On the morning of Day 1 (single-dose period), subjects were fasted for ≥10 hours prior to receiving a single oral dose of peficitinib with 150 mL warm water. Blood samples were collected immediately prior to administration and at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, 60, and 72 hours after administration of peficitinib.
 |
Figure 1 Study flow diagram. |
The multiple-dose period was from Day 8 through Day 13, during which time subjects received peficitinib once daily, in the morning, for 6 consecutive days. On Days 8–13, subjects were fed in unison approximately 30 minutes prior to receiving a single oral dose of peficitinib with 150 mL warm water. Blood samples were collected immediately prior to administration on each day. Blood samples were also collected on Day 8, at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours after administration, and on Day 13, at 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 36, 48, 60, and 72 hours after administration.
Physical examination, vital sign measurements, 12-lead electrocardiogram (ECG), and clinical laboratory evaluations (including hematology, clinical chemistry, serology, urinalysis, and pregnancy test) were conducted at pre-specified times throughout the study at the local laboratory of the clinical center.
Sample Analyses for Peficitinib and Metabolites
Plasma concentrations of peficitinib and metabolite H4 were simultaneously measured using a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) method. Peficitinib, metabolite H4, and the internal standard (IS), which was stable isotope label ([D3]-peficitinib), were extracted by supported liquid extraction (SLE) using Isolute SLE+ 96-well plate (Biotage, Uppsala, Sweden), and separated by a column of BetaSil Silica, 5 μm, 50×3.0 mm (Thermo Fisher Scientific, Waltham, MA, USA). Detection was performed via API5500 mass spectrometer (AB Sciex, Framingham, MA, USA) using positive Turbo ion spray ionization. The lower limit of quantification (LLOQ) was 0.25 ng/mL, when 70 μL of plasma was used. Calibration curves for peficitinib and metabolite H4 in human plasma were linear over the concentration range 0.25–250 ng/mL. The inter-run accuracy of peficitinib and metabolite H4 varied between −2.3% and 4.1%, while the inter-run precision ranged between 2.8% and 4.9%.
Plasma concentrations of metabolites H1 and H2 were simultaneously measured using a validated LC-MS/MS method. Metabolites H1 and H2, and the ISs, which were stable isotope labels (metabolites [D3]-H1 and [D3]-H2), were extracted by protein precipitation and separated by a column of Acquity UPLC BEH Amide, 1.7 µm, 100×2.1 mm (Waters, Milford, MA, USA). Detection was performed via API6500 mass spectrometer (AB Sciex) using positive Turbo ion spray ionization. The LLOQ was 0.25 ng/mL, when 50 μL of human plasma was used. Calibration curves for metabolites H1 and H2 in plasma were linear over the concentration range 0.25–250 ng/mL. The inter-run accuracy of metabolites H1 and H2 varied between 2.5% and 4.7%, while the inter-run precision ranged between 3.4% and 5.6%.
Pharmacokinetic Assessments
The PK profile of peficitinib and its metabolites were assessed based on the following:
Plasma Peficitinib Concentration and PK Parameters
- Day 1 (single-dose): AUCinf, AUClast, AUC24h, CL/F, Cmax, Lambdaz, t1/2, tmax, and Vz/F
- Day 8 (multiple-dose): AUClast, AUC24h, Cmax, and tmax
- Day 9 through Day 12 (multiple-dose): Ctrough
- Day 13 (multiple-dose): AUC24h, CL/F, Ctrough, C24h, Cmax, Lambdaz, t1/2, tmax, peak-trough ratio (PTR), Rac(AUC24h), and Rac(Cmax)
Plasma Metabolite (H1, H2 and H4) Concentrations and PK Parameters
- Day 1 (single-dose): AUCinf, AUClast, AUC24h, Cmax, Lambdaz, t1/2, tmax, and metabolite/parent ratio (MPR)
- Day 8 (multiple-dose): AUClast, AUC24h, Cmax, tmax, and MPR
- Day 9 through Day 12 (multiple-dose): Ctrough
- Day 13 (multiple-dose): AUC24h, Ctrough, C24h, Cmax, Lambdaz, t1/2, tmax, PTR, Rac(AUC24h), Rac(Cmax), and MPR
Safety Assessments
The incidence, nature, and severity of treatment-emergent adverse events (TEAEs), clinical laboratory tests (hematology, chemistry, urinalysis), vital signs, 12-lead ECG, and physical examination findings were evaluated throughout the study, to Day 20 ± 2. TEAEs were defined as any untoward medical occurrence in a subject following administration of the study drug that did not necessarily have a causal relationship with treatment.
Statistical Analyses
Planned Sample Size
The recommended population size for a PK study, according to National Medical Products Administration (NMPA) guidelines, is 8 to 12 subjects per dose group. In this study, a total of 36 healthy subjects (6 males and 6 females per 50, 100, and 150 mg dose groups) were planned to be enrolled to ensure a minimum of 24 subjects (4 males/4 females per dose group). No formal statistical sample size calculation was performed in this study.
Two analysis sets were included in this study: the safety analysis set (SAF), consisting of all subjects who received ≥1 dose of peficitinib; and the PK analysis set (PKAS), consisting of all subjects who received ≥1 dose of peficitinib and provided ≥1 estimable PK parameter.
Pharmacokinetic Analysis
Analyses performed on the PKAS were plasma concentrations and PK parameters for peficitinib and its metabolites, with data summarized descriptively at each time point.
PK parameters were calculated from plasma concentrations of peficitinib and its metabolites, and elapsed times from last dosing, using a non-compartmental analysis method. Half-life (t1/2) was calculated from the slope of the elimination phase of the plasma concentration curve.
Dose proportionality was evaluated by Cmax, AUClast, and AUCinf for single-dose administration (Day 1) and Cmax and AUC24h for multiple-dose administration (Day 13) using the following linearization of the power model:
where β0 was the intercept and β1 was the slope. Dose proportionality was to be declared if the 90% confidence interval (CI) for β1 lay entirely within the critical region (1+[ln 0.5]/[ln r], 1+[ln 2]/[ln r]), where r was the ratio of the highest and the lowest dose used in the model.
Gender differences were examined by treatment group for natural log-transformed Cmax, AUClast, and AUCinf for single-dose administration (Day 1) and Cmax and AUC24h for multiple-dose administration (Day 13). Steady-state was assessed by visual inspection of line plots of individual and mean ± standard deviation (SD) Ctrough of peficitinib and its metabolites from Day 9–13.
Safety Analysis
Safety analyses were performed on the SAF, and the proportions of subjects with TEAEs (including drug-related TEAEs and TEAEs leading to discontinuations) were summarized for single- and multiple-dose periods. Similarly, laboratory, vital sign and 12-lead ECG data (with change from baseline), were also summarized separately for both single- and multiple-dose periods. The proportion of normal/abnormal ECG data were shown for each time point in both single- and multiple-dose periods.
Results
Subject Dispositions
Overall 221 subjects provided informed consent, and 185 failed the screening procedure (subjects had clinically significant abnormalities, as determined by the investigator at screening or Day −1). In total, 36 subjects were enrolled and completed the study, with no discontinuations across the study period. Twelve subjects (6 males and 6 females) were assigned to each peficitinib dose group (50, 100, and 150 mg).
Subject Demographics and Baseline Characteristics
In accordance with the study protocol, 50% of subjects were male, with mean (SD) age 30.4 (7.71) years and mean (SD) BMI 21.5 (1.2) kg/m2 at screening (Table 1). No subjects had high levels of substance abuse (≥10 cigarettes or ≥21 units of alcohol per day within 3 months prior to admission, or any prior history of drug abuse) (data not shown). Additionally, demographic and other baseline characteristics were similar between the peficitinib 50, 100, and 150 mg dose groups.
 |
Table 1 Patient Demographics and Baseline Characteristics (SAF) |
Pharmacokinetics
Peficitinib Plasma Concentration and PK Parameters
In the single-dose period (Day 1), peficitinib demonstrated rapid absorption with a median tmax of 1.0–1.5 hours after administration (fasted state), and plasma levels declined with a mean t1/2: 7.4–13.0 hours across all dose groups (Table 2). Peficitinib also showed a Vz/F of 718–1548 L across all dose groups with high variability (coefficient of variation, CV >59%). In the multiple-dose period (Days 8–13), median tmax was delayed versus the single-dose period, ranging from 1.5–2.0 hours after peficitinib administration (fed state). Peficitinib showed no accumulation by Day 13, with ratios Rac[AUC24h] and Rac[Cmax] close to 1. Mean trough plasma peficitinib concentrations showed a slight increase towards Day 13, with steady state achieved by Day 11 (Supplementary Figure 1).
 |
Table 2 Peficitinib Pharmacokinetic Parameters (PKAS) |
In both single- and multiple-dose periods, a dose-proportional increase in Cmax and AUC24h was observed across all peficitinib dose groups (Supplementary Table 1), with moderate to low variability (CV 14.8–38.7%) (Table 2). Mean concentration-time profiles of peficitinib on Day 1, Day 8, and Day 13 are shown in Figure 2.
 |
Figure 2 Mean (±SD) concentration-time profiles of peficitinib on Day 1, Day 8, and Day 13 (PKAS). SD was not calculated since >50% of values were below the quantitation limit at a given time point. |
Peficitinib Metabolite (H1, H2 and H4) Plasma Concentration and PK Parameters
In both single- and multiple-dose periods, the median tmax for metabolite formation was 3 hours each for H1 and H4, and 2 hours for H2, with a mean t1/2 of 5.3–13.7 hours across all peficitinib dose groups (Table 3). For each metabolite, a dose-proportional increase in Cmax and AUC24h was observed in both single- and multiple-dose periods and across all dose groups (Supplementary Table 1), with minimal to no accumulation (Rac[AUC24h] and Rac[Cmax] of 0.81–1.15 hours).
 |
Table 3 Peficitinib Metabolite Pharmacokinetic Parameters (SAF) |
For metabolite H2, the MPR ranged from 1.52–1.93 indicating systematic exposure of >150% for peficitinib AUC across all peficitinib dose groups (Table 3). However, neither metabolite H1 nor H4 demonstrated MPR exceeding 0.3 in either the single- or multiple-dose periods.
In the multiple-dose period, mean trough concentrations of each metabolite increased from Day 9 until Day 10, with steady state achieved by Day 11 at the latest across almost all peficitinib dose groups (Supplementary Figures 2–4). Mean concentration-time profiles of each metabolite (H1, H2 and H4) on Day 1, Day 8, and Day 13 are shown in Figure 3. Figure 3 Continued. Figure 3 Continued.

Gender Analysis
There were no meaningful gender differences observed in the peficitinib or metabolite H2 PK profiles in either the single- or multiple-dose periods, with GMRfemale/male of 79.7–134.1% and 78.4–129.5%, respectively (Supplementary Table 2).
For metabolites H1 and H4, a higher exposure was observed in females than males following single-dose and multiple-dose administration of peficitinib across all doses, with GMRfemale/male of 124.6–185.8% and 112.1–207.6%, respectively.
Safety
Adverse Events
In the single-dose period, 6 (16.7%) subjects experienced 12 TEAEs, of whom 5 (13.9%) subjects experienced 9 TEAEs considered to be drug related (Table 4). The most frequent drug-related TEAEs were headaches (2 events in 2 [5.6%] subjects) and coughs (2 events in 2 [5.6%] subjects) (Table 5).
 |
Table 4 Summary of Adverse Events for Single- and Multiple-Dose Periods (SAF) |
 |
Table 5 Drug-Related TEAEs During the Single and Multiple-Dose Periods (SAF) |
In the multiple-dose period, 12 (33.3%) subjects experienced 24 TEAEs, of which 22 TEAEs were considered to be drug-related. TEAEs occurred more frequently for peficitinib 100 and 150 mg dose groups than for peficitinib 50 mg, and were recorded in 4 (33.3%), 7 (58.3%), and 1 (8.3%) subjects, respectively (Table 4). The most frequent drug-related TEAEs were nausea (5 events in 5 [13.9%] subjects), dizziness (4 events in 2 [5.6%] subjects), increased blood cholesterol (2 events in 2 [5.6%] subjects), dyspepsia (3 events in 2 [5.6%] subjects) and arthralgia (2 events in 2 [5.6%] subjects) (Table 5).
All TEAEs were mild in severity, with no deaths, severe adverse events or TEAEs leading to discontinuation in this study.
Laboratory Parameters
Overall, five subjects recorded clinically significant abnormalities from clinical laboratory evaluations, vital signs, physical examination, 12-lead ECG or chest X-ray in this study. However, no clinically meaningful findings related to peficitinib were identified in the single-dose period. Three subjects with increased cholesterol or bilirubin were identified from clinical chemistry evaluations in the multiple-dose period, which were considered possibly or probably related to peficitinib (Supplementary Table 3).
Discussion
Ethnicity has been previously shown to be responsible for variability in the pharmacokinetics and pharmacodynamics of drugs, which can lead to alterations in their safety and efficacy.20 As a result, the PK profile of peficitinib has been well explored in the Caucasian and Japanese populations.16–19,21 In this study, the PK profile of peficitinib was investigated extensively in 36 healthy Chinese subjects.
In this study, peficitinib demonstrated rapid absorption after single-dose administration with a median tmax of 1.0–1.5 hours (fasted state), which was slightly delayed to 1.5–2.0 hours following multiple-dose administration (fed state). This is supported by a previous study in healthy Japanese subjects, in which a single dose of peficitinib 150 mg was rapidly absorbed with a mean tmax of 1.6 hours in the fasted state, extending to 2.0 hours in the fed state.17 Elimination of peficitinib from blood plasma after both single- and multiple-dose administration at all dose levels in our study (mean t1/2 7.4–13.0 hours) was similar to that observed after single-dose administration of peficitinib 200 mg (mean t1/2 7.7–12.9 hours) in healthy Caucasian subjects.19 Studies in healthy Caucasian and Japanese subjects have also demonstrated similar mean tmax after both single- and multiple-dose administration of peficitinib.16,18,19,21 Additionally, although t1/2 in this study had high variability, this trend has also been observed between related PK studies. Cao et al, reported t1/2 of 14.2 (±8.9) hours and 17.7 (±9.4) hours following multiple-dose administration of peficitinib 100 mg for male and female subjects respectively;19 Shibata et al, reported t1/2 of 12.0 (±11.3) hours and 9.3 (±7.8) hours following single dose administration of peficitinib 150 mg for fasted and fed subjects respectively.17
Dose-proportional increases in Cmax and AUC24h were observed in the present study for peficitinib and its metabolites, and across all peficitinib dose groups, with minimal or no accumulation. Since t1/2 was also short at all dose levels, the influence of half-life to clinical efficacy was considered to be minor. Clear dose-proportional increases in peficitinib plasma concentration were also observed in studies of healthy Japanese and Caucasian subjects after single-dose administration.16
In general, exposure to peficitinib was higher for multiple- versus single-dose administration (excluding the 50 mg dose group on Day 1 versus Day 8), with low variability in both groups. Metabolites H1 and H4 were minor metabolites, with relative systemic exposures <30% of the parent peficitinib (MPR <0.3). However, metabolite H2 was a major metabolite, with a relative systemic exposure of >150% (MPR >1.5) of the parent peficitinib AUC values. This is supported by single dose peficitinib studies in healthy Japanese subjects, which showed similar MPRs for metabolites H1 and H4, and MPR >1.8 for H2.18,21 However, since the pharmacological activity of metabolites H1, H2 and H4 were very weak, and the active form in vivo is peficitinib,13 exposure of those metabolites was considered to be of no clinical significance.
The impact of gender has been examined previously in healthy Caucasian subjects, with negligible effect observed on pharmacokinetics, pharmacodynamics or safety profiles after either single or multiple doses of peficitinib.19 Similarly, for Chinese subjects in the current study, no marked gender differences were observed in PK parameters after single- and multiple-dose administration of peficitinib across all doses studied.
Overall, peficitinib was well tolerated in Chinese subjects at all doses in the study. Although TEAEs were observed to occur more frequently in higher than lower dose groups, all TEAEs were mild in severity, with no deaths or TEAEs that led to discontinuation. Following single-dose administration of peficitinib, five subjects recorded TEAEs considered to be drug related by the investigator (most frequently headaches and coughs). Following multiple-dose administration of peficitinib, 12 subjects reported TEAEs considered to be drug-related (primarily nausea, increased blood cholesterol, dyspepsia and arthralgia). Decreased neutrophil counts reported in similar studies in healthy Caucasian and Japanese subjects16,19 were not observed in this study. Additionally, no new clinically meaningful abnormal laboratory findings were identified after single-dose administration.
Limitations of this study include the short study duration. Since RA is a chronic disease, peficitinib is typically administered for extended periods, therefore long-term safety assessments would be of greater clinical relevance for Chinese patients. Additionally, these data are restricted to healthy individuals who are relatively young. This is not necessarily representative of the age demographic for the RA patient population in which peficitinib is indicated, many of whom have also comorbidities and are receiving concomitant medications.19 Primary strengths of this study include a study population comprised of both male and female subjects; PK studies commonly have only male subjects. Also, this was the first study to assess the PK profile of peficitinib in Chinese subjects. A further study is also currently ongoing (NCT03660059) investigating the safety and efficacy of peficitinib in patients with RA and an inadequate response to methotrexate in China.
In this study of peficitinib in healthy subjects in China, peficitinib was rapidly absorbed with plasma concentration varying in a dose-dependent manner, for both single- and multiple-dose administration. Several PK parameters (median tmax, t1/2, and dose-proportional increases in Cmax and AUC24h) for single and multiple doses of peficitinib 50, 100, and 150 mg were similar to those reported for Caucasian and Japanese subjects in previous studies. Peficitinib was well tolerated at all doses in the study.
Data Sharing Statement
Researchers may request access to anonymized participant level data, trial level data, and protocols from Astellas sponsored clinical trials at www.clinicalstudydatarequest.com. For the Astellas criteria on data sharing see: https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Astellas.aspx.
Acknowledgments
This study was initiated and supported by Astellas Pharma Inc. Medical writing support was provided by Glen Dorrington, PhD, for Lumanity, funded by Astellas Pharma Inc.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
DM, HO, YO, YK, KK and TW are employees of Astellas Pharma Inc. CC and NS are employees of Astellas Pharma China, Inc. AS, XG and XH have nothing to disclose. The authors report no other conflicts of interest in this work.
References
1. O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med. 2004;350(25):2591–2602. doi:10.1056/NEJMra040226
2. Matcham F, Scott IC, Rayner L, et al. The impact of rheumatoid arthritis on quality-of-life assessed using the SF-36: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):123–130. doi:10.1016/j.semarthrit.2014.05.001
3. Ji J, Zhang L, Zhang Q, et al. Functional disability associated with disease and quality-of-life parameters in Chinese patients with rheumatoid arthritis. Health Qual Life Outcomes. 2017;15(1):89. doi:10.1186/s12955-017-0659-z
4. Taylor PC, Moore A, Vasilescu R, Alvir J, Tarallo M. A structured literature review of the burden of illness and unmet needs in patients with rheumatoid arthritis: a current perspective. Rheumatol Int. 2016;36(5):685–695. doi:10.1007/s00296-015-3415-x
5. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/ANNRHEUMDIS-2019-216655
6. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018;320(13):1360–1372. doi:10.1001/jama.2018.13103
7. Kameda H, Fujii T, Nakajima A, et al. Japan College of Rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod Rheumatol. 2019;29(1):31-40. doi:10.1080/14397595.2018.1472358
8. England BR, Tiong BK, Bergman MJ, et al. 2019 update of the American College of Rheumatology recommended rheumatoid arthritis disease activity measures. Arthritis Care Res. 2019;71(12):1540–1555. doi:10.1002/acr.24042
9. Tian X, Wang Q, Li M, et al. 2018 Chinese guidelines for the diagnosis and treatment of rheumatoid arthritis. Rheumatol Immunol Res. 2021;2(1):1–14. doi:10.2478/rir-2021-0002
10. Takeuchi T, Tanaka Y, Iwasaki M, Ishikura H, Saeki S, Kaneko Y. Efficacy and safety of the oral Janus kinase inhibitor peficitinib (ASP015K) monotherapy in patients with moderate to severe rheumatoid arthritis in Japan: a 12-week, randomised, double-blind, placebo-controlled phase IIb study. Ann Rheum Dis. 2016;75(6):1057–1064. doi:10.1136/annrheumdis-2015-208279
11. Tanaka Y, Takeuchi T, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequate response to conventional DMARDs: a randomised, double-blind, placebo-controlled phase III trial (RAJ3). Ann Rheum Dis. 2019;78(10):1320–1332. doi:10.1136/annrheumdis-2019-215163
12. Takeuchi T, Tanaka Y, Tanaka S, et al. Safety and effectiveness of peficitinib (ASP015K) in patients with rheumatoid arthritis: interim data (22.7 months mean peficitinib treatment) from a long-term, open-label extension study in Japan, Korea, and Taiwan. Arthritis Res Ther. 2020;22(1):47. doi:10.1186/s13075-020-2125-2
13. Pharmaceuticals and Medical Devices Agency (PMDA) Japan. Report on the Deliberation Results: Smyraf tablets® 50 mg and 100 mg. Available from: https://www.pmda.go.jp/files/000233074.pdf. Accessed May 03, 2022.
14. Astellas Pharma Taiwan, Inc. Drug details: 50 mg Smyraf (peficitinib hydrobromide). Available from: https://info.fda.gov.tw/MLMS/H0001D.aspx?Type=Lic&LicId=52027856.
15. Astellas Pharma Taiwan, Inc. Drug details: 100 mg Smyraf (peficitinib hydrobromide); 2020. Available from: https://info.fda.gov.tw/MLMS/H0001D.aspx?Type=Lic&LicId=52027857.
16. Shibata M, Hatta T, Saito M, et al. Pharmacokinetics, pharmacodynamics, and safety of peficitinib (ASP015K) in healthy male Caucasian and Japanese subjects. Clin Drug Investig. 2020;40(5):469–484. doi:10.1007/s40261-020-00910-w
17. Shibata M, Toyoshima J, Kaneko Y, et al. The bioequivalence of two peficitinib formulations, and the effect of food on the pharmacokinetics of peficitinib: two way crossover studies of a single dose of 150 mg peficitinib in healthy volunteers. Clin Pharmacol Drug Dev. 2021;
18. Miyatake D, Shibata T, Shibata M, et al. Pharmacokinetics and safety of a single oral dose of peficitinib (ASP015K) in Japanese subjects with normal and impaired renal function. Clin Drug Investig. 2020;40(2):149–159. doi:10.1007/s40261-019-00873-7
19. Cao YJ, Sawamoto T, Valluri U, et al. Pharmacokinetics, pharmacodynamics, and safety of ASP015K (peficitinib), a new Janus kinase inhibitor, in healthy subjects. Clin Pharmacol Drug Dev. 2016;5(6):435–449. doi:10.1002/cpdd.273
20. Yasuda SU, Zhang L, Huang SM. The role of ethnicity in variability in response to drugs: focus on clinical pharmacology studies. Clin Pharmacol Ther. 2008;84(3):417–423. doi:10.1038/clpt.2008.141
21. Miyatake D, Shibata T, Toyoshima J, et al. Pharmacokinetics and safety of a single oral dose of peficitinib (ASP015K) in Japanese subjects with normal and impaired hepatic function. Clin Pharmacol Drug Dev. 2020;
22. Takeuchi T, Tanaka Y, Tanaka S, et al. Efficacy and safety of peficitinib (ASP015K) in patients with rheumatoid arthritis and an inadequateresponse to methotrexate: results of a phase III randomised, double-blind, placebo-controlled trial (RAJ4) in Japan. Ann Rheum Dis. 2019;78
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.